Abstract
Increasing SET operation speed and reducing RESET operation energy have always been the innovation direction of phase change memory (PCM) technology. Here, we demonstrate that ∼87% and ∼42% reductions of RESET operation energy can be achieved on PCM cell based on stoichiometric Ti1Sb2Te5 alloy, compared with Ge2Sb2Te5 and non-stoichiometric Ti0.4Sb2Te3 based PCM cells at the same size, respectively. The Ti1Sb2Te5 based PCM cell also shows one order of magnitude faster SET operation speed compared to that of the Ge2Sb2Te5 based one. The enhancements may be caused by substantially increased concentration of TiTe2 nano-lamellae in crystalline Ti1Sb2Te5 phase. The highly electrical conduction and lowly thermal dissipation of the TiTe2 nano-lamellae play a major role in enhancing the thermal efficiency of the amorphization, prompting the low-energy RESET operation. Our work may inspire the interests to more thorough understanding and tailoring of the nature of the (TiTe2)n(Sb2Te3)m pseudobinary system which will be advantageous to realize high-speed and low-energy PCM applications.
Similar content being viewed by others
Introduction
Non-volatile phase change memory (PCM), as one of the promising candidates, has great potential to serve as a storage class memory (SCM) to mitigate the widened performance mismatch between dynamic random access memory (DRAM) and non-volatile NAND Flash memory1,2. In the PCM cell, a chalcogenide material, for example, Ge2Sb2Te5, can be switched between the crystalline (c-) and amorphous (a-) phases, corresponding to the SET and RESET states3,4, respectively. The big resistance contrast of such two states is utilized for storing “0” and “1” data states. The RESET operation refers to an amorphization procedure which melts the c-phase and subsequently quenches it into a-phase by applying a short intense electrical pulse on the PCM cell. Conversely, a longer pulse of lower intensity for SET operation can heat the a-phase to a temperature between crystallization temperature (Tc) and melting point (Tm) to obtain the c-phase. To achieve high density SCM application, scaling capability of the PCM cell is largely limited by its high RESET current or energy5 for which the premature degradation of the switching material or the thermal disturbance among nearest cells would happen and cause reliability and endurance issues6.
Since the slow SET speed and high RESET power remain to be important limitations for developing DRAM-like PCM, our previous work demonstrated at least one order of magnitude faster SET speed and as low as one-fifth of the RESET current and energy on a Ti0.4Sb2Te3 based PCM cell compared to those of the Ge2Sb2Te5 based cell with the same size7. The stable c-Ti0.4Sb2Te3 alloy has the nano-scale phase separated morphology that hexagonal (HEX) Sb2Te3 and HEX-TiTe2 crystals coexist8. The triple-layered TiTe2 crystal lamellae locate adjacent to the quintuple-layered Sb2Te3 grains, while there are also some Ti atoms penetrate into Sb2Te3 lattice by occupying Sb sites and constructing Ti-centered octahedrons with surrounding Te atoms8. Such TiTe2 lamellae along with the Ti-centered octahedrons can preserve their ordering configurations even after high temperature RESET operations, which is believed to be responsible for the performance boost of the Ti0.4Sb2Te3 based PCM cell7,8.
