Abstract
We assessed 1-year outcomes in patients with atrial fibrillation enrolled in the EurObservational Research Programme AF General Pilot Registry (EORP-AF), in relation to kidney function, as assessed by glomerular filtration rate (eGFR). In a cohort of 2398 patients (median age 69 years; 61% male), eGFR (ml/min/1.73 m2) calculated using the CKD-EPI formula was ≥80 in 35.1%, 50–79 in 47.2%, 30–49 in 13.9% and <30 in 3.7% of patients. In a logistic regression analysis, eGFR category was an independent predictor of stroke/TIA or death, with elevated odds ratios associated with severe to mild renal impairment, ie. eGFR < 30 ml/min/1.73 m2 [OR 3.641, 95% CI 1.572–8.433, p < 0.0001], 30–49 ml/min/1.73 m2 [OR 3.303, 95% CI 1.740–6.270, p = 0.0026] or 50–79 ml/min/1.73 m2 [OR 2.094, 95% CI 1.194–3.672, p = 0.0003]. The discriminant capability for the risk of death was tested among various eGFR calculation algorithms: the best was the Cockcroft-Gault equation adjusted for BSA, followed by Cockcroft-Gault equation, and CKD-EPI equation, while the worst was the MDRD equation. In conclusion in this prospective observational registry, renal function was a major determinant of adverse outcomes at 1 year, and even mild or moderate renal impairments were associated with an increased risk of stroke/TIA/death.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, and its incidence and prevalence are increasing worldwide1. Given its close association with age and various comorbidities, AF is commonly associated with impairment in renal function, of various degrees2.
AF is associated with an increased risk of stroke and thromboembolic events and risk stratification is essential for appropriate decision making with regard to anticoagulants3,4. There is growing interest on assessing renal function in patients with AF since compromise of renal function has major implications with regard to the risk of stroke and bleeding5. Indeed, a precise estimate of renal function is necessary in patients with non-valvular AF6,7 who are candidates for treatment with the non vitamin K antagonist oral anticoagulants (NOACs)8.
Chronic kidney disease (CKD) is defined as the presence of kidney abnormalities, which can involve its structure and/or its function, for a period longer than 3 months, and glomerular filtration rate (GFR) is widely accepted as the best overall index of kidney function2. The GFR can be estimated from the serum creatinine using a number of different equations to give an estimated GFR (eGFR)2,9. Clinical Practice Guidelines delivered by KDIGO (Kidney Disease: Improving Global Outcomes) group for the evaluation and management of CKD recommended, in 2012, use of the CKD EPI equation for estimation of eGFR, on the basis of standardized serum creatinine, and for staging of kidney function impairment2,9. This recommendation is not concordant with the advice to use Cockcroft-Gault equation for evaluating kidney function for the prescription of NOACs in cardiology practice8.
The objective of this report from the EURObservational Research Programme – Atrial Fibrillation (EORP-AF) General Pilot Registry was to investigate the baseline characteristics and the outcomes at 1 year follow-up of prospectively enrolled AF patients presenting to cardiologists, in relation to kidney function, as assessed by different equations for estimated glomerular filtration rate (eGFR) and 1-year outcomes, in terms of stroke and mortality in European AF patients followed by cardiologists. The analysis had also the aim to assess the concordance between the different equations proposed for estimating GFR and the potential differences in terms of outcome prediction.
The EORP-AF General Pilot Registry10,11,12,13,14,15 is a multicenter European registry which enrolled consecutive in- and out-patients presenting with documented AF to cardiologists, in participating centres from 9 European countries and includes patients with both valvular and non valvular AF with no exclusion criteria on the basis of co-morbidities such as renal or hepatic impairment. An additional objective of our analysis was to compare 4 different equations of common clinical use for calculating eGFR with regard to concordance in estimate of kidney function and in terms of outcome prediction.
