Abstract
MiR399 and its target PHOSPHATE2 (PHO2) play pivotal roles in phosphate signaling in plants. Loss of function mutation in PHO2 leads to excessive Pi accumulation in shoots and growth retardation in diploid plants like Arabidopsis thaliana and rice (Oryza sativa). Here we isolated three PHO2 homologous genes TaPHO2-A1, -B1 and -D1 from hexaploid wheat (Triticum aestivum). These TaPHO2 genes all contained miR399-binding sites and were able to be degraded by tae-miR399. TaPHO2-D1 was expressed much more abundantly than TaPHO2-A1 and -B1. The ion beam-induced deletion mutants were used to analyze the effects of TaPHO2s on phosphorus uptake and plant growth. The tapho2-a1, tapho2-b1 and tapho2-d1 mutants all had significant higher leaf Pi concentrations than did the wild type, with tapho2-d1 having the strongest effect and tapho2-b1 the weakest. Two consecutive field experiments showed that knocking out TaPHO2-D1 reduced plant height and grain yield under both low and high phosphorus conditions. However, knocking out TaPHO2-A1 significantly increased phosphorus uptake and grain yield under low phosphorus conditions, with no adverse effect on grain yield under high phosphorus conditions. Our results indicated that TaPHO2s involved in phosphorus uptake and translocation and molecular engineering TaPHO2 shows potential in improving wheat yield with less phosphorus fertilizer.
Similar content being viewed by others
Introduction
Phosphorus (P) is one of the three macronutrients essential for plant growth and reproduction and phosphate (Pi) is often non-available to plants because of the low abundance and immobile in soils. Therefore, P fertilizers are often required for high yield of crops in modern agriculture. However, due to the high fixation and low diffusion rate in most soils, no more than 30% of the applied P is used by the cultivated plants1. The remains results in eutrophication and nonrenewable phosphate rock waste. Thus improving P use efficiency (PUE) in crops becomes great importance in ensuring sustainable development of agriculture. Exploring the molecular mechanisms in regulation of P uptake and utilization may help us to breed wheat with improved PUE.
Plants have evolved complicated physiological and biochemical responses to adapt to the limiting P conditions. The molecular mechanisms regulating these responses have been well documented in the model plants Arabidopsis thaliana and rice (Oryza sativa)2,3. PHOSPHATE STARVATION RESPONSE 1 (PHR1) in Arabidopsis, a MYB-CC type transcription factor, plays a key role in regulating the expression of Pi starvation-induced (PSI) genes by binding to P1BS (PHR1 binding site) cis-element with an imperfect palindromic sequence4. Overexpression of PHR1 and its homologs activates the expression of many PSI genes including Pi transporters, Phosphate Starvation1 (IPS1) and miR399 and leads to excessive Pi accumulation in shoots of Arabidopsis, rice and wheat (Triticum aestivum)5,6,7,8,9,10. MiR399 is the first reported microRNA specifically induced by Pi starvation11. Under Pi deficiency conditions, miR399 is upregulated by PHR1 and then reciprocal downregulates the transcript level of Phosphate 2 (PHO2) which contains multiple miR399 target sites in the 5′-UTR (untranslated region)11,12,13. However, the miR399 activity in cleaving PHO2 is regulated by IPS1 according to the target mimicry mechanism14. PHO2, encoding an ubiquitin-conjugating E2 enzyme (UBC24), negatively regulates Pi uptake and root-to-shoot translocation. Loss of function of PHO2 and overexpression of miR399 both result in Pi over accumulation in shoots13,15,16. It has been reported that the expression of several Pi transporters were increased in pho2 mutants and miR399-overexpression plants, such as AtPHT1.8 and 1.9 in Arabidopsis12,13 and OsPT1, 2, 4 and 8 in rice16. Further analysis shows that PHO2 genetically interacts with PHOSPHATE 1 (PHO1) and PHT1.117,18. PHO1, encoding an integral membrane protein, is involved in Pi transfer from roots to shoots19,20. The ubiquitination of PHO1 mediated by PHO2 demonstrates that PHO1 is the direct downstream component of PHO217. Several high affinity Pi transporters (PHT1s) were also identified as downstream of PHO2 by using an iTRAQ (for isobaric tags for relative and absolute quantitation)- based quantitative membrane proteomic method18. PHO2 is found to mediate PHT1 proteins degradation and loss of function of PHT1.1 alleviates the Pi toxicity phenotype displayed by pho2 in Arabidopsis18. These results suggest that PHT1s act as downstream components of PHO2. Taking together, PHO2 plays a pivotal role in Pi homeostasis regulation by coordinating the activities of Pi uptake and roots to shoots Pi translocation through PHT1s and PHO118,21,22.