The influence of Ti doping content (x) on the phase change properties of TixSb2Te3 materials was also studied9,10. Although increasing Ti content can enhance 10-year data retention, reduce the volume change upon phase transition, improve interfacial adhesion ability, excessive Ti content (x ≈ 0.56, ∼10.1 at.%) causes Ti segregation which leads to poor endurance characteristic of the PCM cell9,10. This phenomenon may correlate to a low solid solubility of Ti atoms in the HEX-Sb2Te3 lattice, where only limited number of Ti-centered octahedrons could dispersedly distribute inside the quintuple-layered building blocks so as to preserve the ordering configuration8. To inhibit the Ti precipitation after repeated RESET-SET operations, it would be worthwhile to try to concomitantly raise the Te content of the non-stoichiometric TixSb2Te3 materials. Thus, in this paper, we show the better electrical phase change properties based on a stoichiometric Ti1Sb2Te5 material. It is like a pseudobinary compound with 1 : 1 ratio of TiTe2 and Sb2Te3 components. Even containing higher Ti content (12.5 at.%), the Ti1Sb2Te5 based PCM cell has superior endurance characteristic over the Ti0.56Sb2Te3 based one. In addition, without significantly sacrificing the SET speed, the RESET energy of the Ti1Sb2Te5 based PCM cell is further lowered by 42∼47% compared to the Ti0.4Sb2Te3 based one. We argue that the richer concentration of TiTe2 lamellae in c-Ti1Sb2Te5 plays a major role in decreasing the RESET energy, meanwhile the lack of Ti-centered octahedrons resided in the quintuple-layered Sb2Te3 lattice may slow down the nucleation rate, however the increasing TiTe2 lamellae can act as structure-ordering template to enhance the crystal growth rate11. Accordingly, we believe that Ti1Sb2Te5 material is promising for realizing DRAM-like PCM application once advanced fabrication techniques being applied to further shrink the device dimension.
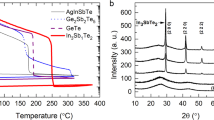
Figure 1 shows the temperature-dependent sheet resistance (Rs) curves of as deposited TiTe2, Sb2Te3, Ti0.4Sb2Te3 and Ti1Sb2Te5 films upon in situ annealing with a heating rate of 10 °C/min. As the annealing temperature increases, a continuous decrease in Rs is observed for each film. Due to the partial crystallization during the sputtering process, compared to Ti0.4Sb2Te3 and Ti1Sb2Te5 films, both TiTe2 and Sb2Te3 films have smaller initial Rs and present a smooth decrement in Rs. By comparison, one can observe the sudden drop in Rs occurs for Ti0.4Sb2Te3 and Ti1Sb2Te5 films when the temperature reaches Tc (both around 186 °C)9. The decrease in Rs with increasing temperature just before the onset of the crystallization indicates a semiconductor-like behavior. The temperature dependence for the Rs in a semiconductor can be expressed by Rs = Rs0exp(−Eσ/kT)12, where Rs0 is a pre-exponential factor and Eσ is the activation energy for electrical conduction. The fitting results of Eσs of Ti0.4Sb2Te3 and Ti1Sb2Te5 are 0.11 eV and 0.13 eV, respectively. The activation energy of electrical transport is simply determined by half of the band gap Eσ = EG/2 + ΔE, where EG/2 is the distance from the Fermi level to the conduction band and ΔE is the depth of the trap states13. In the case of intrinsic conduction with equal amounts of electrons and holes, the Fermi level is situated at the middle of the band gap. Thus we can roughly estimate the optical band gaps (EOPs) of a-Ti0.4Sb2Te3 and a-Ti1Sb2Te5 to be ∼0.22 eV and ∼0.26 eV, respectively, both of which are quite smaller than that of a-Ge2Sb2Te5 (∼0.70 eV)14. Because the carrier density inside the semiconductor is proportional to exp(−EG/2kT), where EG is the electrical band gap which is roughly identical to the EOP, a decrease in the band gap as the temperature approaching to the Tc will lead to the generation of a large number of carriers, which makes a major contribution to the quick drop in film resistivity14. On this view, one may roughly estimate that a-Ti0.4Sb2Te3 and a-Ti1Sb2Te5 could have quite faster crystallization (resistivity decrement) speed than that of a-Ge2Sb2Te5 and a-Ti0.4Sb2Te3 could be a little quicker than a-Ti1Sb2Te5.
