Abstract
This study is a retrospective, nationwide, matched cohort study to investigate the risk of band keratopathy following end-stage renal disease (ESRD). The study cohort included 94,039 ESRD on-dialysis patients identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), code 585 and registered between January 2000 to December 2009 at the Taiwan National Health Insurance Research Database. An age- and sex-matched control group comprised 94,039 patients selected from the Taiwan Longitudinal Health Insurance Database 2000. Information for each patient was collected from the index date until December 2011. In total, 230 ESRD patients and 26 controls had band keratopathy (P < 0.0001) during the follow-up period, indicating a significantly elevated risk of band keratopathy in the ESRD patients compared with controls (incidence rate ratio = 12.21, 95% confidence interval [CI] = 8.14–18.32). After adjustment for potential confounders including sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi, ESRD patients were 11.56 times more likely to develop band keratopathy in the full cohort (adjusted HR = 11.56, 95% CI = 7.70–17.35). In conclusion, ESRD increases the risk of band keratopathy. Close interdisciplinary collaboration between nephrologists and ophthalmologists is important to deal with band keratopathy following ESRD and prevent visual acuity impairments.
Similar content being viewed by others
Introduction
End-stage renal disease (ESRD) is known as a worldwide public health problem because of its increasing incidence and the poor prognosis of morbidity and mortality1,2,3. The prevalence and incidence of ESRD have continued to increase worldwide4,5. Taiwan has the highest incidence and prevalence of ESRD6,7,8.
Band keratopathy is a chronic degenerative condition characterized by the deposition of amorphous, crystalline, grayish to whitish opacities on the corneal surface, most often in the interpalpebral zone9. The opacity is caused by sub-epithelial precipitation of calcium hydroxyapatite salt deposition10. Because of the deposition and accumulation of calcium, the ocular surface may be disrupted, causing irritation, redness, or photophobia. When the whitish opacity extends to the visual axis, band keratopathy may lead to significant glare and impaired vision. The pathogenesis of band keratopathy primarily involves deposition in the epithelial basement membrane, basal epithelium and Bowman’s membrane10. A variety of factors can cause the sub-epithelial calcium salt deposition, such as elevated serum calcium or serum phosphate levels, hyperparathyroidism, iridocyclitis, phthisis bulbi and long- standing eye drop use in cases involving ocular hypertension or irritable red eyes11.
Elevation of serum phosphate levels is a serious complication and one of the most common problems affecting ESRD patients12. Besides elevated serum phosphate levels, secondary renal hyperparathyroidism and the consequent elevated serum calcium levels have long been associated with ESRD13,14. Additionally, ESRD patients are at a higher risk for increased ocular problems such as increased intraocular pressure15,16,17,18 and irritated red eyes9,19,20, which necessitate long-term use of eye drops associated with band keratopathy. Therefore, it is clinically relevant to determine whether ESRD is a predictor of band keratopathy.
Several previous studies have discussed the association between ESRD and red eyes related to calcific deposits in the conjunctivae or corneas9,19,20, but the results of published studies were limited by the small number of patients or the absence of comparative control data. Using a nationwide population-based dataset, we designed a cohort study to investigate the risk of band keratopathy following ESRD in Taiwan. In our cohort study, the ESRD patients were all under dialysis treatment.
Methods
Database
On March 1, 1995, a single-payer National Health Insurance (NHI) scheme was launched in Taiwan, which provides extensive medical care coverage for all residents in Taiwan. About 22.60 million individuals (>98%) of the total Taiwanese population of 22.96 million were enrolled in this program as of 2007. The data of our cohort study were obtained from the Taiwan National Health Insurance Research Database (NHIRD). The NHIRD supplies enciphered patient identification numbers as well as information regarding patient birth date, sex and admission and discharge dates. It also includes the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnoses and procedure codes, prescriptions details and costs covered and paid by NHI. Ethical approval and informed consent were waived off by the Institutional Review Board of Chi-Mei Medical Center because a public database was used for analysis. Because analysis of datasets in a database does not use identifiable personal information, the requirement of informed consent was waived.