Wheat is one of the most important food crops and wheat production consumed 16.1% of the phosphate fertilizer, higher than maize (Zea mays, 15.2%), rice (12.8%) and other cereals (4.4%)23. Therefore, improving PUE of wheat is important in sustainable use of P resources. Understanding the molecular mechanisms regulating P use may facilitate the breeding of wheat with improved PUE. For example, Wang et al.8 proposed that TaPHR1 involves in Pi signaling in wheat. Overexpressing TaPHR1 in wheat up-regulates a subset of PSI genes, stimulates lateral root branching, improves Pi uptake and grain yield8. Overexpression of the Pi transporters TaPT224 and TaPHT1.425 both enchance Pi uptake and plant developmenmt in wheat. Overexpression of the β-expansin gene TaEXPB23 increased lateral root number in transgenic tobacco (Nicotiana tabacum) plants under excess-P and low-P conditions26. By comparing the transcriptome profiles of wheat and rice, an IPS1-mediated signaling cascade (include PHR1-IPS1-miR399-PHO2) and its downstream functions involved in a general response to Pi starvation were revealed27. However, compared with Arabidopsis and rice, molecular mechanisms underlying Pi signaling are still largely unknown in wheat.
Here we cloned three PHO2 homologous genes TaPHO2-A1, B1 and D1 from wheat. The ion beam-induced deletion mutants of these TaPHO2s each showed higher expression of TaPHO1 and TaPHT1s and higher Pi concentrations in shoots than did the wild type. Field experiments exhibited that the tapho2-a1 mutant displayed higher aerial P accumulation and grain yield than did the wild type under low P conditions. These results indicated that TaPHO2 involved in Pi signaling and showed potential in improving PUE and yield in wheat.
Results
Identification of PHO2 genes in wheat
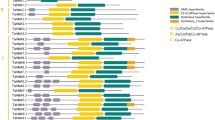
We isolated the full-length cDNA and genomic DNA sequences of three PHO2 homologues from the winter wheat variety Xiaoyan 81 by rapid amplification of cDNA ends (RACE) and genomic PCR amplification. These three TaPHO2 genes were mapped on chromosomes 1A, 1B and 1D by using the Chinese Spring deletion lines (Supplemental Fig. S1) and were named as TaPHO2-A1, TaPHO2-B1 and TaPHO2-D1, respectively. These three TaPHO2 sequences shared highly sequence similarity in the open reading region, but had large variations in the introns, 5′ and 3′-UTRs. Each of them contained 10 exons (including 2 untranslated exons in the 5′-UTR) and had five putative miR399-binding sites in the second exon (Fig. 1a). The deduced protein sequences of these TaPHO2 genes had conserved ubiquitin-conjugating catalytic (UBCc) domain at the C terminus (Supplemental Fig. S2). Phylogenetic analysis revealed that the three TaPHO2s belonged to the same subgroup with OsPHO2 from rice and were more closely related to HvPHO2 from barley (Hordeum vulgare, Supplemental Fig. S3).
Gene structures and expression of the TaPHO2s.
(a) The gene structures of TaPHO2-A1, B1 and D1. The black boxes indicate the ORF of TaPHO2s; the white boxes indicate the UTR regions; the black lines indicate the introns. The blue ticks in the second exon depict the position of five putative miR399 binding sites. Numbers depict the length of exons or introns in the corresponding region. ATG, the start codon; TGA, the stop codon. (b) Overall relative expression levels of TaPHO2 in the spikes, stems, leaf sheaths, flag leaves and top 2~5 leaves (from top to the bottom of the wheat plant) of the field grown wheat plants at the flowering stage. Error bars indicate SE (n = 5). (c) Relative expression levels of TaPHO2–A1, B1 and D1 in roots and shoots of the plants under 10 μM Pi (low P) and 200 μM Pi (high P) conditions at the seedling stage. Error bars indicate SE (n = 3).
Expression profiles of TaPHO2s
We investigated the spatial-temporal expression pattern of TaPHO2 genes in different organs of the field-grown wheat plants at flowering stage. The TaPHO2 transcripts were ubiquitously expressed in all the examined organs including spikes, stems, sheaths and leaves (Fig. 1b). We then analyzed the expression of TaPHO2-A1, -B1 and -D1 in roots and shoots of the wheat plants grown in nutrient solution at seedling stage. All the three TaPHO2 genes had higher expression level in roots than in shoots (Fig. 1c). TaPHO2-D1 displayed much higher transcript abundance than TaPHO2-A1 and -B1 did (Fig. 1c), indicating that TaPHO2-D1 was possibly the primary member of TaPHO2 in wheat. In both roots and shoots, the expression of the three TaPHO2 genes was lower under low P conditions than that under high P conditions (Fig. 1c), suggesting that TaPHO2 was down-regulated by Pi-deficiency.