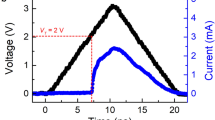
Figure 2a compares the SET speed of Ge2Sb2Te5, Ti0.4Sb2Te3 and Ti1Sb2Te5 based PCM cells with the same size (BEC D = 190 nm), which has the same trend as aforementioned estimation. As the magnitude of applied voltage pulse reaches 1.3 V and 1.5 V, respectively, both the Ti0.4Sb2Te3 and Ti1Sb2Te5 cells show the SET speed of ∼6 ns. Apparently, under lower bias, the Ti0.4Sb2Te3 cell can complete the SET operation more quickly than the Ti1Sb2Te5 cell. In contrast, the SET operation of Ge2Sb2Te5 cell requires ∼75 ns at 1.6 V and ∼35 ns even at 2.1 V. In other words, one order of magnitude faster SET speed can still be achieved even by using the Ti1Sb2Te5 cell. Supplementary Information Figure S1 shows the cell resistance versus required time curves for SET operation of such three cells.
In terms of the RESET operation, even the Ti0.4Sb2Te3 (3.12 nJ) and Ti1Sb2Te5 (1.65 nJ) cells with D = 190 nm BEC have noticeable lower energies compared to the Ge2Sb2Te5 cells with smaller BEC (9.48 nJ for D = 130 nm BEC and 4.20 nJ for D = 80 nm BEC). More significant RESET energy reduction can be achieved on the Ti0.4Sb2Te3 (0.95 nJ) and Ti1Sb2Te5 (0.55 nJ) cells with D = 80 nm BEC. Namely, ∼78% and ∼87% of the energy have been saved via using the Ti0.4Sb2Te3 and Ti1Sb2Te5 cells (D = 80 nm BEC), respectively. Note that the Ti1Sb2Te5 cell achieves a substantial (42∼47%) RESET energy reduction on the basis of the Ti0.4Sb2Te3 cell. The shrinkage of BEC size also remarkably decreases the RESET current as shown in the Supplementary Information Figure S2. An ∼82% reduction of the RESET current is realized for both the Ti0.4Sb2Te3 and Ti1Sb2Te5 cells (∼0.5 mA) compared to that of Ge2Sb2Te5 cell (∼2.8 mA) with the same D = 80 nm BEC. Moreover, the RESET current of the Ti1Sb2Te5 cell (∼1.1 mA) is relatively smaller than that of the Ti0.4Sb2Te3 cell (∼1.3 mA) with the same D = 190 nm BEC. In addition to the improvements in RESET energy and current, the endurance characteristics of the Ti1Sb2Te5 cell (∼107 cycles) is not inferior to that of the Ti0.4Sb2Te3 cell7, which is obviously far more better than that of the Ti0.56Sb2Te3 cell (<106 cycles with severe fluctuation of the RESET state)9, as shown in the Supplementary Information Figure S3.
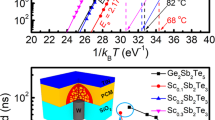
We used Raman spectroscopy (Fig. 3a) and in situ XRD (Fig. 3b) to characterize the c-Ti1Sb2Te5 phase. As a CdI2-like structure with space group P m1 space symmetry, c-TiTe2 has two Raman active modes produced entirely by the Te atoms for both the in-plane, Eg peak (∼122 cm−1) and out of plane, A1g peak (∼143 cm−1), with the Ti atoms at rest15. Since there is no distinctive shoulder near ∼160 cm−1, the c-TiTe2 film can be considered as a stoichiometric compound without noticeable defects or impurities16. Because c-Sb2Te3 crystal belongs to the space group R
