Abstract
Coronary artery disease (CAD) in very young individuals is a rare disease associated with poor prognosis. However, the role of specific lipoprotein subfractions in very young CAD patients (≤45 years) is not established yet. A total of 734 consecutive CAD subjects were enrolled and were classified as very early (n = 81, ≤45), early (n = 304, male: 45–55; female: 45–65) and late (n = 349, male: >55; female: >65) groups. Meanwhile, a group of non-CAD subjects were also enrolled as controls (n = 56, ≤45). The lipoprotein separation was performed using Lipoprint System. As a result, the very early CAD patients have lower large high-density lipoprotein (HDL) subfraction and higher small low-density lipoprotein (LDL) subfraction (p < 0.05). Although body mass index was inversely related to large HDL subfraction, overweight did not influence its association with very early CAD. In the logistic regression analysis, large HDL was inversely [OR 95% CI: 0.872 (0.825–0.922)] while small LDL was positively [1.038 (1.008–1.069)] related to very early CAD. However, after adjusting potential confounders, the association was only significant for large HDL [0.899 (0.848–0.954)]. This study firstly demonstrated that large HDL subfraction was negatively related to very early CAD suggestive of its important role in very early CAD incidence.
Similar content being viewed by others
Introduction
The prevalence of coronary artery disease (CAD) has increased sharply and manifested a younger trend, which has becoming an important public health issue1. Although it has been estimated that less than 10% of all individuals presenting with documented CAD are in very young ages, it can have devastating consequences for these patients, their families and society due to the high morbidity and long-term mortality2.
Till now, the extent of clinical risk factors for CAD occurrence in the young population has been difficult to determine. In terms of traditional risk factors, there is no unique one present in large groups of young adults with CAD3. Previous epidemiological studies indicated that the relatively more important risk factors in young patients are their elevated body mass index (BMI), smoking habits, hypertension and specifically, dyslipidemia4. Currently, the treatment of dyslipidemia has been established as one of the principal targets in clinical practice due to its key role in the development of CAD5,6. However, despite the major advances in the treatment of dyslipidemia, such as the low-density lipoprotein (LDL) cholesterol (LDL-C) lowering7,8 and high-density lipoprotein (HDL) cholesterol (HDL-C) raising9,10 strategies, residual cardiovascular risk remains high in a significant number of patients11. Promisingly, recent studies demonstrated that the cholesterol content of LDL or HDL particles displays a large inter-individual variation12,13.
Although the dysfunction of lipid metabolism is a major contributor for CAD development and progression, lipoprotein subfractions have been suggested to be more precisely reflecting the atherogenity of lipids. Recently, our group demonstrated that patients with CAD have relatively lower large HDL subfraction and higher small HDL and LDL subfraction, providing new perspectives with regard to the role of different lipoprotein subfractions in the CAD prevalence14. In light of the specialization of patients with CAD in young ages, we hypothesized that the distribution and impact of lipoprotein subfractions in younger CAD patients may be varied with those in older ones. However, such data has been unavailable till now.
Therefore, the aim of the present study was to compare LDL and HDL subfractions separated by Lipoprint System among controls without CAD (≤45), very early CAD (≤45), early (male: 45–55; female: 45–65) and late CAD (male: >55; female: >65) patients. Furthermore, we also aimed to assess the influence of different lipoprotein subfractions on very early CAD (≤45 years of age) susceptibility.
Methods
Study design and population
The study complied with the Declaration of Helsinki and was approved by the hospital’s ethical review board (FuWai Hospital & National Center for Cardiovascular Diseases, Beijing, China). Each participant provided written, informed consent before enrollment.
From October 2012 to June 2015, we consecutively recruited 734 patients with angiography proven CAD and a total of 56 non-CAD controls (≤45 years of age) in our institution. All the enrolled CAD patients were classified into three groups: very early CAD (≤45 years of age, n = 81), early CAD (male: 45–55 years of age; female: 45–65 years of age, n = 304) and late CAD (male: >55 years of age; female: >65 years of age, n = 349) groups. Considering the potential influence of lipid lowering drugs on plasma levels of lipid profiles as well as lipoprotein subfractions, we only included patients who were not on the treatment of statins and/or other lipid-lowering drugs at least 3 months before entering the study. Exclusion criteria were subjects over 90 years, pregnancy or lactation, psychiatric disorder, the existence of any infectious or systematic inflammatory disease within 1 month, acute coronary syndrome, serious heart failure or arrhythmia, significant hematologic disorders, thyroid dysfunction, severe liver dysfunction (aspartate aminotransperase or alanine aminotrabsferase three times more than the upper normal limits) and/or renal insufficiency (blood creatinine > 1.5 mg/dL) and malignant tumors.