Regulation of TaPHO2 by tae-miR399 and TaIPS1
Sequence analysis revealed that there were five putative miR399-binding sites in 5′-UTRs of the three TaPHO2 genes (Supplemental Fig. S4). In order to investigate whether TaPHO2 could be degraded by tae-miR399, we used tobacco transient expression system to analyze this possibility. When TaPHO2-A1, -B1 or -D1 was co-transformed with tae-miR399-A1 in the tobacco leaves, the mRNA levels of all three TaPHO2 genes were significantly lower than that transformed with TaPHO2 gene alone (Fig. 2a). Moreover, sequence analysis of TaIPS1s also found that three TaIPS1 genes all contained a motif with sequence complementarity to tae-miR399 (Supplemental Fig. S4). As all the three TaIPS1 genes conferred the conserved complementary sequences with tae-miR399, only TaIPS1.1 was chosen to check if it affected the degradation of TaPHO2-A1, -B1 or -D1 by tae-miR399. TaPHO2 mRNA levels in the tobacco leaves transformed with TaPHO2, tae-miR399-A1 and TaIPS1.1 were higher than that transformed with TaPHO2 and tae-miR399-A1 (Fig. 2a). These results indicated that all the three TaPHO2 genes were able to be degraded by tae-miR399 and TaIPS1.1 inhibited this degradation.
The regulation of the TaPHO2 transcripts by tae-miR399 and TaIPS1.
The relative expression levels of TaPHO2 (a), tae-miR399-A1 (b) and TaIPS1 (c) in the tobacco leaves transiently overexpressed the indicated gene(s). The expression of TaPHO2 was presented as percentage of that in the control leaves which were transformed with TaPHO2-A1, -B1 or -D1 alone, the relative expression levels of tae-miR399-A1 and TaIPS1 were normalized using the expression of NtACTIN. Different letters in (a) indicate significant difference at P < 0.05 level by Student’s t test. Error bars indicate SE (n = 6).
Plant growth and Pi distribution of TaPHO2 deletion mutants in hydroponic culture
To investigate the function of TaPHO2 in wheat, we screened for the TaPHO2 deletion mutants from the ion beam-induced mutants of variety Xiaoyan 81. After screening, the homozygous tapho2-a1, b1 and d1 mutants were obtained by ABI 3730 analysis (Supplemental Fig. S5). Before further analysis, three homozygous tapho2 mutants were backcrossed twice with their wild type progenitor Xiaoyan 81 and homozygous BC2F3 or BC2F4 mutants were used for further analysis. Compared to the wild type plants, the overall TaPHO2 expression levels were significantly declined (Fig. 3a). The tapho2-d1 mutant had lower TaPHO2 expression than did the tapho2-a1 and tapho2-b1 mutants (Fig. 3a). We also did not detect the transcript of TaPHO2-A1, -B1 and -D1 in their corresponding mutant (Fig. 3b), indicating that TaPHO2-A1, -B1 and -D1 were deleted in their corresponding mutant.
Relative expression levels of TaPHO2 genes in roots of the wild type and tapho2 mutant plants at seedling stage.
The plants were grown in the nutrient solution containing 20 μM Pi and the roots were used for gene expression analysis. (a) Overall relative expression levels of TaPHO2s; (b) Relative expression levels of TaPHO2-A1, TaPHO2-B1 and TaPHO2-D1. Error bars indicate SE (n = 5). ND, not detectable. Asterisks indicate the significance of differences between wild type and tapho2 mutants as determined by Student’s t test analysis: **P < 0.01, *P < 0.05.
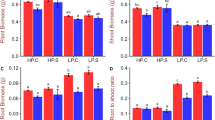
We evaluated the effects of deleting TaPHO2 genes on the growth of wheat seedlings under low P and high P conditions in a hydroponic culture. Under low P conditions, the tapho2-a1 and tapho2-b1 mutants showed significantly higher shoot dry weight (SDW, Fig. 4c), higher root dry weight (RDW, Fig. 4d), lower root/shoot ratio (Fig. 4e) and longer primary root length (Fig. 4f) than did the wild type. Under high P conditions, the tapho2-a1 mutant had significantly higher SDW (Fig. 4c) and lower root/shoot ratio (Fig. 4e) than did the wild type and tapho2-b1 mutant had longer primary root length (Fig. 4f) than did the wild type. Under both low P and high P conditions, the tapho2-d1 mutant had significantly lower SDW, RDW, root/shoot ratio and shorter primary root length than did the wild type (Fig. 4c–f). These results suggested that the seedlings of the tapho2-a1 and tapho2-b1 mutants had advanced adaptive capacity to Pi-deficiency conditions, while significant repression of plant growth occurred in the seedlings of tapho2-d1 mutant under both low P and high P conditions when the plants were grown in nutrient solution.
Growth performance of wild type and tapho2 mutant plants grown in the nutrient solutions containing 10 μM Pi (low P) and 200 μM Pi (high P) at the seedling stage.
(a,b) Images of the plants under low P (a) and high P (b) conditions. Scale bar = 10 cm; (c) Shoot dry weight; (d) Root dry weight; (e) Root/shoot dry weight ratio; (f) Primary root length. Error bars indicate SE (n = 5). Asterisks indicate the significance of differences between wild type and tapho2 mutant plants as determined by Student’s t test analysis: **P < 0.01, *P < 0.05.