Methods
The methods and baseline data from the EORP-AF pilot general registry have previously been published8. The registry was commenced in early 2012. One-year follow-up phase (‘pilot phase’ or Phase 1) data were focused on patients from 9 countries (for a broad representation of European Society of Cardiology member countries) recruited into this database13.
In brief, the registry population comprised consecutive in- and out-patients presenting with AF to cardiologists, enrolled in 67 centres in 9 countries10,11,12,13,14,15. Consecutive patients were screened at the time of their presentation to a cardiologist (hospital or medical centre), and potential patients were approached to obtain written informed consent according to local rules. The protocol of EORP AF was initially approved by the European Society of Cardiology (Sophia Antipolis, France) and then by the Institutional Review Boards, at a national or local level, according to country regulations. The research was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Enrollment required ECG-confirmed diagnosis of AF, with a qualifying episode of AF documented in the 12 months prior to enrollment. Stroke risk was categorised using the CHA2DS2-VASc score10,11,12,13,14,15, whilst bleeding risk was categorised using the HAS-BLED score10,11,12.
In this registry, parameters collected at enrollment included history of CKD, serum creatinine, body weight and the other parameters that permitted us to calculate eGFR according to the CKD Epidemiology Collaboration (CKD-EPI) equation, MDRD equation, Cockcroft-Gault equation, and BSA adjusted- Cockcroft-Gault equation2 (Table w1, web only appendix). Patients were classified on the basis of eGFR in 4 groups, corresponding to eGFR ≥80 ml/min/1.73 m2, between 50 and 79 ml/min/1.73 m2, between 30 and 49 ml/min/1.73 m2 and <30 ml/min/1.73 m2, respectively. The choice of the cuf-offs was in line with the cut-off commonly used for prescribing the appropriate dose of NOACs8. For the main analysis, patient classification according to eGFR calculated with CKD-EPI equation was used.
Outcomes were recorded for all cause mortality, cardiovascular death, thromboembolism (TE) and bleeding. TE refers to stroke, transient ischaemic attack (TIA), acute coronary syndrome (ACS), coronary intervention, cardiac arrest, peripheral embolism and pulmonary embolism – each of these were as recorded by the investigator, in this ‘real world’ observational registry.
Statistical analyses
Univariate analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean ± SD and/or as median and Interquartile Range (IQR), as appropriate. Among-group comparisons were made using a non-parametric test (Kruskal-Wallis test). Categorical variables were reported as percentages. Among-group comparisons were made using a Chi-square test or a Fisher’s exact test if any expected cell count was less than five.
The association between eGFR and stroke/TIA or death at 1 year was analysed by logistic models. Odds ratios of eGFR with CKD-EPI equation were obtained with different models.
The first model included age and sex. The second model included age, sex and co-morbidities like coronary artery disease, chronic heart failure, previous stroke, previous TIA, ischaemic thrombo-embolic complications, haemorrhagic events and malignancy. At the second model, were added in the third model other potential confounding factors (variables with p < 0.10 in univariate, except those with a high number of missing data). And finally, among the last confounding factors a stepwise multiple logistic regression was used to keep only the significant variables. A significance level of 0.05 is required to allow a variable into the model (SLENTRY = 0.05) and a significance level of 0.05 is required for a variable to stay in the model (SLSTAY = 0.05). No interaction was tested. Hosmer and Lemeshow Goodness-of-Fit test was used to verify that the model was optimal.
Plots of the Kaplan-Meier curves for time to all-cause death in relation to eGFR subgroup were performed. The survival distributions have been compared using the log-rank test. Odds ratios [95% confidence intervals (CI)] comparing the categories of eGFR were derived from a logistic model.
Weighted Cohen’s kappa coefficient16 was used to assess the agreement in classification of patients in the different categories of eGFR (≥80 ml/min, between 50 and 79 ml/min, between 30 and 49 ml/min and <30 ml/min) with the 4 different equations used for eGFR.
The relationship between eGFR categories and death prediction was evaluated through the AUCs of the ROC curves and ROC curves were then compared according to De Long, De Long and Clarke-Pearson method17.
