Abstract
There have been notable improvements in survival over the past 2 decades for gastrointestinal (GI) cancer. However, the degree of improvement by age, race, and sex remains unclear. We analyzed data from 9 population-based cancer registries included in the SEER program of the National Cancer Institute (SEER 9) in 1990 to 2009 (n = 288,337). The degree of survival improvement over time by age, race, and sex was longitudinally measured. From 1990 to 2009, improvements in survival were greater for younger age groups. For patients aged 20 to 49 years and diagnosed from 2005 to 2009, adjusted HRs (95% CIs) were 0.74 (95% CI, 0.66–0.83), 0.49 (95% CI, 0.37–0.64), 0.69 (95% CI, 0.65–0.76), 0.62 (95% CI, 0.54–0.69), and 0.56 (95% CI, 0.42–0.76), for cancer of the stomach, small intestine, colon, rectum and anus, respectively, compared with the same age groups of patients diagnosed during 1990 to 1994. Compared with African Americans, whites experienced greater improvement in small intestinal and anal cancer survival. Female anal cancer and regional anal cancer patients experienced no improvement. Our data suggest that different improvement in survival in age, sex and race exists.
Similar content being viewed by others
Introduction
Cancer is a major public health problem in the United States and many other countries. It is currently the second leading cause of death in the United States and gastrointestinal cancer (GI) is one of the most common cancers1. There have been notable improvements in survival over the past 2 decades for gastrointestinal cancer attributed to improvements in cancer screening, along with advances in cancer treatments including surgery, radiotherapy, chemotherapy, and targeted therapies survival2.
Several cross-sectional studies have compared cancer survival rates by race, sex, and age3,4,5,6,7,8. However, these studies did not address the secular trend of cancer survival, which measures the improvement of cancer survival over time. In this study, we analyzed the differences in the improvement of cancer survival by race, age, and sex in the past 2 decades. We analyzed data from 9 population-based cancer registries included in the SEER program of the National Cancer Institute (SEER 9) in 1990 to 2009 to determine whether improvements in cancer survival differ by race, sex, and age in the United States.
Methods
Patient selection in the SEER database
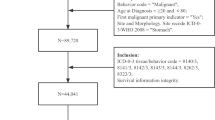
The SEER, a population-based reporting system, was surveyed for the retrospective collection of data used in the analysis. We analyzed data from 9 population-based cancer registries included in the SEER program of the National Cancer Institute (SEER 9). Because no personal identifying information was used in the analysis, this study was granted an exemption from the Institutional Review Board of the study institution on March 30, 2012.
Cases of gastric cancer (C16.0 to C16.9), small intestine cancer (C17.0 to C17.9), colon cancer (C18.0 to C18.9), rectal cancer (C19.9, C20.9) and anal cancer (C21.0, C21.1) from 1990 to 2009 were extracted from the SEER database (SEER*Stat 8.2.1) according to the Site Recode classifications. We excluded patients whose survival months were unknown and SEER historic stage were unknown. Demographic variables (age at diagnosis, year of diagnosis, race, sex, and marital status) and tumor characteristics (stage and histologic types) were obtained from the registry databases.
Statistical Analysis
The primary outcome in this study was a measure of cancer specific death, defined as a death with the specific cancer. Deaths from other causes were treated as censored observations. Survival rates (at 1, 3, and 5 years) by cancer-specific death by age, sex, or race for patients diagnosed between 1990 and 1994 (the baseline period) were calculated using the Kaplan-Meier method. Hazard ratios (HRs) and 95% CIs for cancer-specific death associated with age, sex, or race were calculated using Cox proportional hazards models, for patients diagnosed during the time periods 1995 to 1999, 2000 to 2004, and 2005 to 2009, and were compared with those diagnosed at the baseline. Cox proportional hazards models were also used to calculate trend tests and derive calculate p-values for improvements in cancer survival. In the final analyses, all models were adjusted for marital status, common histologic types, SEER historic stages (localized, regional, and distant), age (20–49, 50–64, 65–74, or 75–84 years), race (white, African American, or other), and sex. The statistical test was two sided and P < 0.05 was considered statistically significant. PASW Statistics 13 (SPSS Inc., Chicago, USA) was used for the statistical analysis.
Results
Analyses included 39,997 gastric cancer patients, 10,652 small intestine cancer patients, 165,577 colon cancer patients, 66,945 rectal cancer patients and 5,166 anal cancer patients from 1990 to 2009. During this period, the percentage of cancer cases diagnosed at a localized stage increased for all gastrointestinal cancer sites (Table 1–5 in the Supplement).
The distribution of other patient characteristics also changed over the 20-year study period (Table 1–5 in the Supplement). In general, the percentage of white patients decreased and the percentage of African American patients increased for all gastrointestinal cancer sites except anal cancer. The percentage of 20–49 years patients increased for gastric cancer and colorectal cancer.
For patients diagnosed from 1990 to 1994, survival rates for African Americans were the lowest for colorectal and anal cancer (Table 1). For all cancer sites, survival rates were lowest in the oldest age group (75–84 years) and were lower among men than among women (Table 1).
