Abstract
Gastro-Oesophageal Reflux (GOR) is a key problem in Cystic Fibrosis (CF), but the relationship between lung and gastric microbiomes is not well understood. We hypothesised that CF gastric and lung microbiomes are related. Gastric and sputum cultures were obtained from fifteen CF patients receiving percutaneous endoscopic gastrostomy feeding. Non-CF gastric juice data was obtained through endoscopy from 14 patients without lung disease. Bacterial and fungal isolates were identified by culture. Molecular bacterial profiling used next generation sequencing (NGS) of the 16S rRNA gene. Cultures grew bacteria and/or fungi in all CF gastric juice and sputa and in 9/14 non-CF gastric juices. Pseudomonas aeruginosa(Pa) was present in CF sputum in 11 patients, 4 had identical Pa strains in the stomach. NGS data from non-CF gastric juice samples were significantly more diverse compared to CF samples. NGS showed CF gastric juice had markedly lower abundance of normal gut bacteria; Bacteroides and Faecalibacterium, but increased Pseudomonas compared with non-CF. Multivariate partial least squares discriminant analysis demonstrated similar bacterial profiles of CF sputum and gastric juice samples, which were distinct from non-CF gastric juice. We provide novel evidence suggesting the existence of an aerodigestive microbiome in CF, which may have clinical relevance.
Similar content being viewed by others
Introduction
Cystic Fibrosis (CF) is the most common recessively inherited condition in the Caucasian population1. Seminal reports from the 1930s described pancreatic abnormalities and steatorrhea, with the disease initially known as “Cystic fibrosis of the pancreas”2.
In the modern era CF is recognised as a multi-system disorder. Most of the morbidity and premature mortality associated with CF can be attributed to chronic lung diseases caused by microbial infections and subsequent inflammation3, most frequently by Pseudomonas aeruginosa (Pa)4. Treatment from dedicated CF centres has been associated with increased survival and treatment of lung disease is an understandable priority. The overall benefits associated with dedicated CF centres however emphasise a multi-disciplinary approach and gastrointestinal problems are recognised as prominent. In particular Gastro-Oesophageal Reflux (GOR) is increased with a prevalence of more than 50%5. Several mechanisms have been proposed including reduced lower oesophageal sphincter pressure (LOS), increased transient LOS relaxation and delayed gastric emptying. Increased GOR might also be due to an increased abdomino-thoracic pressure gradient during cough and physiotherapy6.
A relationship between aspiration of gastric contents during a reflux event and deterioration of lung function is implied by the finding of poorer lung function in CF patients with acid reflux7. Anti-reflux medications have been associated with improved respiratory symptoms and lung function and anti-reflux surgery with a slower lung function decline and significant decrease in CF exacerbations8. However it has also been suggested in a scarce literature that treatment with the proton pump inhibitor esomeprazole (PPI) may be associated with earlier and more frequent exacerbations compared with placebo9.
The potential importance of an aero-digestive microbiome has long been the subject of interest in respiratory and gastrointestinal medicine in situations other than CF. Multiple reports provide evidence that a gastric reservoir is a risk factor for acquiring nosocomial pneumonia in the intensive care unit (ICU) setting10. Studies have also been carried out in elderly, non-CF patients which report both concordance between gut and respiratory bacteria and a high prevalence of bacteria present in the gut prior to their presence in the respiratory tract11. This data is consistent with a link between gastric and lower respiratory tract bacterial colonisation in ICU patients fed by nasogastric tube12.
The microbial colonisation of stomach contents in CF patients and its potential to act as a reservoir for microorganisms known to cause lung infection is not completely understood. We therefore undertook a study of the microbiology of CF lung and gastric contents. Our hypothesis was that bacterial flora in sputum would be related to that in gastric aspirate.
Methods
Ethical approval
This study was adopted by the Hepatopancreatobiliary and Gastroenterology Biobank, Newcastle University and approved by the Newcastle & North Tyneside 1 Research Ethics Committee (UK). All study participants provided written informed consent prior to initiation of the study. All methods were carried out in accordance with relevant guidelines.
