Abstract
Increasing evidences support that metabolically healthy obese (MHO) is a transient state. However, little is known about the early markers associated with the development of metabolic abnormalities in MHO individuals. Serum free fatty acids (FFAs) profile is highlighted in its association with obesity-related insulin resistance, type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD). To examine the association of endogenous fatty acid metabolism with future development of metabolic abnormalities in MHO individuals, we retrospectively analyzed 24 [product FFA]/[precursor FFA] ratios in fasting sera and clinical data from 481 individuals who participated in three independent studies, including 131 metabolic healthy subjects who completed the 10-year longitudinal Shanghai Diabetes Study (SHDS), 312 subjects cross-sectionally sampled from the Shanghai Obesity Study (SHOS) and 38 subjects who completed an 8-week very low carbohydrate diet (VLCD) intervention study. Results showed that higher baseline level of oleic acid/stearic acid (OA/SA) and lower levels of stearic acid/palmitic acid (SA/PA) and arachidonic acid/dihomo-γ-linolenic acid (AA/DGLA) ratios were associated with higher rate of MHO to MUO conversion in the longitudinal SHDS. Further, the finding was validated in the cross-sectional and interventional studies. This panel of FFA ratios could be used for identification and early intervention of at-risk obese individuals.
Similar content being viewed by others
Introduction
Obesity, a global epidemic health problem, is associated with high risks of metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVD)1. However, up to 30% of obese people are metabolically healthy2. These metabolically healthy obese (MHO) individuals have normal levels of circulating lipids, hormones and inflammatory cytokines, their insulin sensitivity, hepatic enzyme function and immune function are also normal and they have no hypertension3. However, the metabolic normality in some of the MHO individuals may be short-lived. Although MHO people may not be at increased short-term risk of metabolic abnormalities4,5,6,7, studies with longer follow-up have found increased likelihood of T2DM, CVD and mortality in the MHO compared with metabolically healthy, normal-weight (MH-NW) subjects8,9,10. To date, factors that are associated with healthy and unhealthy obesity phenotypes remain unclear. Identification of early metabolic markers associated with the risk of developing metabolic disorders in MHO people will help us distinguish at-risk obese individuals and delay or prevent the onset of metabolic diseases with proper interventions.
Free fatty acids (FFAs) are released from adipocytes into the circulation through lipolysis and the concentration of circulating FFAs is usually elevated in obese subjects due to increased amount of adipose tissue. High levels of FFAs in the circulation are implicated in the pathogenesis of obesity-related insulin resistance, T2DM and CVD11, possibly through increased generation of deleterious lipid metabolites12, proinflammatory cytokines13 and inducing oxidative stress and endoplasmic reticulum stress14,15. We have recently shown that several circulating unsaturated FFAs were predictive of future metabolic abnormalities in MHO individuals16. Compared with concentrations of individual FFAs, the ratios of [product FFA/[precursor FFA] may better reflect the status of endogenous fatty acid metabolism. In this study, we measured 24 [product FFA]/[precursor FFA] ratios and assessed the associations between these ratios and the metabolic status of obese individuals, in order to uncover novel biomarkers which could be used to identify MHO individuals who are at-risk for metabolic disorders. This study examined samples collected from participants in 3 independent studies, including a 10-year longitudinal cohort of 131 metabolically healthy individuals from the Shanghai Diabetes Study (SHDS)17, a cross-sectional cohort of 312 subjects from the Shanghai Obesity Study (SHOS)18 and a controlled dietary intervention study of 38 obese subjects who completed 8-week of very low carbohydrate diet (VLCD)19.
Results
FFA ratios as predictive markers for the development of metabolic abnormalities in MHO
The subjects selected from the SHDS consisted of 69 MH-NW and 62 MHO at baseline, with clinical data and sera collected at both baseline and 10-year follow-up. The data were used in the present study to investigate whether MHO had higher risk of developing metabolic abnormalities than MH-NW and to explore the potential role of FFA ratios as early metabolic markers to predict the development of metabolic abnormalities in MHO. At 10-year follow-up, 33 of 69 baseline MH-NW remained metabolically healthy (stable MH-NW) and other 36 became MU-NW (progressors to MU-NW). By contrast, 12 of 62 baseline MHO remained their metabolic health after 10 years (stable MHO), but 50 baseline MHO developed metabolic abnormalities at the 10-year follow-up (progressors to MUO). The incidence rate of metabolic abnormalities was 1.54-fold higher in MHO than in MH-NW during the 10 years.