Study Design
This retrospective, nationwide, matched cohort study involved two groups of participants: a new-onset ESRD group and a matched non-ESRD (control) group.
Study Participants
Patients and controls were recruited in the period 2000–2009. We included 94,039 ESRD patients who started receiving dialysis treatment after 31 December 2000 and who had received a catastrophic illness certificate with the code number 585 between 1 January 2000 and 31 December 2009. Patients with unknown sex or missing data were excluded. Patients diagnosed as having band keratopathy (ICD-9-CM code 371.43) prior to ESRD were also excluded.
For each ESRD case, one control without ESRD was selected from the longitudinal Health Insurance Database 2000 (LHID2000). LHID2000 was a data subset of the NHIRD that contained entire claim data for one million beneficiaries (4.34% of the total population) systemic-randomly selected in 2000. There was no significant difference in age, sex and health care costs between this sample group and all national health insurance enrollees. The 94,039 controls were matched by age, sex and index date. The index date for the ESRD patients was the date of their first dialysis and the index date for the controls was created by matching the date with the ESRD subject’s index date. Moreover, the controls diagnosed with band keratopathy before the index date were also excluded. Each patient was followed up to determine the incidence of band keratopathy until the end of 2011 or censored because of death.
To distinguish all patients who had developed band keratopathy (ICD-9-CM code 371.43), we tracked every patient from his or her index outpatient visit or hospitalization through December 2011. Demographic data (e.g., age and sex) were recorded. Furthermore, we collected information regarding comorbidities including sarcoidosis (ICD-9-CM code 135), hyperparathyroidism (ICD-9-CM codes 252.0, which excluded secondary hyperparathyroidism due to renal disease.), iridocyclitis (ICD-9-CM code 364.0 to 364.3) and phthisis bulbi or degenerated eye (ICD-9-CM code 360.40, 360.41), because these conditions are critical factors that increase the risk of band keratopathy. In this study, the inclusion criteria for sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi were documentation of the condition at least once in the inpatient setting or ≥3 times in the ambulatory setting within 1 year before the initial ESRD on dialysis medical service date.
Statistical Analysis
SAS 9.4 for Windows (SAS Institute, Inc., Cary, NC, USA) was used in this study. Pearson chi-square test was used to compare the demographic characteristics and comorbid disorders between the ESRD and control groups. The incidence rate was calculated as the number of band keratopathy cases identified during follow-up divided by the total person-years (PY) for each group by age, sex and select comorbidities. The Poisson regression analysis was performed to calculate the incidence rate ratio (IRR), which demonstrated the comparison in the risk of developing band keratopathy between the ESRD and control groups. The adjusted hazard ratio (HR) for developing band keratopathy was calculated using Cox proportional hazard regression analysis. Cumulative incidence rates for band keratopathy of ESRD were evaluated by Kaplan–Meier analysis and differences in cumulative-incidence rate curves were analyzed using the log-rank test. Additionally, we subdivided the patients into three age subgroups for further analysis: <50 years, 50–64 years and ≥65 years. Data are presented as mean ± standard deviation (SD) and 95% confidence intervals (CIs) are provided when applicable. Statistical significance was defined as P < 0.05. These statistical assessments were performed in consultation with a statistical expert.
Results
Demographic Data
Between 2000 and 2009, 94,039 ESRD patients and 94,039 controls were recruited after excluding ineligible subjects. Table 1 provides the demographic characteristics and comorbid disorders of ESRD patients and age- and sex-matched controls. The mean age of all participants was 62.22 ± 14.65 years. ESRD patients exhibited a significantly higher prevalence of previously reported comorbidities such as sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi, than did the controls. The mean follow-up periods for the ESRD and control patients were 5.70 (SD, 2.89) and 5.73 (SD, 2.88) years, respectively.