As depicted in our previous studies15, the traditional risk factors were defined as follows. Hypertension was defined as repeated blood pressure measurements ≥140/90 mmHg (at least two times in different environments) or self-reported hypertension and currently taking anti-hypertensive drugs. Diabetes mellitus (DM) was defined as a fasting serum glucose level ≥126 mg/dL in multiple determinations, and/or the current use of medication for diabetes. Dyslipidemia was defined by medical history or fasting total cholesterol (TC) ≥200 mg/dL or triglyceride (TG) ≥150 mg/dL. BMI was calculated as weight (kg) divided by height (m) squared. Overweight was defined as BMI ≥ 25 kg/m2.
Biochemical and clinical analyses
Fasting blood samples were collected in pre-cooled EDTA tubes at baseline from each patient. After centrifugation at 3000 rpm for 15 min at 4 °C, all plasma aliquots were stored in our laboratory at −80 °C and were not thawed until use. The plasma levels of LDL-C and HDL-C were analyzed directly by selective solubilization method (Low density lipid cholesterol test kit or Determiner L HDL, Kyowa Medex, Tokyo). TC and TG were measured by enzymatic methods. All of the lipid profiles were determined using automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan).
LDL and HDL subfraction analysis
The cholesterol contents of LDL and HDL subfractions were determined electrophoretically by the Lipoprint System (Lipoprint LDL System and Lipoprint HDL System, respectively; Quantimetrix Corporation, Redondo Beach, CA, USA) according to the manufacturer’s instructions as described elsewhere16,17. This method was based on electrophoresis of a liquid loading gel with lipophilic dye in the precast linear polyacrylamide gel (stacking gel and separating gel). For LDL particle, a typical Lipoprint profile of decreasing size and increasing density with 1 very low density lipoprotein (VLDL) band, 3 Midbands, up to 7 LDL bands and 1 HDL band were obtained. The various stained bands (lipoprotein subfractions) presented in the sample were identified by their electrophoretic mobility (Rf) using VLDL as the starting reference point (Rf = 0) and HDL as the leading reference point (Rf = 1). Seven LDL subfractions were obtained. Subfraction 1 represented large LDL particles, subfraction 2 indicated medium LDL particles and subfractions 3–7 were defined as small dense LDL particles. Similarly, for HDL particle, the Lipoprint HDL system using VLDL/LDL as the starting reference point (Rf = 0) and albumin as the leading reference point (Rf = 1). Between the two points, 10 HDL subfractions were obtained. Subfractions 1–3 represented large HDL particles, subfractions 4–7 indicated medium HDL particles and subfractions 8–10 meant small HDL particles. The cholesterol concentration (mg/dL) of each lipoprotein subfraction and the mean LDL particle size (Å) were determined by this assay.
Statistical analysis
The data were expressed as the mean ± SD for the continuous variables and the number (percentage) for the categorical variables. The student t test, one-way analysis of variance, or non-parametric test was used for the comparison between/among groups of continuous parameters as appropriate. The categorical variables were compared using the chi-square test. Multivariate logistic regression analysis was used for determining the association of LDL or HDL subfractions with the incident of very early CAD susceptibility. A p value of less than 0.05 was considered statistically significant. Statistical studies were carried out with the SPSS program (version 19.0, SPSS, Chicago, Illinois, USA).