A two-sided p value of <0.05 was considered as statistically significant. All analyses were performed using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
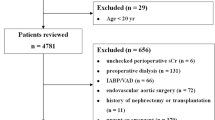
A total of 2398 patients were evaluated in the present analysis, on the basis of availability of baseline data for calculating eGFR, as well as availability of 1-year follow-up data or at least information on vital status at 1 year. This population of 2398 patients corresponds to 90.8% of the 2642 subjects with follow-up data. The distribution of patient population according to eGFR calculated with CKD-EPI equation is shown in Fig. 1. Of the study cohort, 47.2% had mild renal impairment, and 17.6% had moderate-severe renal impairment.
Clinical characteristics associated with different categories of eGFR
The clinical characteristics of enrolled patients according to kidney function, as expressed by eGFR calculated with CKD-EPI, are shown in Table 1. There was a higher prevalence of elderly subjects, heart failure, valvular alterations, diabetes mellitus, hypertension and peripheral artery disease in the lower eGFR groups. Despite this, AF was more frequently asymptomatic (EHRA score I) in patients with severely compromised eGFR.
With regard to thrombotic risk, a history of ischaemic thrombo-embolic complications but not history of prior stroke, was more common in patients with more severely compromised eGFR reflecting the higher mean CHADS2 and CHA2DS2VASc scores. A history of hemorrhagic events, but not major bleeding per se, was more commonly found in patients with severely compromised eGFR and this was associated with higher HAS BLED scores (Table 1).
Prescribed interventions and medications
Interventions and medications at discharge/after consultation according to stages of renal function are shown in Table 2. There was more use of a rate control strategy in patients with progressively worse renal function. Overall use of antithrombotic drugs differed according to eGFR, with significantly lower prescription at discharge of oral anticoagulants (Table 2). Among AF patients with eGFR <30 ml/min/1.73 m2 around 30% did not receive oral anticoagulants.
Outcomes at 1-year follow up and relationships with eGFR
Outcomes at 1-year follow up according to stages of renal function (eGFR with CKD-EPI equation) are shown in Table 3. A progressive increase in the rate of adverse events observed at 1-yr follow up was found, mainly related to a steep increase in all-cause death in subgroups of patients belonging to categories with lower eGFR. For all-cause death and for the composite end-point of ‘stroke/TIA/death’ the event rate at 1-year was almost 10-fold higher in patients with eGFR below 30 ml/min/1.73 m2, as compared to patients with eGFR above 80 ml/min/1.73 m2. Readmissions to hospital for cardiac reasons was also common (approximately one third of patients) with limited differences among eGFR subgroups, while readmission for non cardiac reasons was more common when renal function was more compromised.
In order to assess the potential impact of confounding factors on the relationship between eGFR and outcome, different models were considered with variable adjustments. The evolution of odds ratio for from crude models to fully adjusted models is reported in Table 4. As shown, the impact of confounding factors changes the values of odds ratio, but the odds of CKD stages still remains significant in all models.
Kaplan Meier curves of freedom from all-cause death according to different categories of renal function (eGFR with CKD-EPI equation) are shown in Fig. 2. Survival was significantly worse with eGFR below 30 ml/min (Log rank chi-square = 144.88, p < 0.0001).
eGFR with MDRD, Cockcroft-Gault and adjusted Cockcroft-Gault equations and outcomes
In Table 5 we show other calculations of eGFR using equations based on serum creatinine, and used in clinical practice, that is, MDRD, Cockcroft-Gault, Cockcroft-Gault adjusted for BSA. As expected, some differences were found in the number of patients allocated to each category of eGFR, according to the different formulas.
Using Cohen’s weighted K test for the concordance of attribution to each class of eGFR, we found agreement between the categorisations based on Cockcroft-Gault and Cockcroft-Gault adjusted equations, and those based on either CKD-EPI or MDRD equations were moderate to substantial (weighted K coefficients between 0.5755 and 0.6404)16. Agreement between attributions based on CKD-EPI and MDRD was high and could be interpreted as “almost perfect agreement” (weighted K coefficient of 0.8918)16.
