Abstract
Sulfur chemistry is of great interest to the atmospheric chemistry of several planets. In the presence of water, oxidized sulfur can lead to new particle formation, influencing climate in significant ways. Observations of sulfur compounds in planetary atmospheres when compared with model results suggest that there are missing chemical mechanisms. Here we propose a novel mechanism for the formation of sulfurous acid, which may act as a seed for new particle formation. In this proposed mechanism, the lowest triplet state of SO2 (3B1), which may be accessed by near-UV solar excitation of SO2 to its excited 1B1 state followed by rapid intersystem crossing, reacts directly with water to form H2SO3 in the gas phase. For ground state SO2, this reaction is endothermic and has a very high activation barrier; our quantum chemical calculations point to a facile reaction being possible in the triplet state of SO2. This hygroscopic H2SO3 molecule may act as a condensation nucleus for water, giving rise to facile new particle formation (NPF).
Similar content being viewed by others
Introduction
Sulfur compounds have been observed in the atmospheres of a number of planets and moons in our solar system including Venus, Earth, Mars, and Io1,2,3 and are known to be important to the evolution of planetary atmospheric chemistry and climate. Photochemical models have been used to explain observations of sulfur in the atmosphere of Earth4. Additionally, the models have more recently been applied to ground based observations and measurements from orbiters such as Venus Express5,6,7,8,9, attempting to explain observations of H2SO4 and of sulfur oxides (SO, SO2, SO3) in the middle atmosphere of Venus, a natural laboratory in which to study sulfur chemistry10. However, several discrepancies have arisen between the models and observations. For example, high SO2 mixing ratios are observed above 90 kilometers in the Venusian atmosphere exceeding model predictions by orders of magnitude9. Red light overtone pumping photochemistry of H2SO411 was previously applied to explain sulfur dioxide profiles and aerosol in Earth’s atmosphere12,13 and has been included in the models of the atmosphere of Venus14. While this chemistry was sufficient to explain SO2 profiles on Earth, it does not fully explain the observed sulfur oxide profiles on Venus.
In an oxidizing atmosphere, SO2 is transformed to highly hygroscopic sulfuric acid, H2SO4. Because of its hygroscopicity and ubiquitous nature in Earth’s atmosphere, gas phase sulfuric acid is considered to be a critical agent in much of atmospheric new particle formation (NPF)15,16. Its gas-phase formation is via the reaction of OH with SO2, with the resulting HOSO2 species rapidly reacting with atmospherically abundant O2, to form SO3, whose hydrolysis reaction with water forms H2SO4. Two water molecules, perhaps as a water dimer, are required for this last process, giving rise to a strong temperature (T)- and relative humidity (RH)-dependent hydrolysis rate17,18,19,20. Once formed, the very hygroscopic sulfuric acid molecule provides a good nucleus for the formation of small water clusters, which then may grow by rapid condensation of further water molecules onto this nucleus. The presence of ammonia or small amines in the Earth’s atmosphere is believed to further aid the water condensation process by reducing the water activity in the growing condensation nucleus15,16,21,22.
The importance of sunlight to this process is well established: new particle formation is seen to occur primarily during daylight hours16. Photochemical NPF in Earth’s lower atmosphere, which has available light at wavelengths > ~280 nm only, cannot involve direct SO2 photodissociation because the threshold wavelength for the dissociation SO2 → SO + O lies near 217 nm (551 kJ/mol), Rather, as suggested by the mechanism presented above, photons are required for the production of OH radicals that initiate the oxidation SO2 to H2SO4 in the presence of water. Therefore, according to the standard mechanism, for NPF to take place several requirements must be met: At a minimum, there must be sufficient OH to oxidize the SO2 and there must be a sufficient concentration of water vapor, at a low enough temperature to produce H2O dimers and then hydrolyze SO3 to H2SO4; additionally, the process is aided by a source of ammonia or some other alkaline gas.