The baseline clinical characteristics of MH-NW and MHO groups and four subgroups categorized by follow-up metabolic status were listed in Table 1. The participants in baseline MH-NW group were younger than the MHO. SBP, DBP, 2hPG, fasting insulin (FINS), 2 h INS, TG and HOMA-IR were significantly higher and Matsuda ISI was lower in the MHO group compared with NH-NW. Further, stable MHO had higher levels of 2hPG and TC and lower level of insulin sensitivity relative to the stable MH-NW. However, all of baseline clinical parameters in Table 1 were comparable between the progressors to MU-NW and progressors to MUO. Only TG was significantly higher in both progressors to MUO and progressors to MU-NW compared with their stable MH subjects, respectively (P < 0.05).
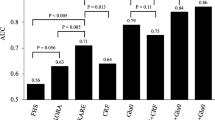
Among 24 [product FFA]/[precursor FFA] ratios, 3 were significantly altered (P < 0.01) in progressors to MUO compared with stable MHO and these 3 ratios were SA/PA (C18:0/C16:0), OA/SA (C18:1 n9/C18:0) and AA/DGLA (C20:4 n6/C20:3 n6) (Fig. 1 and Supplementary Table S1). The predictive powers of the 3 ratios for the risk of developing metabolic abnormalities among the MHO were evaluated using multivariate logistic regression analysis (Table 2). The results showed that baseline level of OA/SA ratio was a positive predictor (adjusted odds ratios 2.38 [95% CI 1.05-5.40]) and SA/PA, AA/DGLA were negative predictors (0.59 [95% CI 0.36-0.98] and 0.85 [95% CI 0.72-0.99], respectively), independent of sex, age, BMI and TG. We also performed receiver operating characteristic (ROC) analysis on the 3 FFAs ratios and conventional predictor of TG and found that the predictive value of 3 FFA ratios were better than it, with an area under the curve (AUC) of 0.755~0.767 compared to the AUC of 0.684 for TG. When the SA/PA, OA/SA, AA/DGLA ratios were combined with TG, the potential to distinguish the progressors to MUO and stable MHO was 0.837 as measured by ROC AUC (Fig. 2). By contrast, none of FFA ratios had significant difference between stable MH-NW and progressors to MU-NW.
Relative contribution of a panel of FFAs ratios to predict metabolic abnormalities after 10-year in MHO individuals.
Presented are ROC AUC comparing TG itself and the combination of TG with SA/PA, OA/SA and AA/DGLA. The combination model of three ratios and TG is computed by 1.748 × TG + 5.447 × OA/SA −0.601 × SA/PA −1.477 × AA/DGLA.
Associations of SA/PA, OA/SA and AA/DGLA with metabolic health and BMI
To further confirm the associations of SA/PA, OA/SA, AA/DGLA ratios with metabolic and BMI, we compared the 3 FFA ratios among the MH-NW, MHO and MUO groups in the 312 subjects selected from the SHOS. Table 3 shows the clinical characteristics of the three study groups. The MHO and MUO subjects had similar BMIs which were significantly higher than that of the MH-NW. Subjects in the MUO group were ~10 years older than those in the other two groups. As expected, FPG, 2hPG and HbA1c concentrations were all significantly higher in the MUO subjects compared with the other two groups. FINS, TG, LDL-C and HOMA-IR were significantly higher and HDL-C was lower in the MHO and MUO subjects compared with NH-NW and these parameters were significantly different between the MHO and the MUO.
The 24 [product FFA]/[precursor FFA] ratios are summarized in Supplementary Table S2. Strikingly, the SA/PA, OA/SA and AA/DGLA ratios were all significantly altered in the MUO (P < 0.01) compared with both MH-NW and MHO groups (Table 3). Correlation analysis showed OA/SA was positively correlated with FPG, 2 h PG, HbA1c, TG, LDL-c, HOMA-IR and negatively correlated with Matsuda ISI, while SA/PA and AA/DGLA were both positively correlated with Matsuda ISI and negatively correlated with FPG, 2 h PG, HbA1c, TG, LDL-c, HOMA-IR (|r| = 0.15 ~ 0.59, P < 0.05) (Supplementary Table S3).