Significant improvements in survival, shown by decreasing HRs over time, were observed for all cancers from 1990 to 2009 in all racial groups except for anal cancer (Fig. 1). White and African American cancer patients experienced different degrees of improvement in survival from 1990 to 2009 for small intestinal and anal cancers.
No apparent racial differences in survival improvements were seen for the other cancers evaluated in this study. During the 20-year study period, there was a statistically significant improvement in small intestinal cancer survival among whites and a slight improvement in survival among African Americans. Over the study period, whites experienced a greater improvement in survival of anal cancer than African Americans and African Americans experienced no improvement. There were only 44, 63, 191and 267 African American anal cases in period of 1990–1994, 1995–1999, 2000–2004 and 2005–2009. This small numbers of cases may make it difficult to obtain statistical significance.
From 1990 to 2009, all age groups demonstrated improved survival for all cancer sites except for anal cancer (Fig. 2). Improvements in survival were greater for younger age groups. For patients aged 20 to 49 years and diagnosed from 2005 to 2009, adjusted HRs (95% CIs) were 0.74 (95% CI, 0.66–0.83), 0.49 (95% CI, 0.37–0.64), 0.69 (95% CI, 0.65–0.76), 0.62 (95% CI, 0.54–0.69), and 0.56 (95% CI, 0.42–0.76), for cancer of the stomach, small intestine, colon, rectum and anus, respectively, compared with the same age groups of patients diagnosed during 1990 to 1994. However, the corresponding HRs (95% CIs) for elderly patients (those 75–84 years old) were only 0.86 (95% CI, 0.80–0.94), 0.78 (95% CI, 0.63–0.97), 0.92 (95% CI, 0.89–0.97), 0.90 (95% CI, 0.84–0.96), and 0.87 (95% CI, 0.63–1.25), for the same 5 cancer sites, respectively. There were no improvements in anal cancer patients ages 50–64 years and 75–84 years (p = 0.08 and p = 0.56).
There was a statistically significant improvement in cancer specific survival for both sex and all stages for all cancers studied except for anal cancer (Figs 3 and 4). Female anal cancer and regional anal cancer patients experienced no improvement in this period (p = 0.08 and p = 0.132, respectively). Regional and distant cancers experienced greater improvements than localized cancers in gastric and colorectal cancer.
Discussion
Using data from the SEER, we have showed a greater improvement in cancer survival over the past 20 years in the United States among younger cancer patients than elderly patients. The widening gap in cancer survival between younger and older patients may be due to differential utilization of newer treatments for elderly patients.
Higher rates of surgical complications, more prevalent co-morbidities, and poorer performance status limit the standard use of multidisciplinary treatment in older patients, and treatment deviation is higher in elderly patients than younger patients. A Surveillance, Epidemiology, and End Results (SEER)–Medicare study of patients more than 65 years of age who were receiving postoperative therapy for rectal cancer showed that 96.6% of Stage III rectal cancer patients completed radiation therapy, only 68.2% and 67.5% completed chemotherapy and both modalities. Among Stage II cancer patients, 91.5% completed radiation therapy but only 49.8% and 47.6% completed chemotherapy and both modalities9. Caitlin et al. also showed that stage III colon cancer patients who were older received chemotherapy less often and increasing age was associated with lower chemoradiation use among stage II/III rectal cancer patients10. In addition, elderly patients were excluded in most clinical trials. Murthy et al. showed that there was a strong relationship between age and enrollment fraction, with trial participants 30 to 64 years of age representing 3.0% of incident cancer patients in that age group, in comparison to 1.3% of 65- to 74-year-old patients and 0.5% of patients 75 years of age and older11. Lewis et al. also found that 32% of participants in clinical trials were elderly, compared with 61% of patients with incident cancers in the United States who were elderly12.
Thus, there is insufficient evidence to determine how they will respond to novel targeted therapies or combinations of chemotherapeutic agents. Our findings demonstrate that age-associated disparities exist and this is particularly pressing because this population constitutes the fastest growing subpopulation of cancer patients in the United States.
It has been reported that women may have lower mortality for almost all non–sex-specific cancers than do men13,14,15,16. Our study showed that improvements in cancer survival over time were similar between men and women except for anal cancer, for which women had no improvement. We observed a widening gap in survival by race only in small intestine and anal cancer. African Americans experienced no improvement in anal cancer during the 20-year study period. Murphy et al. showed that black patients were less likely to receive adjuvant chemotherapy as compared with white patients (risk ratio [RR], .82; 95% CI, .72 to .93)17. White et al. also suggested that blacks and whites had worse survival that Asians in colorectal cancer18. The underlying mechanisms driving this sex and race disparities remain to be investigated.
Regional and distant cancers experienced greater improvements than localized cancers in gastric and colorectal cancer. This is consistent with clinical trial data demonstrating improved survival with improved surgical techniques and novel perioperative treatment for patients with locally advanced and advanced gastric and colorectal cancer19,20,21,22,23,24.