In the following we use results of quantum chemical calculations to outline a novel reaction for SO2 with water and test this proposed mechanism against a laboratory experiment of particle formation. Theoretical approaches have proven useful in exploring the reactions of SOx-H2O systems (see, e.g.)10,14,15. The mechanism involves the near-UV photoexcitation to the lowest forbidden triplet state (3B1) of SO2, either directly, or via intersystem crossing from the allowed excited singlet state (1B1), which lies at somewhat higher energy. The triplet state molecule then reacts with water, along a barrierless pathway, forming ground state H2SO3. A singlet-triplet surface crossing in the entrance channel to the reaction ensures a rapid evolution to ground state products. If this mechanism is correct, it suggests the possibility that this chemistry potentially plays a role in NPF in the atmosphere. The H2SO3 product could conceivably act as a nucleus for water condensation. Experiments in which mixtures of gas phase water and SO2 are illuminated with visible-near UV light (at wavelengths too long, λ > 280 nm, for significant OH formation) show rapid NPF when the gas mixture is illuminated, with no NPF observed in the absence of water or light.
Results
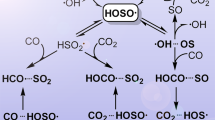
Briefly, we propose that a triplet-state SO2 molecule, containing approx. 3 eV more energy than the ground state, may collide with a water molecule in a way which accesses a surface crossing to the sulfurous acid product on the ground state singlet potential energy surface. The structures and energies of the key chemical species, as calculated at the CCSD/ 6-311++G(3df,3pd) level, are illustrated in Fig. 1. Full results of the calculations at this level, as well as those of a preliminary scan at a lower level of theory are given in Tables S1 (for the lower level) and S2 of the Supplementary Information (SI), with reaction energetics reported in Table S3.
Singlet pathways are shown in black and triplets are in red. The geometries of some key stationary points are shown. The proposed mechanism is illustrated as the green line. It begins on the triplet surface, accessed as indicated by the blue arrows, either by direct excitation or intersystem crossing from a singlet, then switches to the singlet (in the region shown by the circle) then proceeds to the ground state product.
In good agreement with previous studies from this group23,24 and others25,26, the zero-point corrected energetic barrier to the simple hydrolysis reaction on the ground state (singlet) potential energy surface is calculated to lie 34.8 kcal/mol above reagents. Also in agreement with previous work, the overall reaction is calculated to be endoergic, with a (zero-point corrected) △Eo° = +5.6 kcal/mol. These values fall well within the range of those previously reported10,11,12,16, providing some confidence in the energetics calculated at this level of theory.
We calculate that the lowest-energy triplet state of SO2 is of 3B1 symmetry, with a somewhat larger S-O bond length (1.499 Å vs. 1.443 Å) and a somewhat larger O-S-O angle (125.8° vs. 118.7°) than those calculated for in the ground 1A1 state. These geometries are in excellent accord with those reported experimentally27. At its equilibrium geometry the 3B1 state is calculated to lie 71.8 kcal/mol above the ground state; the calculated vertical excitation energy lies at 99.4 kcal/mol (corresponding to a maximum in absorption of 288 nm). The triplet state energy is in very good accord with the experimental adiabatic energy difference (73.8 kcal/mol)27,28. The transition state connecting triplet-state reactants and products lies at low energy – about 6.5 kcal/mol above the reagents – and may be achieved by the approach of a water molecule in such a way that the oxygen and one hydrogen of water lie in the plane of the triplet SO2, and bisect the O-S-O angle, which closes slightly, from 126° to 106°, while the O-S bond lengths increase slightly, to 1.54 Å. The distance between the oxygen of water and the sulfur at the calculated transition state is 2.88 Å, compared to those in ground state H2SO3 of 1.63 Å for the HO-S distances and 1.46 Å for the S = O distance. The fairly low calculated barrier suggests that it might only require a very gentle encounter between a water molecule and a triplet SO2 to achieve this TS geometry. Indeed, whether the 3B1 state is accessed directly, via a vertical excitation, or via intersystem crossing from the (allowed) excited 1B1 singlet state (at 79.8 kcal/mol excitation)29, it will initially contain sufficient energy to overcome the barrier.