Effects of diet-induced weight loss on SA/PA, OA/SA and AA/DGLA ratios
SA/PA, OA/SA and AA/DGLA ratios were analyzed for the 38 obese subjects from the dietary intervention study to examine the effect of weight loss on these FFA ratios. The clinical characteristics of the participants are summarized in Table 4. Eight weeks of VLCD resulted in a significant decrease of BMI) (3.1 kg/m2), significant reduction of subcutaneous and visceral fat areas and improvement of insulin sensitivity. Compared with the baseline levels, OA/SA was significantly decreased while SA/PA and AA/DGLA increased after 8 weeks of VLCD intervention (P < 0.01 or 0.05) (Table 4). Correlation analysis showed the changes of OA/SA and AA/DGLA were negatively (r = −0.35, p = 0.03) and positively (r = 0.52, p < 0.01) correlated with the change of Matsuda ISI, respectively.
Discussion
The present study highlighted the predictive value of SA/PA, OA/SA and AA/DGLA ratios for the development of metabolic abnormalities in MHO, i.e., MHO with higher OA/SA ratio had greater chance of being metabolically unhealthy within a 10-year period, while MHO with higher SA/PA and AA/DGLA ratios had lower chance of developing metabolic abnormalities. The predictive value of these 3 ratios is independent of age, sex, BMI, TG and with higher power (added 0.153 units of ROC area to the TG alone). The finding may contribute to more precise evaluation of the risk of metabolic disease in MHO and enable early intervention to protect the vulnerable population. Prospective studies with long-term follow-ups have shown that MHO individuals had increased risk for developing metabolic abnormalities. For example, 37.1% of baseline MHO subjects became MUO, whereas 15.9% MH-NW were reclassified as MU-NW at 6-year follow-up in Pizarra study20. Similarly, in the North West Adelaide Health study, compared with metabolically healthy normal-weight subjects, the MHO were more likely to develop metabolic risk (15.5% vs. 33.1%) during 5.5–10.3 years of follow-up4. Other studies also reported that 42.1% metabolically healthy abdominal obese subjects developed MetS9 and 64.7% MHO became MUO at 10-year follow-up21. Furthermore, a systematic review evaluating the associations of metabolic health and BMI with CVD events reported that MHO individuals are at increased risk for adverse long-term CVD outcomes compared with MH-NW (RR = 1.24; 95% CI, 1.02 to 1.55), even in the absence of metabolic abnormalities22. In our longitudinal SHDS at 10-year follow-up, we found a higher incidence rate of metabolic abnormalities in MHO (80.6%, n = 62) compared with their MH-NW counterpart (52.2%, n = 69), which further proves that MHO is at higher risk of developing metabolic abnormalities. Factors that benefit metabolic health in obese people are yet to be fully understood. Previous cross-sectional analyses suggested MHO subject were more likely to be younger and female, with higher physical activity and fitness23,24. In addition, greater insulin sensitivity, smaller visceral and ectopic fat accumulation, higher adiponectin level and decreased circulating concentration of high-sensitive C-reactive protein (hsCRP), a surrogate marker of systemic inflammation, were suggested to be the protective factors in MHO phenotype21,25,26. In the analysis of samples collected from the SHDS longitudinal study, we found that, baseline TG was higher in the stable MHO group than the progressors to MUO (p = 0.049), but other baseline markers such as sex, age, plasma glucose and insulin, LDL, HDL and insulin sensitivity were comparable between the two groups. Notably different studies used different criteria to identify MHO. Here we defined MHO as the absence of components of metabolic syndrome by CDS, which was in line with some analyses22, but different from most studies in which allowed MHO to have one4,27, or even two5,21 metabolic syndrome components.
Chronic exposure to elevated concentration of FFAs promotes insulin resistance28 and impairs compensatory β-cell response29, which increased the subsequent risks of MetS, T2DM and CVD. Previous studies confirmed that MHO had lower visceral, liver and muscle fat contents than insulin-resistant obese people did, suggesting that MHO have a better abilities to trap FFAs in the adipose tissue21,30. Consistently, a cross-sectional study by Succurro et al.31 also observed that MUO subjects have increased levels of plasma total FFA compared with the MHO.