We next measured the Pi accumulation in roots and expanded leaves of the tapho2 mutants and wild type. Low P treatment greatly reduced root and leaf Pi concentrations and altered Pi distributions in leaves, as compared to high P treatment (Fig. 5). The leaf Pi concentrations in the mutants and wild type decreased with leaf ages under low P conditions (Fig. 5a); in contrast, they increased with leaf ages under high P conditions (Fig. 5b). The tapho2-a1 and -d1 mutants had significantly higher Pi concentrations in all the examined leaves under both low P and high P conditions and had significantly lower Pi concentrations in roots under high P conditions than did the wild type (Fig. 5). The tapho2-b1 mutant had significantly higher Pi concentrations in the 1st leaf under low P conditions (Fig. 5a) and significantly lower Pi concentrations in roots under high P conditions than did the wild type (Fig. 5b). After comparing the leaf Pi concentrations in the mutants and wild type, we found that tapho2-d1 had the strongest, while tapho2-b1 had the weakest effects on leaf Pi concentrations under both low P and high P conditions (Fig. 5).
Pi concentrations in roots and expanded leaves of the wild type and tapho2 mutant plants grown in nutrient solutions containing 10 μM Pi (low P) and 200 μM Pi (high P) levels at the seedling stage.
(a,b) Pi concentrations in roots and different age leaves of the plants grown in low P (a) and high P (b) nutrient solutions. Error bars indicate SE (n = 5). Asterisks indicate the significance of differences between wild type and tapho2 mutants as determined by Student’s t test analysis: **P < 0.01, *P < 0.05.
To understand the possible mechanisms that tapho2 mutants affected Pi distribution, we analyzed the expression of PHT1 and PHO1 transporters in roots and shoots. All the tapho2-a1, -b1 and -d1 mutants had higher expression of TaPHT1 transporters under low P or high P conditions than did the wild type (Fig. 6a–e). The tapho2-a1 and -d1 mutants had higher expression of TaPHO1 than did the wild type, but the tapho2-b1 mutants showed similar expression of TaPHO1 with the wild type under both low P and high P conditions (Fig. 6f).
Expression levels of TaPHT1s and TaPHO1 in roots and shoots of the wild type and tapho2 mutant plants grown in nutrient solutions containing 10 μM Pi (low P) and 200 μM Pi (high P) levels.
(a) TaPHT1.1/1.9; (b) TaPHT1.2; (c) TaPHT1.10; (d) TaPHT1.3/4; (e) TaPHT1.6; (f) TaPHO1. LP and HP indicate low P and high P treatment, respectively. Error bars indicate SE (n = 5). Asterisks indicate the significance of differences between wild type and tapho2 mutants as determined by Student’s t test analysis: *P < 0.05.
Effects of deleting TaPHO2 on wheat growth and P uptake in field experiments
We measured SDW of the tapho2 mutants and wild type at seedling stage under low P and high P conditions in the field experiment of 2014–2015 growing season. Compared to the wild type plants, the tapho2-a1 mutant had significantly higher SDW under both low P and high P conditions, the tapho2-b1 mutant had significantly higher SDW under high P conditions and the tapho2-d1 mutant had significantly lower SDW under both low P and high P conditions (Fig. 7a). We next analyzed Pi and total P concentrations in roots and shoots. These three mutants all had significant lower Pi and total P concentrations in roots than did the wild type under low P conditions and had significant higher Pi and total P concentrations in shoots than did the wild type under both low P and high P conditions (Fig. 7b,c). These results indicated that the translocation of P from roots to shoots was increased in the tapho2 mutants. Calculation of aerial P accumulations (total P accumulated in shoots) revealed that the tapho2-a1 and -b1 mutants had significantly higher aerial P accumulations than did the wild type both under low P and high P conditions; In contrast, tapho2-d1 mutant had significantly lower aerial P accumulations than did the wild type under both low P and high P conditions (Fig. 7d).
Shoot dry weight and P content of the wild type and tapho2 mutant plants grown under 0 g P m−2 (low P) and 16 g P m−2 (high P) conditions at seedling stage in the field experiment of 2014–2015 growing season.
(a) Shoot dry weight. (b) Pi concentration. (c) Total P concentration. (d) Total P accumulated in shoots. Error bars indicate SE (n = 5). Asterisks indicate the significance of differences between wild type and tapho2 mutants as determined by Student’s t test analysis: **P < 0.01,*P < 0.05.
We then investigated the agronomic traits at maturity in two consecutive field experiments. In both the 2013–2014 and 2014–2015 growing seasons, the tapho2-a1 mutant had significantly higher biomass yield and grain yield than did the wild type under low P conditions and the tapho2-d1 mutant had significantly lower biomass yield and grain yield than did the wild type under both low P and high P conditions (Table 1). In the 2014–2015 growing season, we observed that tapho2-d1 mutant had significantly shorter plant height than did the wild type under both low P and high conditions (Table 1).
The P-use related traits were also analyzed in the 2014–2015 growing season. All the three tapho2 mutants had higher total P concentration in grains than did the wild type under both low P and high P conditions (Table 1). Under both low P and high P conditions, the total P accumulated in the aerial parts in the tapho2-a1 and tapho2-b1 mutants were significantly higher than that in the wild type; in contrast, those in the tapho2-d1 mutant were significantly lower than that in the wild type (Table 1).