At this geometry, the energy of the singlet ground state surface is calculated to lie 5.4 kcal/mol below that of the triplet - a close singlet-triplet encounter. Strong spin-orbit interactions are known to couple singlet and triplet states in SO228,29; since water is known to be an efficient quencher of the SO2 triplet state30 it follows that collisions with water molecules may induce efficient intersystem crossing. Thus it seems likely that the triplet SO2-H2O system could access the singlet surface, which could then evolve towards H2SO3 product. From the triplet transition state a simple rotation of the SO2 moiety “away” from the water will transform the geometry to that of ground state H2SO3. This pathway is illustrated in Fig. 1.
If the system evolves to singlet products as we postulate, the resulting sulfurous acid is calculated to exhibit strong attraction towards water, with zero-point corrected binding energies of 7.7, 7.9 and 6.2 kcal/mol for the addition of one, two and three water molecules, respectively at a lower level of calculation, B3LYP/6-311+G(2df,2p) (see Table S4 for details). The binding energy to a single water molecule is about three times that calculated for the water dimer at the same level of theory (2.65 kcal/mol), also given in the SI. If it is formed, H2SO3 is therefore likely to act as a condensation nucleus for water, and could then participate in NPF.
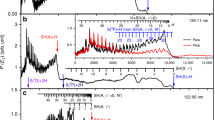
We test this chemistry by performing an experiment in which a mixture of either 3 or 1 Torr of SO2 with 7 Torr of H2O is introduced into a glass flow cell, then illuminated with a Xe arc lamp through a 295 nm long-pass filter. (See Figure S1 in the SI for absorption spectra of the mixture and the filter transmission). Light scattering from aerosols is observed immediately upon illumination of the SO2 + H2O mixture and is recorded by measuring the decrease in transmitted intensity of a 532 nm laser beam passed through the cell. In Fig. 2 we display a typical result from such experiments: Illumination of either SO2 or H2O alone by the arc lamp does not induce light scattering, but illumination of the gas mixture causes a rapid appearance of particles in the flow cell, as indicated by the onset of strong light scattering. No scattering of the probe laser is observed in the absence of illumination.
The scattered light intensity is displayed as a voltage vs. time during the experiment. The red and green traces show the scattering observed from 7.010 Torr of water and 3.015 Torr of SO2, respectively. The blue trace shown is for a mixture of 7.010 Torr of water and 0.891 Torr of SO2, and the black trace shows scattering from a mixture of 7.017 Torr water and 3.3 Torr SO2. No scattering is observed in the absence of light (−20–0 min); only when SO2 and H2O are both present is scattering (indicating particle formation) observed upon illumination (0–90 min). The amount of scattering is greater with higher initial SO2 partial pressure.
It is highly unlikely that NPF is induced in this experiment via OH-mediated oxidation of SO2 in the presence of water. There is no oxygen (or ozone) present in the reaction cell and the 295 nm long-pass optical filter (1% transmission at 276.5 nm) removes any UV radiation with sufficient energy to initiate bond-cleavage in SO2 or H2O; therefore radical formation is highly unlikely. We tested this expectation by introducing cyclohexane, an OH scavenger, into the cell; this is expected to reduce or eliminate any OH-mediated reactions taking place there. In the absence of SO2, the addition of C6H12 did not show any effect, either in the light or the dark, with or without water present, demonstrating the lack of OH chemistry in these experiments. However, when SO2 was also present, particle formation was seen under illumination, even without water (see Figure S2). We suggest that this observation arises from the highly reactive nature of 3SO2 with organics31, which would give rise to radical condensation type reactions in the cell. Any heating due to light absorption in the cell would be expected to mitigate NPF, so small temperature increases are also unlikely to be responsible for the observed particle formation. However, the light transmitted by the filter does overlap with the near-UV absorption spectrum of SO2 (as displayed by Figure S1 in SI), making SO2*-mediated photochemical processes possible.