m1 space symmetry, c-TiTe2 has two Raman active modes produced entirely by the Te atoms for both the in-plane, Eg peak (∼122 cm−1) and out of plane, A1g peak (∼143 cm−1), with the Ti atoms at rest15. Since there is no distinctive shoulder near ∼160 cm−1, the c-TiTe2 film can be considered as a stoichiometric compound without noticeable defects or impurities16. Because c-Sb2Te3 crystal belongs to the space group R m, it has three Raman active modes, including two out of plane vibrations A1g(1) peak (∼69 cm−1) and A1g(2) peak (∼165 cm−1) and one in-plane vibration Eg(1) peak (∼112 cm−1)17. Compared to the Raman curves of the c-TiTe2 and c-Sb2Te3 films, the c-Ti1Sb2Te5 film has five Raman active modes identically peaked corresponding to Eg and A1g of TiTe2 and A1g(1), A1g(2) and Eg(1) of Sb2Te3, respectively, as shown in Fig. 3a. The coexistence of c-TiTe2 and c-Sb2Te3 Raman active modes in c-Ti1Sb2Te5 no doubt shall originate from the two separated phases which is already observed in c-Ti0.4Sb2Te38. In fact, our in situ XRD result of Ti1Sb2Te5 film clearly proves such phase separation phenomenon as shown in Fig. 3b, where both HEX-Sb2Te3 and HEX-TiTe2 diffraction peaks can be identified. Nevertheless we did not observe such distinct phase separation in Ti0.4Sb2Te3 through the same in situ XRD measurement7. The phase separation in Ti0.4Sb2Te3 occurs in nano-scale dimension8, where the TiTe2 lamellae segregate into no more than 10 nm-width belts and most of the TiTe2 lamellae (<2 nm in width) inlay with the Sb2Te3 quintuple-layered blocks. Due to the similar HEX lattice structures of TiTe2 and Sb2Te3 and also considering the lower doping content of Ti (∼7.4 at.%) in Ti0.4Sb2Te3, less concentration of the TiTe2 lamellae may result in undetected XRD signal. Note that the pure TiTe2 alloy has quite high Tm (>1200 °C)18 and the HEX-phase of its thin film can be maintained even at 700 °C (higher than the Tm ∼618 °C of Sb2Te3)8, thus there is no segregated Te or Ti phase being observed in c-Ti1Sb2Te5 as shown in Fig. 3b.
m, it has three Raman active modes, including two out of plane vibrations A1g(1) peak (∼69 cm−1) and A1g(2) peak (∼165 cm−1) and one in-plane vibration Eg(1) peak (∼112 cm−1)17. Compared to the Raman curves of the c-TiTe2 and c-Sb2Te3 films, the c-Ti1Sb2Te5 film has five Raman active modes identically peaked corresponding to Eg and A1g of TiTe2 and A1g(1), A1g(2) and Eg(1) of Sb2Te3, respectively, as shown in Fig. 3a. The coexistence of c-TiTe2 and c-Sb2Te3 Raman active modes in c-Ti1Sb2Te5 no doubt shall originate from the two separated phases which is already observed in c-Ti0.4Sb2Te38. In fact, our in situ XRD result of Ti1Sb2Te5 film clearly proves such phase separation phenomenon as shown in Fig. 3b, where both HEX-Sb2Te3 and HEX-TiTe2 diffraction peaks can be identified. Nevertheless we did not observe such distinct phase separation in Ti0.4Sb2Te3 through the same in situ XRD measurement7. The phase separation in Ti0.4Sb2Te3 occurs in nano-scale dimension8, where the TiTe2 lamellae segregate into no more than 10 nm-width belts and most of the TiTe2 lamellae (<2 nm in width) inlay with the Sb2Te3 quintuple-layered blocks. Due to the similar HEX lattice structures of TiTe2 and Sb2Te3 and also considering the lower doping content of Ti (∼7.4 at.%) in Ti0.4Sb2Te3, less concentration of the TiTe2 lamellae may result in undetected XRD signal. Note that the pure TiTe2 alloy has quite high Tm (>1200 °C)18 and the HEX-phase of its thin film can be maintained even at 700 °C (higher than the Tm ∼618 °C of Sb2Te3)8, thus there is no segregated Te or Ti phase being observed in c-Ti1Sb2Te5 as shown in Fig. 3b.
The Raman spectra of c-TiTe2, c-Sb2Te3 and c-Ti1Sb2Te5 and in situ XRD results of Ti1Sb2Te5.