Discussion
The crucial roles of PHO2 in regulating Pi signaling have been elaborately depicted in Arabidopsis and rice and PHO2 exists in single copy in these diploid plant species12,13,16. As common wheat is an allohexaploid which contains three homoeologous genomes28, theoretically it may have three PHO2 homologous genes. In the wheat variety Xiaoyan 81, we isolated TaPHO2-A1, TaPHO2-B1 and TaPHO2-D1 on chromosomes 1A, 1B and 1D, respectively (Fig. 1A and Supplemental Fig. S1). These three wheat genes seemed to be the orthologues of the PHO2 genes in Arabidopsis and rice. Firstly, the deduced TaPHO2 protein sequences closely related to AtPHO2 and OsPHO2 in the phylogenetic tree of PHO2 proteins (Supplemental Fig. S3) and had conserved UBCc domain (Supplemental Fig. S2). Secondly, all the three TaPHO2s conferred five putative miR399-binding sites in the 5′-UTR (Supplemental Fig. S4) and were able to be degraded by tae-miR399 (Fig. 2), as has been shown in Arabidopsis and rice12,13,16. The degradation of the TaPHO2s by tae-miR399-A1 was able to be inhibited by TaIPS1.1 (Fig. 2). Previously, the miR399 activity in cleaving PHO2 has been found to be reduced by IPS1 according to the target mimicry mechanism in Arabidopsis14. As such, the IPS1-miR399-PHO2 signaling cascade is conserved in plants. Finally, knockout mutants of the three TaPHO2 genes displayed increased Pi translocation from roots to shoots and leaf Pi concentrations (Fig. 5). PHO2 has been demonstrated to regulate PHO1 and PHT1 transporters at post-translational level17,18. As such, further research is needed to explore the PHO1 and PHT1 transporters which may contribute to the increased Pi translocation from roots to shoots and leaf Pi concentrations in the tapho2 mutants. In present study, although we did not investigate the protein abundance of PHO1 and PHT1 transporters in the mutants and wild type plants, we found that the tapho2 mutants had higher expression of TaPHT1 and TaPHO1 transporters than the wild type (Fig. 6). Similar results have been reported in Arabidopsis and rice that loss of function of PHO2 has increased expression of PHO1 and PHT1 transporters12,13,16,17,18.
The three TaPHO2 genes shared similar gene structure, highly similarity in ORF sequences and deduced protein sequences (Fig. 1a and Supplemental Fig. S2) and similar response to Pi-deficiency (Fig. 1c), but they exhibited large difference in expression levels (Fig. 1c). In both roots and shoots, TaPHO2-D1 exhibited much higher transcript abundance than did TaPHO2-A1 and -B1 and TaPHO2-B1 had the lowest expression among these three TaPHO2 genes (Fig. 1c). In consist with these results, the overall expression of TaPHO2 in roots of the tapho2-a1, -b1 and -d1 mutants was reduced to 55.8%, 70.1% and 21.1% of the wild type level (Fig. 3a), respectively. Among the tapho2-a1, -b1 and -d1 mutants, tapho2-d1 had the strongest, tapho2-a1 the moderate and tapho2-b1 the weakest phenotype in term of leaf Pi concentration under both low P and high P conditions when the wheat seedlings were grown hydroponically (Fig. 5). In the field experiment of 2014–2015 growing season, the tapho2-d1 mutant had substantially higher Pi and total P concentrations in shoots at seedling stage (Fig. 7b,c) and total P concentration in grains at maturity than did the tapho2-a1 and -b1 mutants and the wild type as well (Table 1). Taking information together, Pi and total P concentrations in leaves and grains negatively correlated with the expression of TaPHO2. These negative correlations suggested that the increased Pi and total P concentrations in leaves and grains mainly resulted from the deletion of TaPHO2, although some other genes surrounding the interested gene might be deleted in the mutants when the mutation was induced by ion beam29.
In diploid plant species such as Arabidopsis and rice, loss of function of PHO2 has been found to inhibit plant growth, possibly caused by the over-accumulation of Pi in shoots13,16. These results suggest that PHO2 is essential to maintain Pi homeostasis and hence plant growth. Our present study found that a severe reduction in PHO2 expression could also impair Pi homeostasis and plant growth in wheat. Although wheat contains three expressed TaPHO2 genes, the overall expression of TaPHO2 was severely reduced (only 21.1% of the wild type level) when TaPHO2-D1 was deleted in the tapho2-d1 mutant (Fig. 3a). Compared to the wild type plants, the tapho2-d1 mutant displayed inhibited growth at seedling stage (Figs 4c,d and 7a) and at maturity (Table 1) and increased Pi and total P concentrations in leaves and grains (Figs 5 and 7 and Table 1). As such, the tapho2-d1 mutant resembled the phenotypes of pho2 mutants in Arabidopsis and rice. In contrast, the phenotypes of the tapho2-a1 mutant suggested that a moderate reduction in TaPHO2 expression might improve P uptake and grain yield in wheat. Compared to the wild type and tapho2-d1 mutant, Pi and total P concentrations in aerial parts (leaves, shoots and grains) were moderately increased (Figs 5 and 7 and Table 1), these moderately increased P accumulation might benefit to achieve higher grain yield under low P conditions (Table 1). Although both tapho2-a1 and tapho2-d1 mutant had increased leaf Pi concentration (Fig. 5), they showed opposite effects on yield and biomass (Table 1). Furthermore, the shoot growth of tapho2-d1 mutant was inhibited under low P conditions (Fig. 4c); even though the leaf concentrations in the tapho2-d1 mutant under low P conditions were lower than those of the wild type plants under high P conditions (Fig. 5). These results may suggest a role of TaPHO2-D1 in regulating plant development besides its function in controlling Pi homeostasis. Indeed, it has been reported that the miR399-PHO2 module regulates the flowering time in response to different ambient temperatures in Arabidopsis30.