When SO2 is excited into the manifold of states accessible in the near-UV spectral region it is known to undergo rapid and irreversible decay into lower-lying triplet levels29. Photochemistry involving S-O bond cleavage is energetically impossible for a single SO2 molecule at these excitation wavelengths, so any chemical fate must involve a reaction of excited SO2. Although SO3 formation via the reaction of triplet SO2 with ground state SO2 has been reported32, the rate constant for this reaction is 20 times smaller than the quenching rate of triplet SO2 by water30. If NPF in our experiment is due to SO3 hydrolysis one expects a quadratic dependence on the SO2 concentration. Inspection of the results presented in Fig. 2 shows that this is not the case: increasing partial pressure of SO2 by a factor of ~3 results in only a 2-fold increase in the scattering intensity, rather than the order of magnitude change expected for a quadratic dependence.
The rate of collisional quenching of phosphorescence from triplet SO2 by water has been reported only once to our knowledge30. That report gives a collisional quenching rate constant of 1.4 × 10−12cm3 molec−1 s−1 - about 1% of the gas-kinetic collisional rate constant, and at least 10 times greater than quenching by N2, O2 or rare gases30. The products of this quenching reaction were not identified, but our observation of NPF clearly supports a chemical process such as the triplet reaction we propose here. A simple kinetic box model of the illuminated SO2 - H2O system, with relevant rate constants4,20,33, and assuming the quenching of 3SO2 by water30 to be totally due to reaction, shows that H2SO3 formation in our cell (with Torr) dominates over H2SO4 when the SO2 partial pressure is less than about 2.4 Torr. These results are presented in the SI.
Discussion
In the above, we have demonstrated a novel photochemical mechanism for the formation of sulfurous acid, an unstable molecule that has not been isolated in the gas phase. One consequence of this chemistry is the formation of new particles from mixtures of illuminated SO2 and water in the absence of gas phase oxidants. We suggest that the previously reported, efficient quenching of triplet state SO2 by water30 may proceed to some extent via chemical reaction to form hygroscopic sulfurous acid directly. However, this species is highly unstable23,24,25,34, and has not been observed conclusively, either in the gas phase or in solution34. Indeed, a modeling study has demonstrated that hydrolysis of SO3 is more significant than that of SO2 in the atmosphere, in spite of the latter’s much greater abundance35. One important reason for this is that, unlike H2SO4 generated by hydrolysis of SO3, hydrolysis of ground state SO2 to give H2SO3 is an endothermic reaction23,24,25,34,35,36. Additionally, as with the hydration of SO3, there is a high barrier to H2SO3 formation from SO2+H2O; this barrier drops somewhat with the participation of more water molecules23,25,34,36, but is still too high to overcome at normal atmospheric temperatures. One way to overcome the instability in the gas phase has been proposed recently: the participation of ammonia lowers the barrier for the hydrolysis reaction25. However, given that there are at least three molecules required for this process to become energetically viable, the entropic cost of the reaction will increase considerably.
Thus the novel photochemically driven hydrolysis of SO2 mechanism reported here is of interest as a potential synthetic route to the elusive sulfurous acid molecule and may also be important in some planetary atmospheres, such as on Venus. To explore when this might be operational in Earth’s atmosphere, we take the upper limit for the 3SO2 formation rate to be the rate of absorption of actinic photons by ground state SO2 (that is, assume that every excited molecule becomes a triplet), and the loss rate of the triplet to be that of triplet deactivation by air. This yields a steady-state fraction of SO2 in the triplet state of about 10−12 - 10−11. Given a concentration ratio of water vapor to OH radicals on the order of 1010, the collision rate of OH with SO2 is about 10–100 times that of collisions of 3SO2 with water. Therefore we would expect that SO3 formation would dominate under these atmospheric conditions. However, since the hydrolysis of SO3 depends on the square of the water vapor concentration8,19, under conditions of very low humidity, the triplet mechanism may be a substantial contributor to NPF and play some role in Earth’s atmosphere. Indeed, Loerting et al.35 also suggest that the energetically unfavored hydrolysis of ground state SO2 may become as important as SO3 hydrolysis under such conditions.