Symptoms of extraoesophageal reflux
Patients with CF had symptoms of extraoesophageal reflux (EOR) assessed using the Reflux Symptoms Index (RSI) score; score less than 13 classed as not EOR symptomatic13.
CF gastric juice and sputum samples
CF patients receiving percutaneous endoscopic gastrostomy (PEG) feeding represented an important opportunity to directly sample gastric juice, with less potential for contamination with oropharnygeal commensals. Only adult stable CF patients receiving PEG tube feeding, from the 270 CF patients attending the regional CF Centre, were therefore included in this study; potentially 18 patients from the regional clinic population. Following overnight fasting, gastric juice was collected from 15 sequential patients who attended the clinic during the course of the study between 22/05/2013-17-04/2014, 83% of all the potentially available patients with PEG tubes in the region were therefore included in this study. No PEG fed CF patients were excluded or positively selected for the study (Table 1).
In brief, 10 ml of sterile saline was injected through the PEG and aspirated after 2-3 minutes. Spontaneously expectorated sputum (n = 13) and cough swabs (n = 2) were also obtained.
Gastric juice pH was measured using pH strips and sputum samples were homogenised with equal amounts of dithiothreitol for 1 to 3 minutes.
Collection of non-CF gastric juice samples
Following fasting (≥8 hours) gastric juice was collected from 14 patients without CF undergoing routine upper gastro-intestinal endoscopy performed according to British Society of Gastroenterology guidelines, by suctioning gastric juice through the endoscope (Table 2). All patients were requested to stop any acid suppression medication 2 weeks before the endoscopy procedures. Sputum was not collected from the non-CF patients.
Gastric juice and sputum samples microbial study
Gastric juice and sputum samples underwent microbiological culture performed in accordance with UK standard methods. An aliquot was stored at −20 °C for DNA extraction.
10 μL of homogenised sputum and gastric juice were plated for bacterial and fungal cultures. The following media were used: Columbia blood agar supplemented with 5% horse blood, chocolate agar supplemented with 70 mg/L bacitracin, Burkholderia selective agar (for CF sputa and gastric juice, CEP bioMérieux UK), Cysteine lactose electrolyte deficient agar (CLED) and fastidious anaerobic agar (FAA). Plates were incubated according to standard protocol, CEP cultures were incubated for 10 days at 30 °C for isolation of Burkholderia cepacia complex and rapidly growing mycobacterium.
Plates were examined daily for evidence of microbial growth and assessment of the number of distinct colonial variants was recorded. All morphological variants were sub-cultured and used for identification and stored at −20 °C in 10% glycerol skimmed milk. Isolates were identified by matrix assisted laser desorption ionisation time-of-flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, UK) and where necessary, appropriate API kits (bioMérieux, UK)14. Mycobacterium was identified by rpoB, sodA and hsp65 gene sequencing and strain typed using variable number tandem repeat (VNTR), (Colindale, UK15). All isolates of Pa were typed via VNTR profiling16.
DNA extraction
DNA extraction was performed from sputum and gastric juice samples using a PowerSoil™ DNA Isolation Kit (MoBio) in accordance with the manufacturer’s instructions.
Molecular based studies
Bacterial profiling utilised the 16S rRNA gene targeting variable region 4 (V4) based on the Schloss wet-lab MiSeq SOP (http://www.mothur.org/wiki/MiSeq_SOP). Raw fastq data were processed using Mothur (version 1.31.2) as described in the MiSeq SOP17. Chimeric sequences were detected by Chimera.uchime and removed from downstream analysis. Alignment was generated via the Silva database18. A cutoff of 70 was applied to assign sequences to the trainset_ 9_032012 resulting in 2,228,291 reads. All sequences were deposited in MG-RAST under the accession numbers 4603845.3 - 4603893.3.
Statistical analysis
NGS profiles were analysed by multivariate partial least squares discriminant analysis (PLS-DA) (SIMCA 13.0 software, Stockholm, Sweden)19. PLS-DA uses assigned variables to interrogate data for maximum variance. To check data was adhering to multivariate normalities, Hotelling’s T2 tolerance limits were calculated and set at 0.95.