The profile of circulating FFAs is considered as a surrogate marker of fatty acid composition in the adipose tissue32. However, to our best knowledge, few studies have used individual FFA or the ratios of [product FFA]/[precursor FFA] to predict the incidence of metabolic abnormalities in MHO subjects. Challenge may be mainly from the development of easy, fast and reliable method to quantitate individual FFAs in the blood11 which only contain a fairly small fraction of FFAs among the total fatty acids in the circulation (0.2–0.7 mM FFAs vs. 10 mM total fatty acids)33. Our previous work developed a targeted metabolomics approach and quantitatively determined 40 serum FFAs to assess their associations with metabolic health in obese individuals and we found that several unsaturated FFAs were associated with the risk of MetS and T2DM16. Because circulating FFA composition to some extent reflects the function of elongase and desaturase during endogenous fatty acid metabolism, we hypothesized that some key [product FFA]/[precursor FFA] ratios might be better metabolic markers for adverse outcomes of obesity. We found that SA/PA, OA/SA and AA/DGLA ratios, which reflect stearoyl-CoA desaturase 1 (SCD-1), delta 5 desaturase (D5D) and ELOVL 6 activities, respectively, independently associated with the incidence of metabolic abnormalities in baseline MHO at 10-year follow-up. This novel finding was corroborated by a cross-sectional sampling from the SHOS and a dietary intervention study. Our findings highlighted the importance of fatty acid metabolism in the maintenance of metabolic health in overweight/obese subject and warrant future studies to investigate the roles of SCD-1, D5D and ELOVL6 in adipocyte, which may affect FFA metabolism, transportation, storage and utilization.
The effects of some confounding factors such as gender and age on the changes of three key FFAs ratios were examined in this study. We performed gender-based subgroups analyses on MH-NW, MHO and MUO participants in the cross-sectional study, respectively. Results showed that at similar BMI and metabolic status, the levels of the OA/SA, SA/PA and AA/DGLA ratios in male and female participants had not statistically significant difference (p > 0.05). Similarly, none of them had the significant correlation with age in both SHDS and SHOS studies. Considering that the participants in baseline MH-NW group were younger (age range 21~39) than the MHO in the SHDS, we further investigate whether MHO had a higher risk of developing metabolic abnormalities than MH-NW under age-matched condition. Among 29 subjects less than 40 years old selected from 62 baseline MHO subjects, 23 of them (79.3%) developed metabolic abnormalities at 10-year follow-up, similar to the present results ignoring the age in that 50 of 62 baseline MHO (80.6%) progressed to MUO. These results confirmed that the confounding factors of gender and age did not affect our present findings.
There are several limitations in this study. Firstly, the subjects in the longitudinal SHDS were randomly recruited from two urban communities of Shanghai, China, but only ~20% (1250 of 5994) completed 10-year follow-up visit, which may result in selection bias in the cohort used in the present study. Secondly, the size of the cohort selected from the SHDS is moderate and therefore the predictive value of three ratios should be validated larger cohorts, particularly in population-based prospective studies. Thirdly, the FFAs ratios at 10-year follow up were not measured. Fourthly, information on lifestyle and diet were not collected, which may result in covariate imbalance. Fifthly, a recent study reported that insulin sensitivity improved by about 26% in MUO but decreased by about 13% in MHO after a 6-month energy-restricted diet34 and it would be interesting to assess the differences of these key FFA ratios between MHO and MUO individuals after achieving similar diet-induced weight loss. Lastly, due to the potential effect of dietary habit on the fatty acids composition in adipose tissue, whether the findings in this study could translate across into other populations needs further validation. The main strength of this study is that as a retrospective study, we utilized clinical information and serum samples collected in 3 independent studies. Results from the longitudinal SHDS established potential cause-and-effect relationship between the FFA ratios and the incidence of metabolic abnormalities in the MHO, the cross-sectional sampling from the SHOS corroborated the association between the FFA ratios and metabolic abnormalities and the dietary intervention study demonstrated that diet and BMI were influential factors on the FFA ratios.
In conclusion, we showed that MHO individuals had a higher risk of developing metabolic abnormalities over time compared with MH-NW in a communities-based Chinese population. The SA/PA, OA/SA and AA/DGLA ratios were associated with the incidence of metabolic abnormalities in MHO subjects and had relatively high predictive value to identify overweight/obese individuals who are at-risk for metabolic abnormalities. Further study is needed to elucidate whether desaturase and elongase, such as SCD-1, D5D and ELOVL6, play a causal role in metabolic dysregulation and could be novel therapeutic targets for prevention and treatment of metabolic deterioration in obese population.