In summary, we identified three TaPHO2 genes from the homoeologous group 1 of the hexaploid wheat. They displayed large difference in transcription abundance and their knock out mutations exerted different effects on P uptake and distribution and plant growth. Phenotype evaluation in the three tapho2 mutants and wild type revealed the negative correlation between Pi and total P concentrations in aerial parts and TaPHO2 expression levels. A moderate reduction in PHO2 expression by deleting TaPHO2-A1 improved P uptake and grain yield under low P conditions. Our founding provided useful cue to increase wheat yield with less P fertilizer input through conventional breeding by using tapho2-a1 mutant as a parent, or engineering PHO2 expression level by genome editing and RNA interference (RNAi) approach.
Materials and Methods
Plant materials and growth conditions
The wild type and tapho2 mutants were used in this work. The mutants were screened from Xiaoyan 81 deletion mutant library irradiated by nitrogen ions29. Xiaoyan 81 is a winter wheat (Triticum aestivum) variety commercially released in 2006.
Hydroponic culture and field experiments were used to evaluated the phenotypes of the wild type and mutants. For hydroponic culture, the nutrient solution composition, methods for seed sterilization and germination and the growth conditions for the hydroponic experiments were described previously31. Briefly, the plants were grown in a growth chamber with 20 ± 1 °C, 50% to 70% relative humidity, a photon fluence rate of 300 mmol photons m−2 s−1 and a 16 h day/8 h night cycle conditions. The nutrient solution contained 200 μM KH2PO4 was used as high P treatment and the low P treatment was substituted with 10 μM KH2PO4 and 190 μM KCl. The nutrient solution was adjusted to pH 6.0 by 1 M HCl and refreshed every 2 days.
Two consecutive field experiments were conducted in the Beijing experiment station of the IGDB in 2013–2014 and 2014–2015 growing seasons. The experiments consisted of two treatments and a random block design with five replications was used. The high P treatment was applied 16 g P m−2 in the form of calcium superphosphate prior to sowing and the low P treatment had no P application. Both treatments had 18.0 g m−2 of N in the form of urea with 12 g m−2 applied prior to sowing and 6 g m−2 applied at the stem elongation stage. The seeds were sown in the end of September at a seed rate of 108.7 seeds m−2 and the plants were harvested in the middle of the following June. Each genotype in each replication had two 1.5 m long rows spaced with 23 cm.
Gene cloning and phylogenetic analysis
The sequence of AtPHO2 (AT2G33770) was used as query to search the wheat ESTs data base of NCBI. Two pairs of conserved primers (Supplementary Table S1) were designed for the 5′ and 3′ RACE PCR amplification according to the identified ESTs. First-strand cDNA synthesis and RACE PCR were performed with the SMARTerTM RACE cDNA Amplification Kit (Clontech) following the instructions. Thereafter, three full-length of sequences, named as TaPHO2-A1, TaPHO2-B1 and TaPHO2-D1 respectively, were further isolated from cultivar Xiaoyan 81. The gene structures of TaPHO2s were aligned by using ClustalX 2.032. The protein structures and conserved ubiquitin-conjugating catalytic domain were analyzed by CCD analysis (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The phylogenetic analysis was performed by neighbor-joining method and the phylogenetic tree was drawn with MEGA 5.033.
TaPHO2 deletions screening
Conserved forward and reverse primers were designed according to the differences in the seventh intron of the three homologous TaPHO2 genes. This primer set (Supplementary Table S1) amplified three fragments product (with different sizes) in genomic PCR, which were specific for the TaPHO2-A1 (424 bp), TaPHO2-B1 (412 bp) and TaPHO2-D1 (397 bp) of Xiaoyan 81, respectively. For facilitative screening, the forward primer was labeled with 6-FAM fluorophore at the 5′ terminus. PCR was performed in a volume of 20 μL containing 100 ng genomic DNA, 10 μM of each primer, 2 × Taq Mix (GenStar). The amplification program starting at 94 °C 3 min, followed by 35 cycles of 94 °C 30 s, 60 °C 25 s, 72 °C 40 s, with a final extension at 72 °C 5 min. The resulted PCR products were separated by ABI 3730 analysis and the TaPHO2 deletion mutant was confirmed based on the absence of the corresponding fragment. The tapho2 mutants were backcrossed two times with Xiaoyan 81 as recurrent parent. Homozygous tapho2-a1, b1 and d1 deletion lines were obtained from BC2F2.