There were several limitations to this study. The population oversamples urban and foreign-born populations, which may affect the generalizability of our findings to the general US population. Other limitations include those inherent in retrospective database analyses and differences in survival expectations between people of different races. Data on individual socioeconomic status, lifestyle factors, insurance and comorbidities were not available, and thus these variables cannot be adjusted in our study. Finally, the small sample size for anal cancer cases was as an explicit limitation of the study.
In conclusion, our data suggest that different improvement in survival in age, sex and race exists. One of the reasons may be differences in GI cancer care across these subpopulations. Finding factors associated with different improvement in GI cancer survival is a key part of improving cancer care for all.
Additional Information
How to cite this article: Wan, J.-f. et al. Sex, Race, and Age Disparities in the Improvement of Survival for Gastrointestinal Cancer over Time. Sci. Rep. 6, 29655; doi: 10.1038/srep29655 (2016).
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J Clin 65, 5–29 (2015).
Patel, J. D. et al. Clinical Cancer Advances 2013: Annual Report on Progress Against Cancer from the American Society of Clinical Oncology. J Clin Oncol 32, 129–160 (2014).
Cook, M. B., McGlynn, K. A., Devesa, S. S., Freedman, N. D. & Anderson, W. F. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 20, 1629–1637 (2011).
Tannenbaum, S. L., Koru-Sengul, T., Zhao, W., Miao, F. & Byrne, M. M. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 20, 237–245 (2014).
Sun, T., Plutynski, A., Ward, S. & Rubin, J. B. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 72, 3323–3342 (2015).
Walker, G. V. et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol 32, 3118–3125 (2014).
Sineshaw, H. M., Robbins, A. S. & Jemal, A. Disparities in survival improvement for metastatic colorectal cancer by race/ethnicity and age in the United States. Cancer Causes Control 25, 419–423 (2014).
Pulte, D., Redaniel, M. T., Brenner, H. & Jeffreys, M. Changes in survival by ethnicity of patients with cancer between 1992–1996 and 2002–2006: is the discrepancy decreasing? Ann Oncol 23, 2428–2434 (2012).
Dobie, S. A. et al. Survival benefits and trends in use of adjuvant therapy among elderly stage II and III rectal cancer patients in the general population. Cancer 112, 789–799 (2008).
Murphy, C. C., Harlan, L. C., Lund, J. L., Lynch, C. F. & Geiger, A. M. Patterns of Colorectal Cancer Care in the United States: 1990–2010. J Natl Cancer Inst 107 (2015).
Murthy, V. H., Krumholz, H. M. & Gross, C. P. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 291, 2720–2726 (2004).
Lewis, J. H. et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21, 1383–1389 (2003).
Innos, K., Padrik, P., Valvere, V. & Aareleid, T. Sex differences in cancer survival in Estonia: a population-based study. BMC. Cancer 15, 72 (2015).
Najari, B. B. et al. Sex disparities in cancer mortality: the risks of being a man in the United States. J Urol. 189, 1470–1474 (2013).
Wang, Y., Freemantle, N., Nazareth, I. & Hunt, K. Gender differences in survival and the use of primary care prior to diagnosis of three cancers: an analysis of routinely collected UK general practice data. Plos One 9, e101562 (2014).
Jung, K. W. et al. Do female cancer patients display better survival rates compared with males? Analysis of the Korean National Registry data, 2005–2009. PLoS One 7, e52457 (2012).
Murphy, C. C., Harlan, L. C., Warren, J. L. & Geiger, A. M. Race and Insurance Differences in the Receipt of Adjuvant Chemotherapy Among Patients With Stage III Colon Cancer. J Clin Oncol 33, 2530–2536 (2015).
White, A., Vernon, S. W., Franzini, L. & Du, X. L. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer 116, 4622–4631 (2010).
Hubbard, J. M. & Grothey, A. Colorectal cancer in 2014: progress in defining first-line and maintenance therapies. Nat Rev Clin Oncol 12, 73–74 (2015).
Gill, S. et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 22, 1797–1806 (2004).
Ciombor, K. K., Wu, C. & Goldberg, R. M. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 66, 83–95 (2015).
Smyth, E. C. & Cunningham, D. Gastric cancer in 2012: Defining treatment standards and novel insights into disease biology. Nat Rev Clin Oncol 10, 73–74 (2013).
Allum, W. H. et al. Guidelines for the management of oesophageal and gastric cancer. Gut. 60, 1449–1472 (2011).
Fornaro, L. et al. Anti-HER agents in gastric cancer: from bench to bedside. Nat Rev Gastroenterol Hepatol 8, 369–383 (2011).
Author information
Authors and Affiliations
Contributions
J.-f.W., L.-f.Y., Y.-z.S. and Z.Z. conceived of and designed the study. J.-f.W., L.-f.Y., H.-x.J., Y.-z.S., J.Z. and G.-c.L. performed the analyses. J.Z., Y.-z.S. and G.-c.L. prepared all tables. J.-f.W. and L.-f.Y. wrote the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wan, Jf., Yang, Lf., Shen, Yz. et al. Sex, Race, and Age Disparities in the Improvement of Survival for Gastrointestinal Cancer over Time. Sci Rep 6, 29655 (2016). https://doi.org/10.1038/srep29655
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29655
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.