(a) Raman spectra of the c-TiTe2, c-Sb2Te3 and c-Ti1Sb2Te5 films. One can see two main Eg (○) and A1g (□) phonon modes for c-TiTe2 film and three main A1g(1) (■), Eg(1) (●) and A1g(2) (▲) phonon modes for c-Sb2Te3 film. The c-Ti1Sb2Te5 film shows all the five phonon modes. (b) In situ XRD curves of Ti1Sb2Te5 film at different temperatures, where HEX lattice planes of Sb2Te3 (♦) and TiTe2 (◊) can be identified.
Since the Ti dopants in c-Ti0.4Sb2Te3 either segregate in the form of TiTe2 lamellae or construct Ti-centered octahedrons scattered in the quintuple-layered blocks of the Sb-Te lattice8, this non-stoichiometric composition can be chemically regarded as (TiTe2)xTi0.4−xSb2Te3−2x (0 < x < 0.4), where (TiTe2)x part corresponds to the TiTe2 lamellae and in Ti0.4−xSb2Te3−2x part the Ti0.4−xTe0.8−2x accounts for the scattered Ti-centered octahedrons. The Ti0.4-xSb2Te3-2x part behaves more like the Sb-rich Sb-Te compound contributing to the faster recrystallization8. Extending this analysis to the stoichiometric Ti1Sb2Te5 = (TiTe2)yTi1-ySb2Te5-2y (0 < y < 1), where (TiTe2)y part also stands for the segregated TiTe2 lamellae, one can easily find that, in Ti1−ySb2Te5−2y part (=Ti1−yTe2−2ySb2Te3), if Ti1−yTe2−2y is assigned as the Ti-centered octahedrons accommodated in Sb-Te quintuple-layered blocks, it will be contradictory to keep the rest Sb-Te part in a ratio of 2 : 3 without Te deficiency. In other words, since there is no Ti or Te phase separation, it is more appropriate to describe the c-Ti1Sb2Te5 as (TiTe2)1(Sb2Te3)1 in which all the Ti atoms are supposed to be contained in the separated TiTe2 lamellae. Of course, this is a rough estimate, however, we may still get a reasonable inference that compared to the c-Ti0.4Sb2Te3, c-Ti1Sb2Te5 should have a higher concentration of the c-TiTe2 lamellae but a lower concentration of the “solid-solute” Ti-centered octahedrons.
Note that the quasi-two-dimensionalc-TiTe2 is semimetallic19 (see Fig. 1 also) with a quite low thermal conductivity (∼0.12 W/mK)20. The TiTe2 lamellae could act like the embedded nano-electrodes in c-Ti1Sb2Te5 to conduct the electrical current to the adjacent Sb2Te3 grains to generate Joule heat. The heat dissipation could be concomitantly refrained by those low thermal-conductive TiTe2 lamellae so as to effectively enhance the thermal efficiency of the RESET operation. Not surprisingly the c-Ti1Sb2Te5 (∼1/2 = 50.0% concentration of TiTe2 lamellae) based PCM cell can accomplish substantial reduction of the RESET energy compared to the c-Ti0.4Sb2Te3 (< ∼0.4/1.4 ≈ 28.6% concentration of TiTe2 lamellae) based PCM cell. On the contrary, the lack of survived Ti-centered octahedrons in Sb-Te rich amorphous matrix after RESET operation may slow down the nucleation rate for recrystallization process8,9, leading to a relatively slower SET speed for the Ti1Sb2Te5 based PCM cell as compared to that of the Ti0.4Sb2Te3 based one.
We also used the two-dimensional finite element method (FEM) to simulate and compare the RESET operations of the Ge2Sb2Te5, Ti0.4Sb2Te3 and Ti1Sb2Te5 based PCM cells, as shown in Fig. 4. The Joule heat is mainly generated in the phase change films. The thermal transfer obeys the standard heat conduction equation:21
The two-dimensional finite element method simulations for the RESET operation.