Quantitative RT-PCR analysis
Total RNA was extracted with TRIzol reagent (Ambion). Reverse transcription was performed with the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo) using 1 μg total RNA. Quantitative RT-PCR analysis was carried out with a LightCycler 480 engine (Roche) by using the LightCycler480 SYBR Green I Master Mix (Roche). The relative transcript level of each cDNA sample was calculated from triplicates using the formulate 2−ΔCt after normalization to TaACTIN (in common wheat) or NtACTIN (in tobacco) control. The primers used for qRT-PCR analysis were listed in Supplementary Table S2. As the three TaPHO2 homologous genes displayed highly sequence similarities, it is difficult to design gene specific primer pair in the similar regions. The designed three pairs of gene specific primers for TaPHO2-A1, TaPHO2-B1 and TaPHO2-D1 located at 1281~1462 bp, −215~−58 bp (spanning the last two miR399 binding sites) and 2377~2521 bp (the ATG start codon defined as 1), respectively. The primer pair for detecting the overall expression of TaPHO2 located at 2220~2341 bp (relative to the ATG start codon of TaPHO-A1).
Vector construction and plant transformation
To investigate the regulation of tae-miR399 and TaIPS1 on TaPHO2 degradation, a 1.6 Kb cDNA fragment containing the five putative miR399 binding sites of the three TaPHO2 genes were cloned into pRI101-AN vector (Takara). The full length of tae-miR399 and TaIPS1.1 were also cloned into pRI101-AN vector. All the resulting binary vectors were introduced into the GV3101 strain of Agrobacterium tumefaciens. The Agrobacterium mediated transient expression assays in tobacco leaves were conducted as described by Liu et al.17. The Primers used for vector construction were listed in Supplementary Table S1.
Measurements of P concentration
Plant samples were frozen after measured fresh weight or dried at 80 °C for 3 days to a constant dry weight. The Pi and total P concentrations were measured using the method described by Wang et al.34 with some modified. For Pi concentration measurement, a frozen sample was homogenized with 400 μL of 5 M H2SO4 and 4 mL H2O, using an ice-cold mortar and pestle. After the mixture was centrifuged at 12000 g for 10 min at 4 °C, the supernatant was diluted appropriately and carried out with a SmartChem200 analyzer (Alliance) by following the instruction of P analysis. Pi concentration was calculated by normalization of fresh weight.
For total P concentration measurement, the dried plant samples (about 0.2 g) were milled and subsequently digested with 5 mL H2SO4. The digested solution was cooled and diluted to 100 mL. The solution was diluted appropriately and Pi content was analyzed as described above. The total P content was calculated by normalization of dry weight.
Statistical analysis
One-way ANOVA was performed with SPSS19 for mean comparisons between the wild type and mutant plants.
Additional Information
How to cite this article: Ouyang, X. et al. Knock out of the PHOSPHATE 2 Gene TaPHO2-A1 Improves Phosphorus Uptake and Grain Yield under Low Phosphorus Conditions in Common Wheat. Sci. Rep. 6, 29850; doi: 10.1038/srep29850 (2016).
References
Lopez-Arredondo, D. L., Leyva-Gonzalez, M. A., Gonzalez-Morales, S. I., Lopez-Bucio, J. & Herrera-Estrella, L. Phosphate nutrition: improving low-phosphate tolerance in crops. Annu Rev Plant Biol 65, 95–123 (2014).
Wu, P., Shou, H., Xu, G. & Lian, X. Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Curr. Opin. Plant Biol. 16, 205–212 (2013).
Zhang, Z., Liao, H. & Lucas, W. J. Molecular mechanisms underlying phosphate sensing, signaling and adaptation in plants. J Integr Plant Biol. 56, 192–220 (2014).
Rubio, V. et al. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 15, 2122–2133 (2001).
Zhou, J. et al. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol. 146, 1673–1686 (2008).
Nilsson, L., Muller, R. & Nielsen, T. H. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ. 30, 1499–1512 (2007).
Valdes-Lopez, O. et al. Essential role of MYB transcription factor: PvPHR1 and microRNA: PvmiR399 in phosphorus-deficiency signalling in common bean roots. Plant Cell Environ 31, 1834–1843 (2008).
Wang, J. et al. A phosphate starvation response regulator Ta-PHR1 is involved in phosphate signalling and increases grain yield in wheat. Ann. Bot. 111, 1139–1153 (2013).
Ren, F. et al. Brassica napus PHR1 gene encoding a MYB-like protein functions in response to phosphate starvation. PLoS One 7, e44005 (2012).
Bustos, R. et al. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 6, e1001102 (2010).
Fujii, H., Chiou, T. J., Lin, S. I., Aung, K. & Zhu, J. K. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol. 15, 2038–2043 (2005).