Results
CF Patients
We sampled over 80% of all the PEG patients potentially available in the North East Region of England. This patient cohort had severity of CF lung disease in keeping with this population (median FEV1, 1.55 L range 0.45–3.5 L) along with long term antibiotic exposure and use of acid suppression medication for all patients (Table 1).
Microbial culture
Routine microbial culture was positive for bacteria and/or fungi in all CF gastric juice and sputum samples. All samples had more than one organism isolated except a gastric juice sample from CF-8 that only had Candida albicans. See table E1 and E2 in the online data supplement for CF gastric juice and CF sputum samples culture results.
Symptoms of extraoesophageal reflux in CF patients
Symptom scores for extraoesophageal reflux were available in 12 of the 15 patients with CF (Table 1). All patients were EOR symptomatic, with an RSI score >12; median RSI score 17 (range 13–36).
CF gastric juice vs sputum samples culture results
Nine of the 15 CF patients included in this study had one or more microorganism (fungal or bacteria) common between sputum and gastric juice samples, 5 had at least one bacteria taxa common between sputum and gastric juice while 4 had one or more fungal species. Among the bacterial common between sputum and gastric juice were, Pa (4/9), Streptococcus spp (2/9) and Achromobacter spp. (2/9). Conversely, 6 patients had microorganisms isolated in both gastric juice and sputum, with no organism common to both samples.
All CF patients had fungal species isolated in either sputum or gastric juice samples, this included 8 patients with Candida spp. One patient had Aspergillus spp. isolated in both gastric and sputum samples.
CF gastric juice vs non-CF gastric juice culture results
Microorganisms, either bacterial or fungal, were isolated from all 15 CF gastric juice samples. No microorganisms were isolated from 5 of the 14 non-CF gastric juice samples. (see Table E3).
Bacterial species were detected in 11/15 CF gastric juice samples (mean 2.2 bacterial isolates/patient). Streptococcus spp. (4/15), Pa (4/15), Lactobacillus spp. (4/15) and Staphylococcus spp. (3/15) were the most frequent bacteria isolated. Bacterial species were detected in 8/14 non-CF gastric juice samples (mean 2.2 bacteria isolates/patient). Streptococcus spp (4/14), Lactobacillus spp (2/14) Staphylococcus spp (2/14) were the most frequently isolated. Pa was detected in only one patient, without CF or other underlying lung disease. Fungal pathogens were detected in all CF gastric juice samples (mean of 2 fungi /patient), Candida spp were isolated from only 3 out of 14 non-CF gastric juice samples.
Pseudomonas aeruginosa
Pa was detected in 11 of the 15 CF sputa (73%) (CF-1, CF-5, CF-6 and CF8-15) and in 4 of the 15 CF gastric juice samples (26%) (CF-1, CF-5, CF-12 and CF-15). CF-1 and CF-12 had both mucoidal and non-mucoidal Pa in both sputum and the gastric juice, CF-5 had mucoidal and non-mucoidal Pa in sputum and only mucoidal Pa in gastric juice, CF-15 had only mucoidal Pa in sputum and gastric juice.
The median age of patients with and without Pa was 26 (range 20–27) and 23 (range 18–41) respectively. The median FEV1 for patients with Pa was 1.55L (range 0.45–3.5L) compared to 1.25L (range 0.8–2.7L) in non Pa infected patients.
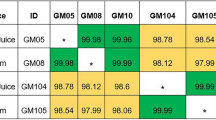
Variable number tandem repeat (VNTR) analysis of Pa showed that Pa strains isolated from gastric juice and sputum samples were identical in 3 of the 4 patients who cultured Pa in both gastric juice and sputum. The remaining CF patient with Pa in gastric juice and sputum (CF-1) also appeared to have matched strains of Pa in both sputum and gastric juice. However, in addition, this patient also had other strains of Pa present only in sputum samples and not isolated in gastric juice.