Simulated RESET temperature distributions in PCM cells with (a) c-Ge2Sb2Te5, (b) c-Ti0.4Sb2Te3 and (c) c-Ti1Sb2Te5 layers. In (a) all the square grids represent the small Ge2Sb2Te5 crystal grains, while in (b,c) the grids marked with slash lines stand for the TiTe2 crystal grains and other non-marked ones are belonged to the Sb2Te3 crystal grains. The isothermal curves with corresponding temperatures in the PCM cells are also shown.

where κ, is the thermal conductivity, c, the specific heat, ρ, the density, t, the time, T, the temperature and Q, the Joule heat per unit volume and per unit time, which is called the heat density. The key material parameters for FEM simulations include κ of c-Ge2Sb2Te5 (∼0.46 W/mK)21, c-Sb2Te3(∼0.78 W/mK)22 and c-TiTe2 (∼0.12 W/mK)20, c of c-Ge2Sb2Te5 (∼1.20 J/cm3K)23, c-Sb2Te3(∼1.02 J/cm3K)24 and c-TiTe2 (assumed to be ∼1.80 J/cm3K of c-TiSe2)25 and ρ of c-Ge2Sb2Te5 (∼6.2 g/cm3)21, c-Sb2Te3 (∼6.5 g/cm3)26 and c-TiTe2 (∼6.3 g/cm3)27. The phase change film layers of the models are divided into grid shape to represent the poly-crystalline morphology. Each grid denotes the small crystal grain. ∼29% and ∼50% of the grids in the c-Ti0.4Sb2Te3 and c-Ti1Sb2Te5 layers are randomly chosen to be the c-TiTe2 grains, respectively, as shown in Fig. 4b,c. Constant voltage pulse is applied to the axis-symmetric mushroom-type (T-shaped) cells. It can be observed the highest peak temperature is achieved in the Ti1Sb2Te5 based cell (Fig. 4c) while the Ge2Sb2Te5 based cell (Fig. 4a) has the lowest peak temperature. Apparently, more heat can be generated and confined in the phase change film layer as c-TiTe2 concentration increases, therefore lower energy is needed for the RESET operation.
In summary, the pseudobinary Ti1Sb2Te5 phase change alloy shows drastically decreased RESET energy, while increasing the SET speed, of the PCM cell compared to the Ge2Sb2Te5 based one. Without significantly reducing the SET speed as compared to the Ti0.4Sb2Te3 based PCM cell, nearly half of the RESET power can be saved on the Ti1Sb2Te5 based one. These improvements are achieved by introducing more nano-scale separated TiTe2 lamellae. We believe with more thermally stable TiTe2 lamellae the efficiency of electric conduction and heat inhibition could be greatly enhanced for the low-energy RESET operation. We expect the speed/power to be further increased/decreased significantly on thorough investigations of the (TiTe2)n(Sb2Te3)m pseudobinary system and device dimension scaling techniques. In this regard, for example, a superlattice or multilayered structure constructed by alternate TiTe2/Sb2Te3 stacking film instead of the co-sputtering one will be a great help. It may also be possible to search topological superconducting properties on a finely-tuned TiTe2/Sb2Te3 superlattice sample.
Methods
Ti0.4Sb2Te3 films were deposited by co-sputtering of pure Ti and Sb2Te3 targets. By adding an additional pure Te target, three-target co-sputtering technique was used to fabricate the Ti1Sb2Te5 films. Similarly, TiTe2 films were obtained by co-sputtering of pure Ti and Te targets. For Sb2Te3 and Ge2Sb2Te5 films, respective pure alloy target was used for sputtering. The compositions of all films were measured by X-ray fluorescence spectroscopy using a Rigaku RIX 2100 system. The temperature-dependent sheet resistance changing trends of TiTe2, Sb2Te3, Ti0.4Sb2Te3 and Ti1Sb2Te5 films with the same 150 nm thickness were studied by Linkam LMP 95 hot stage. For real-time observation of structure transition in Ti1Sb2Te5 film, vacuum in situ X-ray diffraction (XRD) measurement with a 20 °C/min heating rate was performed on 300-nm-thick film (deposited on Si substrate at room temperature) using PANalytical X’Pert PRO diffractometer with a Cu Kα (λ = 0.15418 nm) radiation source. The diffraction data were collected in the 2θ range of 10°–60° with a scanning step of 0.02°. Raman spectroscopy (Thermo Fisher DXR) was performed on 300-nm-thick film samples at room temperature using an Ar+ laser (wavelength 532 nm) with ∼1 μm2 beam spot.