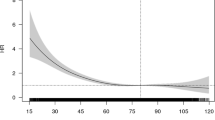
Kaplan Meier curves of freedom form all-cause death according to different categories of renal function, by calculating eGFR with MDRD, Cockcroft-Gault and Cockcroft-Gault adjusted for BSA equations are shown in the Supplementary web-only material (Figures w1, w2, w3). Irrespective of eGFR equation used, survival at 1 year significantly differed according to eGFR categorisation. According to AUCs of the ROC curves, the best discriminant capability for death prediction according to eGFR categories was found for the Cockcroft-Gault equation adjusted for BSA (p < 0.0001 vs. MDRD equation and p = 0.0238 vs. CKD-EPI equation), followed by Cockcroft-Gault equation (p = 0.0002 vs. MDRD equation and p = 0.0676 vs. CKD-EPI equation), and CKD-EPI equation (p = 0.0023), while the worst was found for the MDRD equation (Fig. 3). No statistically significant difference was found between Cockcroft-Gault equation and the same equation adjusted for BSA (p = 0.9205).
Discussion
We found that in a group of patients with AF presenting to cardiologists across Europe evaluation of renal function with eGFR has important implications with regard to 1-year outcome (all-cause death or the combined end-point Stroke/TIA/death). Our principal findings indicate that only around 35% of patients have a normal eGFR and that AF is more frequently asymptomatic when it occurs in patients with severely compromised eGFR. Second, with regard to outcomes at 1-year follow up, we found that lower eGFR categories are associated with a steep increase in all-cause death, and with more hospital readmission for non cardiac reasons. Indeed, a reduced eGFR category appears to be a strong independent predictor of the end point of stroke/TIA or death and even mild or moderate impairments in renal function are associated with a significantly increased risk. Third, the concordance between the different equations for estimating GFR was variable, and the best discriminant capability for the risk of death was found for the Cockcroft-Gault equation adjusted for BSA, followed by Cockcroft-Gault equation, and CKD-EPI equation, while the MDRD equation had the worst performance.
EORP-AF is a “real world” cardiology registry exploring all the spectrum of patients with AF presenting to cardiologists, with no exclusions due to characterization of AF as “valvular” or “non valvular”, extent of renal or hepatic dysfunction, age, malignancy or other co-morbidities10,11,12,13,14,15. Only around one third of the patients enrolled in EORP-AF had a normal eGFR (i.e. >80 ml/min/1.73 m2) and therefore the finding that any category of eGFR below this normal value is associated with a worse outcome has clinical implications applicable to a large proportion of AF patients.
In our study, significant differences in outcome at 1 year were found between different categories of renal function with regard to all-cause death and the combined end-point of stroke/TIA/death, while limited differences were found in the occurrence of stroke. The percentage of patients treated with oral anticoagulants, according to guidelines was relatively high (around 83–84%), with the only exception seen in patients with eGFR < 30 ml/min/1.73 m2, where the use of oral anticoagulants dropped below 70%. The overall high use of anticoagulants may explain the relatively low incidence of strokes in a 1-year follow up period. As shown by Marijon et al. with regard to patients with non-valvular AF, the majority of deaths that occur in a contemporary anticoagulated AF population are not related to stroke, which per se only accounted for 7% of the cases of deaths observed18.
In EORP-AF, hospitalizations for non cardiac causes markedly increased in relation to decreased renal function and this finding, related to the frequent association between CKD and other co-morbidities, such as hypertension, coronary disease and diabetes mellitus, has important implications in terms of organization of health care (need for a multidisciplinary approach) and cost of care2,19.
Indeed, even mild to moderate reduction of eGFR have an impact on patients outcome in terms of increased risk of the combined end-point corresponding to TIA/stroke or death at 1 year. Of note, the increase in risk related to renal function can be more appropriately appreciated by considering allocation to categories of eGFR, rather than by considering more generic information such as “history of CKD”.