Incidence Rates of Band Keratopathy
During the follow-up period, 256 (256/188,078 [0.14%]) patients developed band keratopathy. A significantly higher proportion of ESRD patients (230/94,039 [0.24%]) than control patients (26/94,039 [0.05%]) developed band keratopathy (Table 2). In addition, there was a significant difference in the incidence of band keratopathy between the groups (ESRD patients = 5.20/10000 PY; control = 0.43/10000 PY) and the IRR between the ESRD and control groups was statistically significant (12.21, 95% CI = 8.14–18.32, P < 0.0001; Table 2).
After the two groups were divided by age, we found that ESRD patients <50 years old had the highest incidence rate (8.37/10000 PY), followed by patients aged 50 to 64 years and patients ≥65 years old. We found significantly higher IRRs for all ESRD age groups compared with their age-matched controls (Table 2).
Male ESRD patients had a band keratopathy incidence of 5.30/10000 PY, whereas male control patients had a band keratopathy incidence of only 0.27/10000 PY, leading to a significant IRR between male ESRD patients and their controls (IRR = 19.71, 95% CI = 9.62–40.37, P < 0.0001). For female patients, a significant difference was also noted between female ESRD patients and their controls (IRR = 8.89, 95% CI = 5.14–14.60, P < 0.0001; Table 2).
In the ESRD group, the incidence rates of band keratopathy, from the highest to the lowest, were in the order of patients with phthisis bulbi (61.41/10000 PY), iridocyclitis (25.54/10000 PY) and hyperparathyroidism (3.09/10000 PY). However, the IRR for band keratopathy associated with comorbidities could not be determined because no band keratopathy was observed in patients with sarcoidosis, hyperparathyroidism, iridocyclitis, phthisis bulbi in the control group. (Table 2)
Table 3 provides the crude and adjusted HRs for band keratopathy, by cohort, during the follow-up period. After adjusting for age, sex and select comorbid conditions, ESRD remained an independent risk factor for band keratopathy (adjusted HR = 11.56, 95% CI = 7.70–17.36). The comorbidities that were significant risk factors for band keratopathy in both groups included iridocyclitis (adjusted HR = 4.31, 95% CI = 1.07–17.39, P < 0.05) and phthisis bulbi (adjusted HR = 10.02, 95% CI = 2.48–40.49, P < 0.05) after adjusting for age, sex and select comorbid conditions.
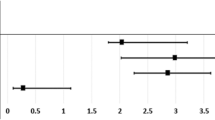
The Kaplan–Meier survival analyses revealed higher band keratopathy cumulative incidence rates in the ESRD patients than in the control patients and the log-rank test was also significant (P < 0.001; Fig. 1).
Discussion
To the best of our knowledge, our study is the largest-scale population-based study that has been conducted to explore the relationship between ESRD and subsequent band keratopathy. We analyzed 94,039 ESRD patients and 94,039 control subjects. We found that the incidence rate of band keratopathy in ESRD patients was 12.21 times higher than that in controls and that the relative risk of band keratopathy for patients with ESRD was 11.56 times higher in the full cohort after adjusting for age, sex, sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi.
Band keratopathy is a frequent chronic degenerative condition that presents with deposition of grayish to whitish opacities on the corneal surface, most commonly in the interpalpebral zone11. These opacities are the result of precipitation of calcium hydroxyapatite crystals in the superficial layers of the cornea, including the epithelial basement membrane, basal epithelium and Bowman’s membrane10. The pathophysiology is multifactorial; besides the well-known chronic ocular conditions such as uveitis and phithsis bulbi, other contributing factors may include elevated serum phosphate levels and increased serum calcium levels that are possibly related to hyperparathyroidism and long-term eye drop use11. Although many previous reports drew attention to the link between ESRD and red eyes resulting from calcific deposits in the conjunctivae or corneas9,19,20, there are few studies that directly evaluated the association of band keratopathy and ESRD. Mullaem suggested that band keratopathy, which typically involves amorphous, white, crystalline and sub-epithelial calcific deposits, is one of the most frequent ocular problems in ESRD patients21. Our study is the largest nationwide population-based cohort study to investigate the risk of band keratopathy following ESRD in Taiwan.