T-shaped PCM cells with diameter (D) = 190 (80) nm tungsten plug bottom electrode contact (BEC) were fabricated using 0.13 μm complementary metal-oxide semiconductor technology. In all the PCM cells, the thickness of the switching material films is around 170 nm. The 15-nm-thick TiN and 300-nm-thick Al films were used as top electrode for all cells. All the electrical measurements were performed by using the Keithley 2600C source meter (measuring cell resistance), the Tektronix AWG5002B pulse generator (generating voltage pulse with a minimum width of ∼6 ns), the homemade constant current driver (generating current pulse with a maximum magnitude of ∼10 mA) and the Tektronix 7054 digital phosphor oscilloscope (measuring transient voltage drop across the cell when current pulse is applied).
Additional Information
How to cite this article: Ding, K. et al. Low-Energy Amorphization of Ti1Sb2Te5 Phase Change Alloy Induced by TiTe2 Nano-Lamellae. Sci. Rep. 6, 30645; doi: 10.1038/srep30645 (2016).
References
Lam, C. H. In Storage Class Memory. 10th IEEE International Conference onSolid-State and Integrated Circuit Technology. 1080–1083 (2010).
Frietas, R. F. & Wilcke, W. W. Storage-class memory: the next storage systemtechnology. IBM J. Res. Dev. 25, 439 (2008).
Atwood, G. Phase-change materials for electronic memories. Science 321, 210–211 (2008).
Wuttig, M. Phase-change materials: towards a universal memory? Nat. Mater. 4, 265–266 (2005).
Kang, M. J. et al. PRAM cell technology and characterization in 20 nm node size. IEEE International Electron Devices Meeting 3, 1.1–3.1.4 (2011).
Ahn, S. J. et al. Reliability perspectives for high density PRAM manufacturing. IEEE International Electron Devices Meeting 12, 6.1–12.6.4 (2011).
Zhu, M. et al. One order of magnitude faster phase change at reduced power in Ti-Sb-Te. Nat. Commun. 5, 4086 (2014).
Rao, F. et al. Direct observation of titanium-centered octahedral in titanium-antimony-tellurium phase-change material. Nat. Commun. 6, 10040 (2015).
Xia, M. J. et al. Ti–Sb–Te Alloy: A candidate for fast and long-life phase-change memory. ACS Appl. Mater. Inter. 7, 7627–7634 (2015).
Zhu, M. et al. The micro-structure and composition evolution of Ti-Sb-Te alloy during reversible phase transition in phase change memory. Appl. Phys. Lett. 104, 063105 (2014).
Simpson, R. E., Fons, P., Kolobov, A. V., Krbal, M. & Tominaga, J. Enhanced crystallization of GeTe from an Sb2Te3 template. Appl. Phys. Lett. 100, 021911 (2012).
Friedrich, I., Weidenhof, V., Njoroge, W., Franz, P. & Wuttig, M. Structural transformations of Ge2Sb2Te5 films studied by electrical resistance measurements. J. Appl. Phys. 87, 4130–4134 (2000).
Chung, K. M., Wamwangi, D., Woda, M., Wuttig, M. & Bensch, W. Investigation of SnSe, SnSe and Sn2Se3 alloys for phase change memory applications. J. Appl. Phys. 103, 083523 (2008).
Rao, F. et al. Investigation of changes in band gap and density of localized states on phase transition for Ge2Sb2Te5 and Si3.5Sb2Te3 materials. Acta Mater. 60, 323–328 (2012).