Our findings emphasise that assessment of eGFR, in daily practice should be done not only for calculating the appropriate dosing of drugs eliminated by the kidney, such as NOACs, but also for identifying those patients who have a worse prognosis and require more strict clinical surveillance. Detection and treatment of associated conditions like uncontrolled hypertension, diabetes or significant coronary disease are mandatory, as well as detection of (micro)albuminuria and assessment by a nephrologist to prevent further deterioration of renal function. Previous registries that investigated the outcome of patients with non valvular AF, including the ATRIA study20 and the Loire Valley Atrial Fibrillation Project21 did not analyse differences in outcome above a value of eGFR ≥ 60 ml. Our study provides the clinically important information that the risk of death or TIA/stroke/or death at 1 year is elevated even in patients in whom renal function is considered only slightly decreased, i.e. the risk is two-fold for patients with eGFR calculated with CKD-EPI formula between 50 and 79, as compared to patients with eGFR ≥80 ml/min/1.73 m2.
In clinical practice, many equations have been proposed for calculating eGFR on the basis of serum creatinine2,9, but it is unclear what formula is more commonly used or should be used. The KDIGO document recommends use of the CKD-EPI equation9, while randomized trials that validated NOACs versus warfarin used the Cockcroft-Gault equation for dosing NOACs through eGFR2,8,22 and many laboratories report eGFR calculated with MDRD formula anytime serum creatinine is measured. In general, as already reported2, the clinician should remain aware of caveats for any estimating equation.
The MDRD23 equation uses 4 variables (age, gender, serum creatinine and ethnicity) to calculate eGFR and is widely used, especially in routine reports by many laboratories. The CKD-EPI equation24, which uses the same 4 variables as the MDRD, is becoming more widely adopted25, according to recommendations9, and appears to have greater accuracy and precision than the MDRD equation, with less bias at GFR > 60 ml/min/1.73 m226. The Cockcroft-Gault equation, proposed around 40 years ago27, has a series of bias in patients with a high body weight or BMI and its overall accuracy is lower than that of the two other formulas28. In this context, our analysis on the concordance in eGFR values and in allocation to different classes of renal dysfunction according to the different formulas used for eGRF, as done in our analysis, is of clinical value. While the discordance between MDRD, CKD-EPI and Cockcroft-Gault formulas was previously reported by Manzano-Fernandez et al.29 with regard to dosing of NOACs,no prior analysis has compared these formulas with regard to prognostic implications in AF patients. In our study, the concordance in allocation to eGFR categories was lower when considering the Cockcroft-Gault equation versus the other two equations, similarly to what found in patients with heart failure30.
With regard to the risk of death significant differences according to allocation to different eGFR categories were found. Even if the predicting capabilities may show some variations according to the equation used for eGFR calculation, the prediction of worse outcome in the presence of a low eGFR category is maintained for every specific eGFR formula adopted. Of note, in our study, the best discriminant capability for death at 1 year was found for the Cockcroft-Gault equation adjusted for BSA, suggesting that this should perhaps be the method of choice when using eGFR for predicting the risk of death.
Limitations
The patients were enrolled in EORP AF through cardiology clinics and therefore the study findings cannot be generalized to patients treated by internists, or general practitioners. Albuminuria, which is an important component of assessment of kidney dysfunction was not evaluated, similarly to many other studies performed in the cardiology setting2,21. Finally, as in any observational study we cannot exclude the presence of some residual confounding, persisting despite adjustment for a series of variables, due to unmeasured factors, or binarily categorized factors.
Conclusions
In this one-year follow-up analysis of a registry of “real world” patients with AF followed by cardiologists, renal function was a major determinant of adverse outcomes, and even mild or moderate impairments in renal function were associated with an increased risk of stroke/TIA/death. The best discriminant capability for death according to eGFR categories was found for the Cockcroft-Gault equation adjusted for BSA.