The box model analysis of our experimental results bears this out. Taking an upper limit for the reaction of 3SO2 with water to be the quenching rate, the box model results in Figure S3 show that sulfurous acid formation dominates over sulfuric acid formation in our experimental setup. Even assuming the reactive quenching to be only 10% of the total only reduces the amount of H2SO3 to be about half of that of the H2SO4 produced in the model; that is, the triplet hydrolysis mechanism is still significant. Of course, in Earth’s atmosphere the SO2*+SO2 reaction will not be important, due to the much lower concentrations of sulfur dioxide. We conclude that, although under some circumstances SO3 formation (and thus NPF initiated by sulfuric acid) is certainly possible via reaction of ground-state with excited-state SO2 molecules, H2SO3 is likely a significant contributor to the NPF we observed here, and may play an important role in NPF in other planetary atmospheres where water may be limited such as in the Earth’s upper stratosphere or the middle atmosphere of Venus. In these cases H2SO3 formation may be favored over the water-catalyzed formation of H2SO4.
Methods
Density-functional theory was used to perform initial energy scans of the singlet and triplet reactions, using the Spartan 14 series of programs37,38 running on a Mac under the OS-X operating system. For this, the geometries were optimized and the energies were calculated at the B3LYP 6-311 + G(2df, 2p) level. Following the identification of the important stationary points, further geometry optimizations were carried out at the B3LYP/6-311++G(3df,3pd) level, with zero-point energies calculated from the predicted harmonic vibrational frequencies without application of a correction factor. Ab-initio single-point energies including configuration interaction were then calculated at these reoptimized geometries using CCSD and the 6-311++G(3df, 3pd) basis set. The overall accuracy of the energetics is assumed to be similar to that reported in Fu et al.39.
The experimental setup consisted of a glass cell with quartz windows evacuated using an Edwards 12 E2-M12 dual stage rotary vane mechanical pump. The cell was a 90 degree angle square cross with a 2.5 inch diameter and 8 inch long pathlength. It was pumped out overnight to remove impurities or potential contaminants and then filled with the sample of interest (SO2, H2O or a mixture of the two) to p = 7.010 Torr water, degassed by 3–5 freeze pump thaw cycles. A sulfur dioxide (>99.9% purity, Sigma Aldrich) pressure of p = 3.015 Torr was used alone, or a mixture of the two (water p = 7.010 Torr with 3.3 Torr of SO2 or water p = 7.017 Torr with 0.891 Torr of SO2) was introduced to the cell. The cell was allowed to equilibrate for at least 20 minutes and then was exposed to light from a 450 watt Xe arc lamp (Newport) filtered using a N-WG-295 50 mm diameter, 3 mm thick, longpass optical filter (Edmond Optics) with a stopband limit (0.001% transmittance) of 250 nm, passband limit of 400 nm, and a cut-off position (50% transmittance) of 300 ± 6 nm. Scattering data was collected using a Brightline Pro green dot projecting alignment laser (Laserglow Technologies) detected by a PDA155 amplified silicon photodetector (Thorlabs). The laser was directed through an iris and along the 8 inch pathlength of the cell, through a second iris, and then detected using the photodetector.
Additional Information
How to cite this article: Donaldson, D. J. et al. Gas-phase hydrolysis of triplet SO2: A possible direct route to atmospheric acid formation. Sci. Rep. 6, 30000; doi: 10.1038/srep30000 (2016).
References
Voegele, A. F. et al. Sulfurous acid (H2SO3) on Io? Icarus 169, 2422–2249 (2004).
Tian, F. et al. Photochemical and climate consequences of sulfur outgassing on early Mars. Earth and Planetary Science Letters 295, 412–418 (2010).
Hu, R., Seager, S. & Bains, W. Photochemistry in Terrestrial Exoplanet Atmospheres. I. Photochemistry Model and Benchmark Cases. The Astrophysical Journal 761, 166 (2012).
Rinsland, C. P. et al. H2SO4 Photolysis: A source of sulfur dioxide in the upper stratosphere. Geophys. Res. Lett. 22, 1109–1112 (1995).
Belyaev, D. et al. Vertical profiling of SO2 and SO above Venus’ clouds by SPICAV/SOIR solar ocultation. Icarus 217, 740–751 (2012).
Marcq, E. et al. A latitudinal survey of CO, OCS, H2O, and SO2 in the lower atmosphere of Venus: Spectroscopic tudies using VIRTIS-H. J. Geophys. Res.- Planets 113, E00B07 (2008).