Methods
Study design and participants
This retrospective study utilized clinical data and samples collected from participants enrolled in the following studies:
-
1
SHDS17: a 10-year community-based epidemiological survey initiated in 1998–2001, designed to assess the prevalence of diabetes and other metabolic disorders. Totally 5,994 individuals older than 15 years were enrolled from Shanghai, China and 1,250 subjects completed the follow-up examination between 2010 and 2011. A total of 10.5% (131 out of 1250) subjects (mean age 37 years, 72.5% women) from SHDS met our inclusion criteria and were selected for the present study. The inclusion criteria included: 1) metabolically healthy at baseline; 2) no significant changes of body mass index (BMI) level between the baseline and follow-up (significant changes include NW → OW/OB, or OW/OB → NW). Among the 131 participants, 69 had normal weight and other 62 were overweight or obese.
-
2
SHOS18: a prospective study designed to investigate the occurrence and development of metabolic syndrome and related disease, started in 2009 and enrolled 5000 participants older than 20 years from four communities in Shanghai, China. The present study included clinical data and samples from 312 adult participants in SHOS (mean age 49 years, 60.6% women). The criteria for selecting participants for the present study were as follow: 1) Those metabolically healthy participants with normal BMI (MH-NW, n = 132);2) Those metabolically healthy participants with overweight or obese state (MHO, n = 107); 3) Those metabolically unhealthy participants with overweight or obese state (MUO, n = 73). Any participants who had received antihypertensive therapy, lipid-lowering therapy, or a combination of antihypertensive and lipid-lowering therapy were excluded.
-
3
A controlled dietary intervention study19: an 8-week VLCD intervention study on 53 obese volunteers. The present study involved 38 subjects (mean age 33 years, 39.5% women, mean BMI 32.9 kg/m2) who completed the 8-week VLCD and their serum samples collected at baseline and eight weeks after intervention.
The protocol for this metabolomics study using samples from the three aforementioned studies was approved by the ethics committee at the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from all participants.
Definitions of overweight, obesity and metabolic health
According to the Working Group on Obesity in China, for Chinese adults, obesity, overweight and normal weight are defined as BMI ≥28 kg/m2, 28 kg/m2 >BMI ≥24 kg/m2 and BMI <24 kg/m2, respectively35. Being metabolically healthy means complete absence of any metabolic abnormalities that meet the Chinese Diabetes Society (CDS), metabolic syndrome diagnostic criteria36, including 1) systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; 2) fasting plasma triglyceride (TG) >1.7 mmol/L; 3) fasting plasma glucose (FPG) >6.1 mmol/L or 2-hour after meal plasma glucose (2hPG) >7.8 mmol/L; 4) High-density lipoprotein (HDL) ≥0.9 mmol/L in male or ≥1.0 mmol/L in female.
Based on the changes of metabolic parameters between baseline and follow-up, participants in the SHDS longitudinal study were separated into four groups: 1) stable MH-NW: BMI <24 kg/m2 and no metabolic abnormalities at baseline and follow-up; 2) progressors to MU-NW: BMI <24 kg/m2 no metabolic abnormalities at baseline, remained normal weight but became metabolically unhealthy at follow-up; 3) stable MHO, BMI ≥24 kg/m2 and no metabolic abnormalities at baseline and follow-up; 4) progressors to MUO: BMI ≥24 kg/m2 and no metabolic abnormalities at baseline, remained being overweight or obese and became metabolically unhealthy at follow-up. Participants selected from the SHOS were divided into three group: 1) MH-NW: BMI <24 kg/m2 and no metabolic abnormalities; 2) MHO: BMI ≥24 kg/m2 and no metabolic abnormalities; 3) MUO: BMI ≥24 kg/m2 and met at least two CD metabolic syndrome diagnostic criteria.
Anthropometric indices and laboratory measurements
Each participant had a physical examination including measurement of height, weight, waist circumference and blood pressure. BMI was calculated as weight (kg) divided by squared height (m2). Waist circumference (WC) was measured at the horizontal plane between the inferior costal margin and the iliac crest on the mid-axillary line. Blood pressure was the average of three measurements made with a sphygmomanometer at two minute intervals.