Additional Information
How to cite this article: Boriani, G. et al. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci. Rep. 6, 30271; doi: 10.1038/srep30271 (2016).
Change history
22 February 2017
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Boriani, G., Diemberger, I., Martignani, C., Biffi, M. & Branzi, A. The epidemiological burden of atrial fibrillation: a challenge for clinicians and health care systems. Eur Heart J. 27, 893–894 (2006).
Boriani, G. et al. Chronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: clinical significance and implications for decision making-a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 17, 1169–1196 (2015).
Lip, G. Y. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J. 34, 1041–1049 (2013).
Lip, G. Y. H. & Lane, D. A. Stroke Prevention in Atrial Fibrillation: A Systematic Review. JAMA. 313, 1950–1962 (2015).
Olesen, J. B. et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 367, 625–635 (2012).
De Caterina, R. & Camm, A. J. What is ‘valvular’ atrial fibrillation? A reappraisal. Eur Heart J. 35, 3328–3335 (2014).
Boriani, G. et al. Non-valvular atrial fibrillation: potential clinical implications of the heterogeneous definitions used in trials on new oral anticoagulants. J Cardiovasc Med (Hagerstown). 16, 491–496 (2015).
Heidbuchel, H. et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 17, 1467–1507 (2015).
Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney international. 3 (Suppl): 1–150 (2013).
Lip, G. Y. et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace. 16, 308–319 (2014).
Lip, G. Y. et al. ‘Real-world’ antithrombotic treatment in atrial fibrillation: The EORP-AF pilot survey. Am J Med. 127, 519–529 (2014).
Lip, G. Y. et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J. 35, 3365–3376 (2014).
Lip, G. Y. et al. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. 17, 24–31 (2015).
Lip, G. Y. et al. Regional differences in presentation and treatment of patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Atrial Fibrillation (EORP-AF) Pilot General Registry. Europace. 17, 194–206 (2015).
Boriani, G. et al. Asymptomatic atrial fibrillation: clinical correlates, management and outcomes in the EORP-AF Pilot General Registry. Am J Med. 128, 509–518 (2015).
Viera, A. J. & Garrett, J. M. Understanding interobserver agreement: the kappa statistic. Fam Med. 37, 360–363 (2005).
Delong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two ro more correlated receiver operating characteristic curves: A nonparametric approach Biometrics. 44, 837–845 (2008).
Marijon, E. et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 128, 2192–2201 (2013).
Boriani, G. et al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European Heart Rhythm Association. Eur Heart J. 34, 1869–1874 (2013).
Go, A. S. et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 119, 1363–1369 (2009).
Banerjee, A. et al. A prospective study of estimated glomerular filtration rate and outcomes in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest. 145, 1370–1382 (2014).
January, C. T. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 64, e1–76 (2014).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modifi-cation of Diet in Renal Disease Study Group. Ann Intern Med 130, 461–470 (1999).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150, 604–612 (2009).
Kornej, J. et al. Changes in renal function after catheter ablation of atrial fibrillation are associated with CHADS2 and CHA2DS2-VASc scores and arrhythmia recurrences. Heart. 101, 126–131 (2015).
Earley, A., Miskulin, D., Lamb, E. J., Levey, A. S. & Uhlig, K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 156, 785–795 (2012).
Cockcroft, D. W. & Gault, M. H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41 (1976).
Michels, W. M. et al. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 5, 1003–1009 (2010).
Manzano-Fernández, S. et al. Comparison of estimated glomerular filtration rate equations for dosing new oral anticoagulants in patients with atrial fibrillation. Rev Esp Cardiol (Engl Ed). 68, 497–504 (2015).
Zamora, E. et al. Estimated glomerular filtration rate and prognosis in heart failure: value of the Modification of Diet in Renal Disease Study, Chronic Kidney Disease Epidemiology collaboration, and Cockroft-Gault formulas. J Am Coll Cardiol. 59, 1709–1715 (2012).