Marcq, E. et al. An investigation of the SO2 above the clouds of Venus using SPICAV-UV in nadir mode. Icarus 211, 58–69 (2011).
Sandor, B. J., Clancy, R. T. & Moriarty-Schievena, G. H. Upper limits for H2SO4 in the mesosphere of Venus. Icarus 217, 839–844 (2012).
Sandor, B. J., Clancy, R. T., Moriarty- Schieven, G. & Mills, F. P. Sulfur chemistry in the Venus mesosphere from SO2 and SO microwave spectra. Icarus 208, 49–60 (2010).
Mills, F. P. & Allen, M. A review of selected issues concerning the chemistry in Venus’ middle atmosphere. Planetary and Space Science 55, 1729–1740 (2007).
Vaida, V., Kjaergaard, H. G., Hintze, P. E. & Donaldson, D. J. Photolysis of sulfuric acid vapor by visible solar radiation. Science 299, 1566–1568 (2003).
Mills, M. J., Toon, O. B. & Thomas, G. E. Mesospheric sulfate aerosol layer. J. Geophys. Res. 110, D24208 (2005).
Mills, M. J. et al. Photolysis of sulfuric acid vapor by visible light as a source of the polar stratospheric CN layer. J. Geophys. Res.-Atmos 110, D08201 (2005).
Zhang, X. et al. Photolysis of sulphuric acid as the source of sulphur oxides in the mesosphere of Venus. Nature Geoscience 3, 834–837 (2010).
Curtius, J. Nucleation of atmospheric aerosol particles. Comptes Rendus Physique 7, 1027–1045, 10.1016/j.crhy.2006.10.018 (2006).
Nieminen, T. et al. Trends in atmospheric new-particle formation: 16 years of observations in a boreal-forest environment. Boreal Env. Res. 19 (suppl. B), 191–214 (2014).
Kolb, C. E. et al. Gas-Phase Reaction of Sulfur-Trioxide with Water-Vapor. Journal of the American Chemical Society 116, 10314–10315 (1994).
Lovejoy, E. R., Hanson, D. R. & Huey, L. G. Kinetics and products of the gas-phase reaction of SO3 with water. Journal of Physical Chemistry 100, 19911–19916, 10.1021/jp962414d (1996).
Morokuma, K. & Muguruma, C. Ab-Initio Molecular-Orbital Study of the Mechanism of the Gas- Phase Reaction SO3 + H2O - Importance of the 2nd Water Molecule. Journal of the American Chemical Society 116, 10316–10317 (1994).
Larson, L. J., Kuno, M. & Tao, F. M. Hydrolysis of sulfur trioxide to form sulfuric acid in small water clusters. Journal of Chemical Physics 112, 8830–8838, 10.1063/1.481532 (2000).
Larson, L. J., Largent, A. & Tao, F. M. Structure of the sulfuric acid-ammonia system and the effect of water molecules in the gas phase. Journal of Physical Chemistry A 103, 6786–6792, 10.1021/jp991529p (1999).
Larson, L. J. & Tao, F. M. Interactions and reactions of sulfur trioxide, water, and ammonia: An ab initio and density functional theory study. Journal of Physical Chemistry A 105, 4344–4350, 10.1021/jp004354o (2001).
Kahan, T. F., Ardura, D. & Donaldson, D. J. Mechanism of Aqueous-Phase Ozonation of S(IV). Journal of Physical Chemistry a 114, 2164–2170, 10.1021/jp9085156 (2010).
Bishenden, E. & Donaldson, D. Ab initio study of SO2 + H2O. Journal of Physical Chemistry a 102, 4638–4642, 10.1021/jp980160l (1998).
Liu, J. J. et al. Mechanism of the Gaseous Hydrolysis Reaction of SO2: Effects of NH3 versus H2O. Journal of Physical Chemistry A 119, 102–111, 10.1021/jp5086075 (2015).
Li, W. K. & McKee, M. L. Theoretical study of OH and H2O addition to SO2 . Journal of Physical Chemistry A 101, 9778–9782, 10.1021/jp972389r (1997).