After a fasting venous blood sample was collected, each participant received a 75 g oral glucose tolerance test. Plasma glucose levels were measured by the glucose oxidase method. Serum insulin was assayed using bio-antibody technique (Linco, St Louis, MO, USA). Serum lipid profiles including total cholesterol (TC), serum total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), were determined by standard commercial methods on a parallel, multichannel analyser (Hitachi 7600-020, Tokyo, Japan). An experienced technician measured glycated haemoglobin A1c (HbA1c) of de-identified samples using high performance liquid chromatography (HLC-73G7, Tosoh, Japan). Insulin resistance and insulin sensitivity were evaluated using the following formulas: (1) homeostasis model assessment of insulin resistance (HOMA-IR) = fasting insulin×fasting glucose/22.5; (2) Matsuda insulin sensitivity index (Matsuda ISI) = 10,000/√ (fasting insulin×fasting glucose×mean insulin during OGTT×mean glucose during OGTT)37.
Subcutaneous and visceral fat areas were assessed by Philips Achieva 3.0T MRI system (Philips Medical Systems, Eindhoven, The Netherlands) using standard array coils with the subject in a supine position as previously reported19.
Sample preparation and FFAs analysis
All of serum samples were stored at −80 °C until use. Sample preparation were performed according to a modified protocol based on Püttmann et al.33. Forty FFAs were analyzed using an ACQUITY ultra-performance liquid chromatography (UPLC) system (Waters Corporation, Milford, USA) equipped with a binary solvent delivery system and an auto-sampler (Waters Corporation, Milford, USA), coupled to a tandem quadrupole-time-of-flight (Q-TOF) mass spectrometry (Waters Corporation, Milford, USA)16. For details, see the Supplementary information. A mixture of all the reference standards at an appropriate concentration was prepared and run after every ten serum samples for quality control. Twenty-four FFAs ratios were calculated by the absolute concentrations of product- to-precursor.
Statistical analysis
The raw data produced by UPLC-QTOFMS were initially processed using TargetLynx applications manager version 4.1 (Waters Corp., Milford, MA) to detect peak signals, obtain calibration equations and calculate the absolute concentration of each FFA16. Manual examination and correction were needed to ensure data quality.
Data are presented as mean ± SD or median ± SE. Group values were compared using the Mann-Whitney test for continuous variables or χ2 for nominal variables. Simple associations were tested by spearman correlation coefficients (r) between calculated FFAs ratios and clinical measures. Logistic regression was used to predict metabolic abnormalities risk; odds ratios and 95% CIs were presented. Performance of different logistic models was assessed as the receiver operating characteristic area under the curve (ROC AUC). Statistical significance was defined as two-tailed P < 0.05. All statistical computing and graphics were carried out using SPSS 16.0 (SPSS, Chicago, IL, USA).
Additional Information
How to cite this article: Zhao, L. et al. A panel of free fatty acid ratios to predict the development of metabolic abnormalities in healthy obese individuals. Sci. Rep. 6, 28418; doi: 10.1038/srep28418 (2016).
References
Mokdad, A. H. et al. Prevalence of obesity, diabetes and obesity-related health risk factors, 2001. JAMA 289, 76–79 (2003).
Karelis, A. D., St-Pierre, D. H., Conus, F., Rabasa-Lhoret, R. & Poehlman, E. T. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 89, 2569–2575 (2004).
Brochu, M. et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab 86, 1020–1025 (2001).
Appleton, S. L. et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype a cohort study. Diabetes Care 36, 2388–2394 (2013).
Meigs, J. B. et al. Body mass index, metabolic syndrome and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 91, 2906–2912 (2006).
Calori, G. et al. Prevalence, metabolic features and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34, 210–215 (2011).
Ogorodnikova, A. D. et al. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity 20, 651–659 (2012).
Twig, G. et al. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care 37, 2989–2995 (2014).
Eshtiaghi, R., Keihani, S., Hosseinpanah, F., Barzin, M. & Azizi, F. Natural course of metabolically healthy abdominal obese adults after 10 years of follow-up: the Tehran Lipid and Glucose Study. Int J Obes 39, 514–519 (2014).
Kuk, J. L. & Ardern, C. I. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 32, 2297–2299 (2009).
Boden, G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 18, 139–143 (2011).
Boden, G., Lebed, B., Schatz, M., Homko, C. & Lemieux, S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50, 1612–1617 (2001).
Boden, G. et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κB pathway in rat liver. Diabetes 54, 3458–3465 (2005).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114, 1752–1761 (2004).