Our findings demonstrate an association between band keratopathy and ESRD. The common pathogenic mechanisms for band keratopathy and ESRD include elevated serum phosphate levels, increased serum calcium levels that are possibly related to hyperparathyroidism and long-term eye drop use in cases involving elevated intraocular pressure or irritated red eyes; these three conditions are discussed separately as follows.
The most well-known pathogenic mechanism common to both conditions is increased serum phosphate and calcium levels. Elevated serum phosphate level is a serious complication affecting ESRD patients receiving hemodialysis12. Increased serum calcium levels occur in conjunction with the secondary renal hyperthyroidism13,14. When the elevated serum phosphate and calcium levels result in calcium phosphate salt precipitation, ESRD patients are susceptible to band keratopathy associated with the deposition of calcium phosphate salts in the form of microcrystalline hydroxyapatite20,21,22. To prevent ongoing hydroxyapatite deposition, elevated levels of phosphate or calcium have to be aggressively controlled.
Another possible pathogenic cause of band keratopathy and ESRD is the greater frequency of long-standing eye drop use in ESRD patients with ocular hypertension and irritable red eyes. There is controversy regarding the effect of hemodialysis on intraocular pressure. Although some studies have demonstrated that intraocular pressure may decrease or remain stable after hemodialysis23, most studies describe an increase in IOP during hemodialysis15,16,17,18. Various theories about the relationship between elevated IOP and hemodialysis have been postulated and the most well-accepted theory suggests an influx of volume into the posterior chamber via the ciliary body due to an imbalance in osmolality between the ocular chamber and the blood during hemodialysis24. When the intraocular pressure is elevated, ESRD patients usually need long-standing eye drop treatment. Band keratopathy might result from the chronic or excessive use of glaucoma medications with mercury-containing preservatives or the use of pilocarpine, an anti-hypertension agent11,25. Another condition related to long-standing eye drop use in ESRD patients is the dryness and irritable red eyes related to inflammation of calcific deposition in the conjunctiva9,19,20. Band keratopathy might be associated with the chronic use of symptom-releasing medications manufactured with phosphate-containing preservatives26.
We found that the incidence of band keratopathy was greater in younger ESRD patients. ESRD patients aged ≥65 years exhibited the lowest incidence of band keratopathy in the ESRD groups (Table 2) and this was an independent protective factor after adjusting for other confounding factors in both groups (adjusted HR = 0.30, 95% CI = 0.21–0.43, P < 0.05, Table 3). The age-dependent incidence trend was found in the control group. However, paradoxically, the incidence of band keratopathy in ESRD patients aged ≥65 years was the lowest. We have attempted to explain the phenomenon by proposing that the death censoring might play a role in explaining the higher incidence rate in the younger ESRD patients and the low incidence rate in ESRD patients aged ≥65 years. Among elder ESRD populations, there might be a higher proportion of patients who died before band keratopathy development than those in the control group.
Band keratopathy is a common and vision-threatening corneal disorder with a characteristic of deposition with gray to white opacity in the surface of the cornea. Many comorbidities have been associated with band keratopathy, including sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi. In this cohort study, we evaluated these comorbidities in ESRD patients and controls and found that iridocyclitis and phthisis bulbi are associated with higher incidences of band keratopathy in the ESRD patients compared with that in the controls (Table 2) and are significant risk factors for band keratopathy in the cohort (Table 3). Many reports demonstrated the link between iridocyclitis and band keratopathy11,27,28. They suggested that although the exact mechanism of calcium-phosphate precipitation in the superficial layers of cornea is unknown, it may result from deposition left as degeneration and necrosis from chronic inflammation related to iridocyclitis11,27,28. ESRD patients with iridocyclitis should be advised to control their inflammation through regular follow-up and treatment by ophthalmologists because of significant association with subsequent band keratopathy.