Results
Summary of Study Subjects
The baseline demographics and clinical characteristics of the study population at baseline were shown in Table 1. Overall, the enrolled subjects were classified into four groups according to the presence of CAD and the age of CAD onset. Significantly, compared with the non-CAD controls (with a mean age of 41.1 ± 2.9 years old), the very early CAD patients have higher BMI levels, higher percentage of hypertension and dyslipidemia. Meanwhile, in comparison with the relatively older CAD patients, the very early cases were more likely to be more male gender, to have a higher BMI level, diastolic blood pressure, current smokers and family history of CAD. However, the levels of inflammatory markers such as white blood cell count, fibrinogen and high-sensitivity C reactive protein (all p > 0.05) were similar among groups except for the lower concentrations of D-dimer (p < 0.001) and erythrocyte sedimentation rate (p = 0.004).
The angiographic characteristics of CAD participants according to age were presented in Table 2. The left anterior descending artery was less frequently involved (86.1% vs. 87.2% vs. 92.9%, p = 0.030) while the other related arteries were similar (p > 0.05) in the very early patients compared with early and late cases. In addition, compared to the early and late CAD patients, the very early cases have higher percentage of single artery disease (39.0% vs. 31.4% vs. 24.6%, p = 0.023) and less total number of diseased vessels (2.04 ± 0.98 vs. 2.15 ± 0.96 vs. 2.34 ± 0.95, p = 0.009).
HDL and LDL subfractions in very early CAD patients
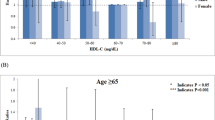
To exclude the potential impact of age on the distribution of lipoprotein subfractions, we analyzed these parameters in very young patients with and without CAD (≤45 years of age). We finally observed that the very early CAD patients have relatively lower large HDL and higher medium and small LDL subfractions (p < 0.05, all) (Fig. 1). Moreover, by contrast to early and old CAD patients, as shown in Table 3, the very early CAD cases have higher mean concentrations of TG, TC and LDL-C but lower levels of HDL-C (all p < 0.01). Regarding to the lipoprotein subfraction analysis, we found that the concentrations of large and medium HDL subfraction (both cholesterol levels and percentages) were significantly lower in the very early CAD group (cholesterol levels of large HDL: 10.16 ± 4.31 vs. 12.47 ± 6.23 vs. 14.34 ± 7.30 mg/dL, p < 0.001; large HDL percentage: 26.77 ± 7.52 vs. 29.10 ± 8.03 vs. 32.04 ± 7.99%, p < 0.001; cholesterol levels of medium HDL: 19.01 ± 3.93 vs. 20.75 ± 6.57 vs. 21.01 ± 6.44 mg/dL, p = 0.035; medium HDL percentage: 51.42 ± 5.58 vs. 50.04 ± 5.17 vs. 48.95 ± 4.80%, p < 0.001). On the contrary, the concentrations of small HDL subfraction (percentage) were significantly higher in the very early CAD group (21.75 ± 7.47 vs. 20.63 ± 7.54 vs. 18.95 ± 6.22%, p < 0.001). Meanwhile, the very early CAD group has markedly higher medium LDL subfraction (cholesterol levels: 22.24 ± 9.23 vs. 20.77 ± 8.95 vs. 19.20 ± 8.98 mg/dL, p = 0.039) and small LDL subfraction (cholesterol levels: 10.80 ± 12.41 vs. 10.27 ± 10.00 vs. 7.45 ± 8.33 mg/dL, p = 0.003; percentages: 4.99 ± 4.97 vs. 5.02 ± 4.51 vs. 3.79 ± 3.93%, p = 0.008) as well as smaller mean LDL particle size (265.54 ± 6.13 vs. 265.42 ± 5.93 vs. 267.03 ± 5.54 Å, p = 0.011).
In the current analysis, the very early CAD patients have significantly high BMI levels. Specifically, we found that BMI was negatively associated with cholesterol levels of large HDL (r = −0.297, p < 0.001, Fig. 2A) while positively related to cholesterol levels of small LDL (r = 0.133, p = 0.003, Fig. 2B). Next, we further investigated the differences in lipoprotein subfractions by comparing the lean (BMI < 25 kg/m2) and the overweight CAD patients (BMI ≥ 25 kg/m2). As a result, the cholesterol levels of small LDL was highest in the very early CAD group only in the lean but not in the overweight cases (Fig. 3A,B) while the cholesterol levels of large HDL was lowest both in the lean and overweight cases (Fig. 3C,D).