Khan, J. M., Nolen, C. M., Teweldebrhan, D. & Balandin, A. A. Properties of quasi-two-dimensional crystals of titanium ditelluride. ECS Transactions 33, 211–217 (2010).
Khan, J. M. et al. Anomalous electron transport in back-gated field-effect transistors with TiTe2 semimetal thin-film channels. Appl. Phys. Lett. 100, 043109 (2012).
Sosso, G. C., Caravati, S. & Bernasconi, M. Vibrational properties of crystalline Sb2Te3 from first principles. J. Phys.: Condens. Matter. 21, 095410 (2009).
Cordes, H. & Schmid-Fetzer, R. Phase equilibria in the Ti-Te system. J. Alloy.Compd. 216, 197–206 (1994).
Claessen, R. et al. Complete band-structure determination of the quasi-two-dimensional Fermi-liquid reference compound TiTe2 . Phys. Rev. B 54, 2453–2465 (1996).
Chiritescu, C., Mortensen, C., Cahill, D. G., Johnson, D. & Zschack, P. Lower limit to the lattice thermal conductivity of nanostructured Bi2Te3-based materials. J. Appl. Phys. 106, 073503 (2009).
Kang, D. H., Ahn, D. H., Kim, K. B., Webb, J. F. & Yi, K. W. One-dimensional heat conduction model for an electrical phase change random access memory device with an 8F2 memory cell (F = 0.15 μm). J. Appl. Phys. 94, 3536–3542 (2003).
Chen, J. et al. Sb2Te3 nanoparticles with enhanced Seebeck coefficient and low thermal conductivity. Chem. Mater. 22, 3086–3092 (2010).
Kim, S. S. et al. Simulation for Reset operation of Ge2Sb2Te5 phase-change random access memory. Jpn. J. Appl. Phys. 44, 5943–5948 (2005).
Yáñez-Limón, J. M., González-Hernández, J., Alvarado-Gil, J. J., Delgadillo, I. & Vargas, H. Thermal and electrical properties of the Ge:Sb:Te system by photoacoustic and Hall measurements. Phys. Rev. B 52, 16321–16324 (1995).
Craven, R. A., Di Salvo, F. J. & Hsu, F. S. L. Mechanisms for the 200 K transition in TiSe2: A measurement of the specific heat. Solid State Commun. 25, 39–42 (1978).
Anderson, T. L. & Krause, H. B. Refinement of the Sb2Te3 and Sb2Te2Se structures and their relationship to nonstoichiometric Sb2Te3−ySey compounds. Acta Crystallogr. B 30, 1307–1310 (1974).
McTaggart, F. K. & Wadsley, A. D. The sulphides, selenides and tellurides of titanium, zirconium, hafnium and thorium. I. Preparation and characterization. Aust. J. Chem. 11, 445–457 (1958).
Acknowledgements
This work was supported by Strategic Priority Research Program of Chinese Academy of Sciences (XDA09020402), National Integrate Circuit Research Program of China (2009ZX02023-003), National Natural Science Foundation of China (61076121, 61176122, 61106001, 61261160500, 61376006), Science and Technology Council of Shanghai (13ZR1447200, 13DZ2295700).
Author information
Authors and Affiliations
Contributions
K.D. prepared the film samples and carried out the in situ XRD, Raman measurements and so on. K.D., L.W. and Y.C. fabricated the PCM cells and carried out electrical measurements. S.L. prepared PCM cell TEM samples by using FIB technique. F.R. carried out theoretical analysis and wrote this paper with help from all co-authors. The project was initiated and conceptualized by F.R. and Z.S.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ding, K., Rao, F., Lv, S. et al. Low-Energy Amorphization of Ti1Sb2Te5 Phase Change Alloy Induced by TiTe2 Nano-Lamellae. Sci Rep 6, 30645 (2016). https://doi.org/10.1038/srep30645
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30645
This article is cited by
-
Novel nanocomposite-superlattices for low energy and high stability nanoscale phase-change memory
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.