Band keratopathy in ESRD is an interdisciplinary important issue and close collaboration between nephrologists and ophthalmologists is essential for its management. Nephrologists should be aware of the potential for irritation and visual impairment, which typically presents as white, amorphous and subepithelial hydroxyapatite crystalline deposition, in ESRD patients under chronic dialysis. The most important concerns for ophthalmologists are evaluating the necessity of treatment in various band keratopathy conditions. Although band keratopathy usually does not impair visual acuity or induce irritation, especially in the early stages, there are some indications for intervention if the condition has progressed. The two major indications for treatment in band keratopathy are decreased vision, which occurs as the calcific precipitation spreads centrally and mechanical irritation, which occurs because of broken epithelium or a disrupted corneal surface related to calcium accumulation11,21. Multiple therapies have been attempted for band keratopathy, including mechanical debridement to remove the calcific deposition, EDTA chelation to remove the calcium only and keep the corneal surface smooth29,30 and excimer laser phototherapeutic keratectomy to remove wide areas of cornea precisely while avoiding trauma to adjacent tissue31,32,33. To avoid additional calcium phosphate deposition, ophthalmologists should maintain caution while prescribing phosphate-containing eye drops to treat irritation26 and old mercury-containing eye drops to control the elevated ocular hypertension in ESRD with band keratopathy patients11,25. Once the diagnosis of band keratopathy is confirmed by an ophthalmologist, prevention of ongoing hydroxyapatite crystalline deposition by aggressive treatment of the increased serum calcium or phosphate levels in ESRD patients on dialysis is of outmost importance for nephrologists. These modalities include dietary recommendations such as substitution of animal protein with vegetarian sources12,34,35, more frequent and more prolonged sessions of dialysis treatments36,37, dual binder therapy – the use of two phosphorus-binding medications38, drug treatment in the patients with secondary renal hyperparathyroidism with agents such as calcitriol analogues13 or calcimimetic agents14,39 and recommending the patients with secondary renal hyperparathyroidism to undergo parathyroidectomy. When dealing with band keratopathy in ESRD patients on dialysis, close cooperation between nephrologists and ophthalmologists is important to reduce the risk of further irritation and visual impairment.
There are several strengths in our study. Based on a nationwide and population-based dataset including a large sample of ESRD patients, the study showed increased precision in risk appraisal and elevated statistical power. In addition, because patients with visual disturbances visit an ophthalmologist rather than a general practitioner in Taiwan, the selection bias in referral centers and chances of misdiagnosis are reduced. Furthermore, this study is a cohort study monitoring the band keratopathy incidence in ESRD and in comparison cohorts with maximum longitudinal data of 10 years. Finally, because sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi were taken into account as confounding factors to adjust the hazard ratio of band keratopathy in ESRD patients, our results are reliable.
There are some limitations in our study. Because the sampled patients’ medical history can only be traced back to the year 1996, we cannot confirm that the controls had no ESRD history before January 1996. Additionally, the diagnosis of ESRD, band keratopathy and other comorbidity disorders relied on ICD-9-codes, which may lead to disease misclassification. Furthermore, some bias may have been introduced because the insurance claims data did not include laboratory data on serum calcium or phosphate levels, or information regarding vitamin D treatment. Besides, intraocular pressure changes after hemodialysis are a controversial topic, since they may increase, remain stable, or decrease. Therefore, one of the mechanisms explaining the increased incidence of band keratopathy based on an increased use of eye drops for the treatment of elevated intraocular pressure in ESRD patients is not very solid. Finally, the evaluation of many comorbidities associated with band keratopathy, including sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi in ESRD patients and controls showed that the absence of these comorbidities in the control group compromised the significant incidence ratios of these comorbidities between ESRD and control patients.