Relation of lipoprotein subfractions to very early CAD incidence
After observed the association of lipoprotein subfractions with very early CAD cases, logistic regression analysis was performed in the current study. In unadjusted analysis (Table 4), among different HDL subfractions, large and medium HDL measures were inversely [OR 95%CI: large HDL: 0.872 (0.825–0.922); medium HDL: 0.935 (0.889–0.983)] associated with the incident of very early CAD. Therefore, in the following multivariate logistic regression analysis, we further adjusted for BMI as well as other potential risk factors covering sex, hypertension, dyslipidemia, DM, current smoking and family history of CAD. We finally found that only the cholesterol levels of large HDL [OR 95%CI: 0.899 (0.848–0.954)] remained negatively related to very early CAD susceptibility (Table 4).
Discussion
The current study is the first to document the relationship between lipoprotein subfractions and very early CAD occurrence involving 734 consecutive CAD patients and 56 non-CAD controls who were not treated with lipid-lowering drugs. Specifically, we found that CAD patients in younger ages have significantly lower large HDL subfractions, higher small HDL and LDL subfractions and relatively smaller mean LDL particle size. In the logistic regression analysis, large HDL subfraction was associated with lower risk while small LDL subfraction was related to higher risk of very early CAD. In addition, we found that overweight was not only related to large HDL and small LDL subfraction but also the age of CAD incidence. However, only large HDL subfraction remained negatively associated with CAD in younger ages after adjusting for BMI and other potential confounders. Our data may provide novel information with regard to the potential role of different lipoprotein subfractions in the incident of very early CAD.
Although the CAD occurrence in very young ages has a relatively low prevalence rate, it can have devastating consequences. During the past decades, multiple studies have tried to address the issue why it happens in these very young individuals18 and finally emphasized BMI, smoking habits, hypertension, family history of CAD and dyslipidemia as more relevant risk factors4. However, it has been difficult to define risk factors unique to this population because all of these are traditional risk factors for common CAD patients. Undoubtedly, the elevated lipid and lipoprotein levels remain one of the most pivotal risk factors for the development of CAD in young ages. Previous study has revealed that the role of higher TC and LDL-C and lower HDL-C levels appeared to be important factors in the process of very early CAD process19,20. However, the concept of lipoprotein particle or subfraction has recently challenged the relevance of the cholesterol content of lipoproteins13,21. The small dense LDL-C has been demonstrated to be associated with the incident CAD in the Atherosclerosis Risk in Communities study involving 11,419 participants22. A variety of mechanisms have been proposed to explain the enhanced atherogenicity of small dense LDL, such as the higher penetration into the arterial wall, prolonged plasma half-life and lower affinity for the LDL-receptor23. Recently, Martin et al. reported that low HDL3-C (small HDL-C) subclasses, but not HDL2-C (large HDL-C) was associated with increased long-term hard clinical events in two cohorts of secondary prevention24. In our recent study involving 591 un-treated patients, large HDL has been proven to be associated with lower rate of future cardiovascular events25. Till now, the relationship between HDL subfraction and cardiovascular risk remains in debate and the potential mechanisms have not been elucidated yet. In light of the preceding discussion, we tentatively investigate the distribution and potential impact of LDL and HDL subfractions with very early CAD presence.
Actually, LDL and HDL particles are comprised of a variety of different subfractions that can be separated by several methods. Among the various lipoprotein separation methods, Lipoprint system, nuclear magnetic resonance (NMR) spectroscopy and Vertical Auto Profile method (VAP) are most commonly applied in clinical research. In this study, we applied the Lipoprint system and 10 HDL and 7 LDL subclasses (large HDL: 1–3, medium HDL: 4–7 and small HDL: 8–10; large LDL: 1; medium LDL: 2; small LDL: 3–7) have been separated. This method was based on decreasing size and increasing density by electrophoresis of a liquid loading gel with lipophilic dye in the precast linear polyacrylamide gel (stacking gel and separating gel)26. In addition, NMR was the currently common used method in clinical and laboratory research. In this way, HDL and LDL subclasses were quantified using the amplitudes of their spectroscopically distinct lipid methyl group NMR signals27. The VAP separates lipoproteins on the base of density using single vertical-spin density gradient ultracentrifugation24. The existing of diverse lipoprotein separation methodologies may mainly contribute to discrepancies. As early in 1991, Salonen et al. reported that large HDL-C levels were inversely associated with the risk of acute myocardial infarction and may thus be protective factors28. Besides that, our recent studies have revealed that large HDL subfraction was negatively associated with several cardiovascular risk factors, such as serum uric acid29 and hypertension30. In the current study, we found that only large HDL subfraction was negatively associated with very early CAD susceptibility, which may provide additive information regarding the different role of specific subfraction on the very early CAD incidence.