Bari, R., Datt Pant, B., Stitt, M. & Scheible, W. R. PHO2, microRNA399 and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 141, 988–999 (2006).
Aung, K. et al. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 141, 1000–1011 (2006).
Franco-Zorrilla, J. M. et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39, 1033–1037 (2007).
Lin, S. I. et al. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 147, 732–746 (2008).
Hu, B. et al. LEAF TIP NECROSIS 1 plays a pivotal role in the regulation of multiple phosphate starvation responses in rice. Plant Physiol. 156, 1101–1115 (2011).
Liu, T. Y. et al. PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24, 2168–2183 (2012).
Huang, T. K. et al. Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25, 4044–4060 (2013).
Arpat, A. B. et al. Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J. 71, 479–491 (2012).
Hamburger, D., Rezzonico, E., MacDonald-Comber Petetot, J., Somerville, C. & Poirier, Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14, 889–902 (2002).
Liang, C., Wang, J., Zhao, J., Tian, J. & Liao, H. Control of phosphate homeostasis through gene regulation in crops. Curr Opin Plant Biol. 21, 59–66 (2014).
Liu, T. Y., Lin, W. Y., Huang, T. K. & Chiou, T. J. MicroRNA-mediated surveillance of phosphate transporters on the move. Trends Plant Sci. 19, 647–655 (2014).
Heffer, P. Assessment of Fertilizer Use by Crop at the Global Level 2010–2010_11. International Fertilizer Industry Association, Paris (2013).
Guo, C. J. et al. TaPT2, a high-affinity phosphate transporter gene in wheat (Triticum aestivum L.), is crucial in plant Pi uptake under phosphorus deprivation. Acta Physiologiae Plantarum 36, 1373–1384 (2014).
Liu, X. M. et al. TaPht1.4, a high-affinity phosphate transporter gene in wheat (Triticum aestivum), plays an important role in plant phosphate acquisition under phosphorus deprivation. Funct. Plant Biol. 40, 329–341 (2013).
Han, Y. Y. et al. The involvement of expansins in responses to phosphorus availability in wheat and its potentials in improving phosphorus efficiency of plants. Plant Physiol. Biochem. 78, 53–62 (2014).
Oono, Y. et al. Characterisation of the wheat (Triticum aestivum L.) transcriptome by de novo assembly for the discovery of phosphate starvation-responsive genes: gene expression in Pi-stressed wheat. Bmc Genomics 14, 77–90 (2013).
Petersen, G., Seberg, O., Yde, M. & Berthelsen, K. Phylogenetic relationships of Triticum and Aegilops and evidence for the origin of the A, B and D genomes of common wheat (Triticum aestivum). Mol Phylogenet Evol 39, 70–82 (2006).
Yang, Y. et al. Efficient isolation of ion beam-induced mutants for homoeologous loci in common wheat and comparison of the contributions of Glu-1 loci to gluten functionality. Theor. Appl. Genet. 127, 359–372 (2014).
Kim, W., Ahn, H. J., Chiou, T. J. & Ahn, J. H. The role of the miR399-PHO2 module in the regulation of flowering time in response to different ambient temperatures in Arabidopsis thaliana. Molecules and Cells 32, 83–88 (2011).
Ren, Y. et al. Major quantitative trait loci for seminal root morphology of wheat seedlings. Mol. Breed. 30, 139–148 (2011).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Wang, C. et al. Involvement of OsSPX1 in phosphate homeostasis in rice. Plant J. 57, 895–904 (2009).
Acknowledgements
We thank Professor Daowen Wang for kindly providing the Xiaoyan 81 deletion mutant library. This research was supported by the Ministry of Science and Technology of the People’s Republic of China (2016ZX08002-005) and the Chinese Academy of Sciences (XDA0801040401).
Author information
Authors and Affiliations
Contributions
X.O., X.Z. and Y.T. designed the experiments. X.O., Xia Hong and W.Z. performed the experiments. X.O. and Xia Hong analyzed the data and wrote the paper. Y.T., Xue He, W.M. and W.T. supervised and edited the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ouyang, X., Hong, X., Zhao, X. et al. Knock out of the PHOSPHATE 2 Gene TaPHO2-A1 Improves Phosphorus Uptake and Grain Yield under Low Phosphorus Conditions in Common Wheat. Sci Rep 6, 29850 (2016). https://doi.org/10.1038/srep29850
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29850
This article is cited by
-
Physiological traits and expression profile of genes associated with nitrogen and phosphorous use efficiency in wheat
Molecular Biology Reports (2023)
-
Unraveling Metabolic Profile of Wheat Plants Subjected to Different Phosphate Regimes
Journal of Soil Science and Plant Nutrition (2023)
-
Integrated mRNA and miRNA Expression Analyses of Pinus massoniana Roots and Shoots in Long-Term Response to Phosphate Deficiency
Journal of Plant Growth Regulation (2022)
-
Narrowing down molecular targets for improving phosphorus-use efficiency in maize (Zea mays L.)
Molecular Biology Reports (2022)
-
Wheat genomic study for genetic improvement of traits in China
Science China Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.