Heicklen, J., Kelly, N. & Partymillert, K. The photophysics and photochemistry of SO2 . Rev. Chem. Intermed. 3, 315–404 (1980).
Mai, S. The Role of Triplet States in the Excited States Dynamics of Sulphur Dioxide diploma thesis thesis, Friedrich-Schiller-Universität, (2012).
Wilkinson, I. et al. Excited state dynamics in SO2. I. Bound state relaxation studied by time-resolved photoelectron-photoion coincidence spectroscopy. Journal of Chemical Physics 140, 10.1063/1.4875035 (2014).
Sidebottom, H. W. et al. Photooxidation of sulfur dioxide. Environmental Science & Technology 6, 72–79, 10.1021/es60060a001 (1972).
Whitehill, A. R. et al. Vibronic origin of sulfur mass-independent isotope effect in photoexcitation of SO2 and the implications to the early earth’s atmosphere. Proceedings of the National Academy of Sciences 110, 17697–17702, 10.1073/pnas.1306979110 (2013).
Chung, K., Calvert, J. G. & Bottenheim, J. W. The photochemistry of sulfur dioxide excited within its first allowed band (3130 Å) and the “forbidden” band (3700–;4000 Å). International Journal of Chemical Kinetics 7, 161–182, 10.1002/kin.550070202 (1975).
Whitehill, A. R. & Ono, S. Excitation band dependence of sulfur isotope mass-independent fractionation during photochemistry of sulfur dioxide using broadband light sources. Geochimica Et Cosmochimica Acta 94, 238–253, 10.1016/j.gca.2012.06.014 (2012).
Voegele, A. E. et al. About the stability of sulfurous acid (H2SO3) and its dimer. Chemistry-a European Journal 8, 5644–5651, 10.1002/1521-3765(20021216)8:24<5644::aid-chem5644>3.0.co;2-9 (2002).
Loerting, T., Kroemer, R. T. & Liedl, K. R. On the competing hydrations of sulfur dioxide and sulfur trioxide in our atmosphere. Chemical Communications 999–1000, 10.1039/b002602f (2000).
Voegele, A. F. et al. About the Stability of Sulfurous Acid (H2SO3) and Its Dimer. Chemistry–A European Journal 8, 5644–5651, 10.1002/1521-3765(20021216)8:24<5644::aid-chem5644>3.0.co;2-9 (2002).
Spartan’ 14 (Irvine, CA, 2014).
Shao, Y. et al. Advances in methods and algorithms in a modern quantum chemistry program package. Physical Chemistry Chemical Physics 8, 3172–3191, 10.1039/b517914a (2006).
Fu, H. et al. Photosensitized production of atmospherically reactive organic compounds at the air-aqueous interface” J. Amer. Chem. Soc. 137, 8348–8352 10.1021/jacs.5b04051 (2015).
Acknowledgements
D.J.D. acknowledges ongoing support from NSERC. V.V. gratefully acknowledges financial support from the NSF (grant number CHE-1306386) and from NASA (award number NNX15AP20G). J.A.K. gratefully acknowledges partial financial support from the John B. Ekeley fellowship awarded by the Department of Chemistry and Biochemistry at the University of Colorado, Boulder.
Author information
Authors and Affiliations
Contributions
The idea about triplet state SO2 chemistry arose following discussions between D.J.D. and V.V. and D.J.D. carried out the quantum calculations. J.A.K. carried out the experiments, under the direction of V.V. and D.J.D. wrote the manuscript, with input from V.V. and J.A.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Donaldson, D., Kroll, J. & Vaida, V. Gas-phase hydrolysis of triplet SO2: A possible direct route to atmospheric acid formation. Sci Rep 6, 30000 (2016). https://doi.org/10.1038/srep30000
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep30000
This article is cited by
-
The structure, stability, thermochemistry, and bonding in SO3-(H2O)n (n = 1–7) clusters: a computational analysis
Structural Chemistry (2023)
-
Hygroscopic Coating of Sulfuric Acid Shields Oxidant Attack on the Atmospheric Pollutant Benzo(a)pyrene Bound to Model Soot Particles
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.