In summary, our study showed that after adjusting for age, sex, sarcoidosis, hyperparathyroidism, iridocyclitis and phthisis bulbi, ESRD patients showed a significantly higher risk of developing band keratopathy during the follow-up period. The association between ESRD and band keratopathy is possible based on the elevated serum phosphate and increased serum calcium levels related to secondary renal hyperparathyroidism and long-term eye drop use in patients with ocular hypertension or irritable red eyes. We recommend that ophthalmologists should provide adequate treatment modalities in ESRD patients with band keratopathy including observation only, avoidance of phosphate-containing or mercury-containing eye drops, mechanical removal, chelation treatment and phototheraputic keratectomy. Nephrologists should be aware of the link between the elevated serum phosphate, increased serum calcium levels related to secondary renal hyperparathyroidism and band keratopathy and aggressively control the elevated levels of phosphate or calcium by dietary recommendation, more frequent and more prolonged hemodialysis, dual binder therapy and medical control (e.g., calcitriol analogues or calcimimetic agents) or recommendations for surgery (e.g., subtotal or total parathyroidectomy) in patients with secondary renal hyperparathyroidism. Close cooperation between nephrologists and ophthalmologists is necessary when dealing with band keratopathy following ESRD and to reduce the irritation and visual impairment development.
Additional Information
How to cite this article: Weng, S.-F. et al. Risk of Band Keratopathy in Patients with End-Stage Renal Disease. Sci. Rep. 6, 28675; doi: 10.1038/srep28675 (2016).
References
Levey, A. S. et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney international 72, 247–259 (2007).
Meguid El Nahas, A. & Bello, A. K. Chronic kidney disease: the global challenge. Lancet 365, 331–340 (2005).
Hwang, S. J., Tsai, J. C. & Chen, H. C. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology (Carlton) 15 Suppl 2, 3–9 (2010).
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. Jama 298, 2038–2047 (2007).
McClellan, W. et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. Journal of the American Society of Nephrology : JASN 17, 1710–1715 (2006).
Kuo, H. W., Tsai, S. S., Tiao, M. M. & Yang, C. Y. Epidemiological features of CKD in Taiwan. American journal of kidney diseases : the official journal of the National Kidney Foundation 49, 46–55 (2007).
Wen, C. P. et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371, 2173–2182 (2008).
Hsu, C. C. et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. American journal of kidney diseases : the official journal of the National Kidney Foundation 48, 727–738 (2006).
Klaassen-Broekema, N. & van Bijsterveld, O. P. Red eyes in renal failure. The British journal of ophthalmology 76, 268–271 (1992).
O’Connor, G. R. Calcific band keratopathy. Transactions of the American Ophthalmological Society 70, 58–81 (1972).
Jhanji, V., Rapuano, C. J. & Vajpayee, R. B. Corneal calcific band keratopathy. Current opinion in ophthalmology 22, 283–289 (2011).
Waheed, A. A., Pedraza, F., Lenz, O. & Isakova, T. Phosphate control in end-stage renal disease: barriers and opportunities. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 28, 2961–2968 (2013).
Negri, A. L. & Brandenburg, V. M. Calcitriol resistance in hemodialysis patients with secondary hyperparathyroidism. International urology and nephrology 46, 1145–1151 (2014).
Ballinger, A. E., Palmer, S. C., Nistor, I., Craig, J. C. & Strippoli, G. F. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. The Cochrane database of systematic reviews 12, CD006254 (2014).
Nongpiur, M. E. et al. Chronic kidney disease and intraocular pressure: the Singapore Malay Eye Study. Ophthalmology 117, 477–483 (2010).
Tawara, A., Kobata, H., Fujisawa, K., Abe, T. & Ohnishi, Y. Mechanism of intraocular pressure elevation during hemodialysis. Current eye research 17, 339–347 (1998).
Leiba, H., Oliver, M., Shimshoni, M. & Bar-Khayim, Y. Intraocular pressure fluctuations during regular hemodialysis and ultrafiltration. Acta ophthalmologica 68, 320–322 (1990).
Levy, J., Tovbin, D., Lifshitz, T., Zlotnik, M. & Tessler, Z. Intraocular pressure during haemodialysis: a review. Eye (Lond) 19, 1249–1256 (2005).
Abrams, J. D. Corneal and other ocular findings in patients on intermittent dialysis for renal failure. Proceedings of the Royal Society of Medicine 59, 533–534 (1966).