There were several limitations of the present study. First, the cross-sectional design was a limitation. Therefore, the results should be evaluated with some degree of caution. Second, this was a single center study with relatively small sample size (the very early CAD group, n = 81), the data should be confirmed by large scale studies. Finally, the lipoprotein subclassification was performed by Lipoprint system, NMR and other methodologies may be necessary in the future studies.
In summary, the CAD patients in younger ages have relatively lower large HDL subfractions, smaller mean LDL particle size and higher small HDL and LDL subfractions. Only large HDL subfraction was inversely and independently associated with incident of very early CAD after adjusting for potential confounders. Our data for the first time revealed the distribution of lipoprotein subfractions in very early CAD status, suggesting the potential role of large HDL subfraction in the very early CAD susceptibility.
Additional Information
How to cite this article: Zhang, Y. et al. HDL subfractions and very early CAD: novel findings from untreated patients in a Chinese cohort. Sci. Rep. 6, 30741; doi: 10.1038/srep30741 (2016).
References
Strong, J. P. et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 281, 727–735 (1999).
Fournier, J. A. et al. Long-term prognosis of patients having acute myocardial infarction when </ = 40 years of age. Am J Cardiol 94, 989–992, doi: 10.1016/j.amjcard.2004.06.051 (2004).
Cole, J. H. & Sperling, L. S. Premature coronary artery disease: clinical risk factors and prognosis. Curr Atheroscler Rep 6, 121–125 (2004).
Allen, J., Markovitz, J., Jacobs, D. R. Jr. & Knox, S. S. Social support and health behavior in hostile black and white men and women in CARDIA. Coronary Artery Risk Development in Young Adults. Psychosomatic medicine 63, 609–618 (2001).
Grundy, S. M. et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110, 227–239, doi: 10.1161/01.cir.0000133317.49796.0e (2004).
Reiner, Z. et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 32, 1769–1818, doi: 10.1093/eurheartj/ehr158 (2011).
Koren, M. J. et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation 129, 234–243, doi: 10.1161/circulationaha.113.007012 (2014).
Baigent, C. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681, doi: 10.1016/s0140-6736(10)61350-5 (2010).
Tuteja, S. & Rader, D. J. High-density lipoproteins in the prevention of cardiovascular disease: changing the paradigm. Clinical pharmacology and therapeutics 96, 48–56, doi: 10.1038/clpt.2014.79 (2014).
Fazio, S. & Linton, M. F. Elevated high-density lipoprotein (HDL) levels due to hepatic lipase mutations do not reduce cardiovascular disease risk: another strike against the HDL dogma. J Clin Endocrinol Metab 94, 1081–1083, doi: 10.1210/jc.2009-0344 (2009).
Blacher, J. et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens 24, 19–26, doi: 10.1038/jhh.2009.34 (2010).
Berneis, K. K. & Krauss, R. M. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 43, 1363–1379 (2002).
Superko, H. R. et al. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol 6, 496–523, doi: 10.1016/j.jacl.2012.03.001 (2012).
Xu, R. X. et al. Analysis of Lipoprotein Subfractions in Chinese Han Patients with Stable Coronary Artery Disease. Heart, lung & circulation 24, 1203–1210, doi: 10.1016/j.hlc.2015.05.002 (2015).
Zhang, Y. et al. Risk Factors, Coronary Severity, Outcome and ABO Blood Group: A Large Chinese Han Cohort Study. Medicine 94, e1708, doi: 10.1097/md.0000000000001708 (2015).
Zhu, C. G. et al. Circulating non-HDL-C levels were more relevant to atherogenic lipoprotein subfractions compared with LDL-C in patients with stable coronary artery disease. J Clin Lipidol 9, 794–800, doi: 10.1016/j.jacl.2015.08.010 (2015).