Wei, Y., Wang, D., Topczewski, F. & Pagliassotti, M. J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 291, E275–E281 (2006).
Ni, Y. et al. Circulating unsaturated fatty acids delineate the metabolic status of obese individuals. EBioMedicine 2, 1513–1522 (2015).
Jia, W. et al. Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia 50, 286–292 (2007).
Bao, Y. et al. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J Clin Endocrinol Metab 98, 345–351 (2012).
Gu, Y. et al. Beneficial effects of an 8-week, very low carbohydrate diet intervention on obese subjects. Evid Based Complement Alternat Med 2013, 760804, 10.1155/2013/760804 (2013).
Soriguer, F. et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab 98, 2318–2325 (2013).
Hwang, Y. et al. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes 39, 1365–1370 (2015).
Kramer, C. K., Zinman, B. & Retnakaran, R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med 159, 758–769 (2013).
Stefan, N., Häring, H.-U., Hu, F. B. & Schulze, M. B. Metabolically healthy obesity: epidemiology, mechanisms and clinical implications. Lancet Diabetes Endocrinol 1, 152–162 (2013).
Primeau, V. et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 35, 971–981 (2011).
Aguilar-Salinas, C. A. et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab 93, 4075–4079 (2008).
Jung, C. H. et al. The risk of incident type 2 diabetes in a Korean metabolically healthy obese population: The role of systemic inflammation. J Clin Endocrinol Metab 100, 934–941 (2015).
Aung, K., Lorenzo, C., Hinojosa, M. A. & Haffner, S. M. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab 99, 462–468 (2014).
Roden, M. et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97, 2859–2865 (1996).
Lee, Y. et al. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA 91, 10878–10882 (1994).
Stefan, N. et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 168, 1609–1616 (2008).
Succurro, E. et al. Insulin secretion in metabolically obese, but normal weight and in metabolically healthy but obese individuals. Obesity 16, 1881–1886 (2008).
Hodson, L., Skeaff, C. M. & Fielding, B. A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47, 348–380 (2008).
Püttmann, M., Krug, H., von Ochsenstein, E. & Kattermann, R. Fast HPLC determination of serum free fatty acids in the picomole range. Clin Chem 39, 825–832 (1993).
Karelis, A., Messier, V., Brochu, M. & Rabasa-Lhoret, R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 51, 1752–1754 (2008).
Zhou, B. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases–report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci 15, 245–252 (2002).
Pang, C. et al. The significance of screening for microvascular diseases in Chinese community-based subjects with various metabolic abnormalities. PLoS One 9, e97928, 10.1371/journal.pone.0097928 (2014).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Acknowledgements
This study was supported by grants from International Science & Technology Cooperation Program of China (2014DFA31870) and China Scholarship Council (201408310049).
Author information
Authors and Affiliations
Contributions
W.J. and W.P.J. are the guarantors of this work. W.J. and W.P.J. designed the research. X.M., Y.B., C.W. and C.H. collected the samples and performed clinical measurements. L.Z., A.Z. and J.L. performed serum FFAs targeted metabolomics analysis. L.Z., T.C. and M.S. did the data processing and quality control assessment. L.Z. wrote the manuscript. G.X., J.P., Y.N. and H.Y. provided valuable suggestions in preparing the manuscript. W.J. and W.P.J. revised the manuscript. All authors approved the final version to be published.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhao, L., Ni, Y., Ma, X. et al. A panel of free fatty acid ratios to predict the development of metabolic abnormalities in healthy obese individuals. Sci Rep 6, 28418 (2016). https://doi.org/10.1038/srep28418
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28418
This article is cited by
-
Combination of TRP channel dietary agonists induces energy expending and glucose utilizing phenotype in HFD-fed mice
International Journal of Obesity (2022)
-
Estimated Elovl6 and delta-5 desaturase activities might represent potential markers for insulin resistance in Japanese adults
Journal of Diabetes & Metabolic Disorders (2022)
-
Lipid droplet biogenesis and COX-2 pathway activation are triggered by Barrett’s esophagus and adenocarcinoma, but not esophageal squamous cell carcinoma risk factors
Scientific Reports (2021)
-
A comprehensive metabolic profiling of the metabolically healthy obesity phenotype
Lipids in Health and Disease (2020)
-
Fatty acid profile and estimated desaturase activities in whole blood are associated with metabolic health
Lipids in Health and Disease (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.