Klaassen-Broekema, N. & van Bijsterveld, O. P. The red eye of renal failure: a crystal induced inflammation? The British journal of ophthalmology 76, 578–581 (1992).
Mullaem, G. & Rosner, M. H. Ocular problems in the patient with end-stage renal disease. Seminars in dialysis 25, 403–407 (2012).
Berkow, J. W., Fine, B. S. & Zimmerman, L. E. Unusual ocular calcification in hyperparathyroidism. American journal of ophthalmology 66, 812–824 (1968).
Liakopoulos, V. et al. Intraocular pressure changes during hemodialysis. International urology and nephrology 47, 1685–1690 (2015).
Evans, R. D. & Rosner, M. Ocular abnormalities associated with advanced kidney disease and hemodialysis. Seminars in dialysis 18, 252–257 (2005).
Pavicic-Astalos, J. et al. Eye drops preservative as the cause of corneal band keratopathy in long-term pilocarpine hydrochloride treatment. Acta clinica Croatica 51, 107–111 (2012).
Popiela, M. Z. & Hawksworth, N. Corneal calcification and phosphates: do you need to prescribe phosphate free? Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 30, 800–802 (2014).
Moisseiev, E. et al. Acute calcific band keratopathy: case report and literature review. Journal of cataract and refractive surgery 39, 292–294 (2013).
Nascimento, H. et al. Uveitic band keratopathy: child and adult. Journal of ophthalmic inflammation and infection 5, 35 (2015).
Najjar, D. M., Cohen, E. J., Rapuano, C. J. & Laibson, P. R. EDTA chelation for calcific band keratopathy: results and long-term follow-up. American journal of ophthalmology 137, 1056–1064 (2004).
Arjamaa, O. EDTA chelation for calcific band keratopathy. American journal of ophthalmology 139, 216; author reply 216 (2005).
Stewart, O. G. & Morrell, A. J. Management of band keratopathy with excimer phototherapeutic keratectomy: visual, refractive and symptomatic outcome. Eye (Lond) 17, 233–237 (2003).
Najjar, D. M. Management of band keratopathy with excimer phototherapeutic keratectomy. Eye (Lond) 20, 252 (2006).
Rapuano, C. J. Phototherapeutic keratectomy: who are the best candidates and how do you treat them? Current opinion in ophthalmology 21, 280–282 (2010).
Moe, S. M. et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN 6, 257–264 (2011).
Karp, H. J., Vaihia, K. P., Karkkainen, M. U., Niemisto, M. J. & Lamberg-Allardt, C. J. Acute effects of different phosphorus sources on calcium and bone metabolism in young women: a whole-foods approach. Calcified tissue international 80, 251–258 (2007).
Rocco, M. V. et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney international 80, 1080–1091 (2011).
Daugirdas, J. T. et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. Journal of the American Society of Nephrology : JASN 23, 727–738 (2012).
Huml, A. M., Sullivan, C. M., Leon, J. B. & Sehgal, A. R. The adequacy of phosphorus binder prescriptions among American hemodialysis patients. Renal failure 34, 1258–1263 (2012).
Fukagawa, M. et al. Prescription patterns and mineral metabolism abnormalities in the cinacalcet era: results from the MBD-5D study. Clinical journal of the American Society of Nephrology : CJASN 7, 1473–1480 (2012).
Acknowledgements
Data from the National Health Insurance Research Database were provided by the Taiwan Bureau of National Health Insurance and Department of Health. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Author information
Authors and Affiliations
Contributions
All authors conceived the study. S.-F.W., R.-L.J., C.C. and Y.-S.C. conducted the study. S.-F.W., R.-L.J., C.C., J.-J.W. and Y.-S.C. analyzed the results. J.-J.W., S.-B.S., C.C.H. and S.-H.T. provided materials. S.-F.W., R.-L.J., C.C. and Y.-S.C. wrote the article. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Weng, SF., Jan, RL., Chang, C. et al. Risk of Band Keratopathy in Patients with End-Stage Renal Disease. Sci Rep 6, 28675 (2016). https://doi.org/10.1038/srep28675
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28675
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.