Xu, R. X. et al. Relation of plasma PCSK9 levels to lipoprotein subfractions in patients with stable coronary artery disease. Lipids Health Dis 13, 188, doi: 10.1186/1476-511x-13-188 (2014).
Berenson, G. S. et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338, 1650–1656, doi: 10.1056/nejm199806043382302 (1998).
Mohan, V., Deepa, R., Rani, S. S. & Premalatha, G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: The Chennai Urban Population Study (CUPS No. 5). J Am Coll Cardiol 38, 682–687 (2001).
Sharma, M. & Ganguly, N. K. Premature coronary artery disease in Indians and its associated risk factors. Vasc Health Risk Manag 1, 217–225 (2005).
Arsenault, B. J. et al. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis 206, 276–281, doi: 10.1016/j.atherosclerosis.2009.01.044 (2009).
Hoogeveen, R. C. et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol 34, 1069–1077, doi: 10.1161/atvbaha.114.303284 (2014).
Diffenderfer, M. R. & Schaefer, E. J. The composition and metabolism of large and small LDL. Curr Opin Lipidol 25, 221–226, doi: 10.1097/mol.0000000000000067 (2014).
Martin, S. S. et al. HDL cholesterol subclasses, myocardial infarction and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J 36, 22–30, doi: 10.1093/eurheartj/ehu264 (2015).
Li, J. J. et al. Large HDL Subfraction But Not HDL-C Is Closely Linked With Risk Factors, Coronary Severity and Outcomes in a Cohort of Nontreated Patients With Stable Coronary Artery Disease: A Prospective Observational Study. Medicine 95, e2600, doi: 10.1097/md.0000000000002600 (2016).
Hoefner, D. M. et al. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem 47, 266–274 (2001).
Mackey, R. H. et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes care 38, 628–636, doi: 10.2337/dc14-0645 (2015).
Salonen, J. T., Salonen, R., Seppanen, K., Rauramaa, R. & Tuomilehto, J. HDL, HDL2 and HDL3 subfractions and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation 84, 129–139 (1991).
Zhang, Y. et al. Lipoprotein subfractions partly mediate the association between serum uric acid and coronary artery disease. Clin Chim Acta 441, 109–114, doi: 10.1016/j.cca.2014.12.030 (2015).
Zhang, Y. et al. Distribution of High-Density Lipoprotein Subfractions and Hypertensive Status: A Cross-Sectional Study. Medicine 94, e1912, doi: 10.1097/md.0000000000001912 (2015).
Acknowledgements
We are grateful to the field staff and the participants of our study. This work was partly supported by National Natural Science Foundation (81070171, 81241121), Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), Capital Special Foundation of Clinical Application Research (Z121107001012015), Capital Health Development Fund (2011400302, 2016-1-4035) and Beijing Natural Science Foundation (7131014) awarded to Dr. Jian-Jun Li, MD, PhD.
Author information
Authors and Affiliations
Contributions
Y.Z., C.-G.Z. and R.-X.X. completed the project, analyzed the data and wrote the manuscript. J.-J.L. conceived the study, interpreted the data and contributed to reviewing/editing the manuscript. S.L., X.-L.L., Y.-L.G., N.-Q.W., Y.G., P.Q., C.-J.C. and J.S. were responsible for acquisition and selection of all serum samples and performed all laboratory assays. All authors have read and approved the final version of this manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Y., Zhu, CG., Xu, RX. et al. HDL subfractions and very early CAD: novel findings from untreated patients in a Chinese cohort. Sci Rep 6, 30741 (2016). https://doi.org/10.1038/srep30741
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30741
This article is cited by
-
The profile of HDL-C subfractions and their association with cardiovascular risk in the Hungarian general and Roma populations
Scientific Reports (2022)
-
Neck circumference is associated with non-traditional cardiovascular risk factors in individuals at low-to-moderate cardiovascular risk: cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil)
Diabetology & Metabolic Syndrome (2018)
-
Lipoprotein-associated phospholipase A2 and oxidized low-density lipoprotein in young patients with acute coronary syndrome in China
Scientific Reports (2017)
-
Novel and traditional lipid-related biomarkers and their combinations in predicting coronary severity
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.