Acknowledgements
The work of a series of persons was important for coordination of the registry, data collection and data management: Executive steering committee of the EurObservational Research Programme – Atrial Fibrillation (EORP-AF) Pilot General Registry of the European Society of Cardiology (ESC): Gregory Y.H. Lip, Luigi Tavazzi, Aldo P. Maggioni, Harry JGM Crijns, Paulus Kirchhof, and Panos Vardas. Steering Committee (National Coordinators): Gheorghe-Andrei Dan, Dan Atar, Emmanuel Simantirakis, Massimo Santini, Zbigniew Kalarus, Lars Hvilsted Rasmussen, Mário Martins Oliveira, and Georges Mairesse. Data monitor and technical support team: Data collection was conducted by the EurObservational Research Programe Department from the ESC by Viviane Missiamenou. Statistical analyses were performed by Cecile Laroche with the support of Renato Urso. Overall activitieswere coordinated by Aldo P. Maggioni (Scientific Coordinator EORP) and Thierry Ferreira (Head of Department EORP). EORP Sponsors: Since the start of EORP, the following companies have supported the programme: Abbott Vascular Int. (2011–2014), Amgen (2012–2018), AstraZeneca (2014–2017), Bayer (2013–2018), Boehringer Ingelheim (2013–2016), Boston Scientific (2010–2012), The Bristol Myers Squibb and Pfizer Alliance (2014–2016), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Gedeon Richter Plc. (2014–2017), Menarini Int. Op. (2010–2012), MSD-Merck & Co. (2011–2014), Novartis Pharma AG (2014–2017), ResMed (2014–2016), Sanofi (2010–2011), SERVIER (2012–2018). A full list of EORP consortium members appears in the Supplementary Information.
Author information
Authors and Affiliations
Contributions
G.B. conception and design, interpretation of data, writing of the manuscript. C.L. analysis and interpretation of data. I.D. interpretation of data, critical review of the manuscript. M.I.P. critical review of the manuscript. L.H.R. critical review of the manuscript. L.P. critical review of the manuscript. H.J.G.M.C. supervision of the research plan and of the manuscript. L.T. supervision of the research plan and of the manuscript. A.P.M. conception and design, supervision of the research plan and of the manuscript. G.Y.H.L. conception and design, writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.Boriani—received small speaker’s fees from Boehringer, Medtronic Inc and Boston Scientific. L.H.Rasmussen—speaker bureaus for Bayer, BMS/Pfizer, Janssen Pharmaceuticals, Takeda, Roche Diagnostics, and Boehringer Ingelheim. L.Tavazzi—consultant and Speakers bureau member for Servier; Committee Member for Servier, Medtronic, St Jude Medical, CVIE Therapeutics, Boston Scientific, Vifor Pharma, Cardiorentis. G.Y.H.Lip—consultant for Bayer, Medtronic, Sanofi, BMS/Pfizer, Daiichi-Sankyo, and Boehringer Ingelheim, and has been a speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo and Medtronic. Other authors—none declared in relation to this manuscript.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Boriani, G., Laroche, C., Diemberger, I. et al. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci Rep 6, 30271 (2016). https://doi.org/10.1038/srep30271
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30271
This article is cited by
-
Changes in estimated glomerular filtration rate before and after the first visit for atrial fibrillation
BMC Nephrology (2024)
-
Prescription of DOACs in Patients with Atrial Fibrillation at Different Stages of Renal Insufficiency
Advances in Therapy (2023)
-
Kidney function monitoring and trajectories in patients with atrial fibrillation
Clinical and Experimental Nephrology (2023)
-
Characterization of baseline clinical factors associated with incident worsening kidney function in patients with non-valvular atrial fibrillation: the Hokuriku-Plus AF Registry
Heart and Vessels (2023)
-
Impact of baseline renal function on the efficacy and safety of different Anticoagulants in Atrial Fibrillation Patients – A cohort study
Thrombosis Journal (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.