Next Generation Sequencing
Fourteen non-CF gastric juice (GJ 1-14), 13 CF gastric juice (CF-GJ 1, 2, 4-12, 14, 15) and 12 CF sputum samples (CFS 4-15) had microbiome analysis using NGS. Non-CF gastric juice showed higher diversity compared to the CF gastric juices and sputum samples (Fig. 1A). Proteobacteria were abundant in both CF gastric juice and CF sputum samples (72% and 74% of relative abundance, respectively) whereas Firmicutes were more abundant in non-CF gastric juice (48%).
The average Shannon diversity indices (H’) was significantly higher in non-CF gastric juice than CF gastric juices (P = 0.002) and CF sputum (P = < 0.001). The H’ of CF gastric juice and sputum samples were not different (P = 0.93: Fig. 1B).
PLS-DA of all samples demonstrated that both gastric juice and sputum samples from patients with CF had comparable profiles, which was distinct from non-CF gastric juice (Fig. 2). CF samples showed a relative low average of Shannon diversity making them cluster near the origin of PLS-DA plots, while non-CF gastric juice samples showed high diversity. Further analysis of the matched gastric juice and sputum samples from the same patient showed these samples generally grouped together (see Figure E1 in the data supplement). The loading plot from the PLS-DA of the overall bacterial community revealed that Prevotella, Rothia, Streptococcus, Staphylococcus, Herminiimonas and Pseudomonas were closely associated with CF samples while Faecalibacterium, Roseburia, Bacteroides, and Lactobacillus were more associated with non-CF gastric juices (see Figure E2 in the data supplement).
Partial least square discriminant analysis (PLS-DA) score scatter plot of all samples.
Non-CF gastric juice – GJ; CF gastric juice – CFGJ; Sputum samples – CFS. The Axes represent a % of variance. The CF gastric juice and sputum samples show a close relationship. In contrast the Gastric Juice samples from control patients are widely variable and clearly distinct from the CF sputum and gastric juice clustering.
Discussion
We believe this is the first study directly comparing microorganisms isolated from the airways and gastric juice in the same PEG fed CF patients. We showed that the digestive tract and airways were populated by bacteria which were sometimes identical and of known importance in CF lung disease, including biofilm forming strains of Pa. This highlights the possibility that the stomach constitutes a viable bacterial reservoir, relevant to the overall pathophysiology of CF20.
We found that gastric juice samples from adult CF patients were significantly different from non-CF gastric juice samples. At a molecular level, NGS of CF gastric juice showed a much lower abundance of bacteria than typically found in the normal stomach, with CF gastric juice containing Bacteroides, Faecalibacterium and increased amounts of Pa compared with the non-CF controls21. The results obtained from our non-CF gastric juice samples were in accordance with the known make-up of the normal gastric microbiota22.
In all cases where Pa was found concordantly in CF gastric juice and sputum we demonstrated genetically identical strains by VNTR. If lung and stomach were colonised by Pa from unrelated, stochastic sources, the organisms would be highly unlikely to be identical at the molecular level. In CF patients with intermittent colonization or recently acquired chronic Pa infection, there are high levels of genotype diversity23, suggesting that CF patients acquire unique Pa strains independently, from different environmental sources24. For intermittently colonized patients, following initial eradication by inhaled antibiotic treatment, recolonization by Pa can occur. This can be with a different genotype, suggesting new environmental sources, but in approximately 25% of patients the same genotype is identified, suggesting either undetectable persistent colonisation or local unknown environmental sources of recolonization23. Careful longitudinal eradication studies in CF have observed patients being re-colonized with the original Pa strains after several years of no demonstrable Pa in sputa25. The findings reported in this study might be consistent with the notion that gastric contents may represent a niche, relatively protected from inhaled antibiotics used in best practice eradication protocols26.
Our PEG tube gastric juice sampling eliminated the possibility of contamination by upper gastro intestinal tract and respiratory flora. These are potential considerations for other sampling procedures, such as endoscopy. Our data unambiguously confirms the ability of bacteria to survive in the stomach, constituting a potential reservoir of viable pathogens relevant to CF disease. Reflux and aspiration events have been widely documented in CF patients27 and our CF patients had symptoms of extraoesophageal reflux beyond the normal range. Our data might therefore be consistent with the possibility that microorganisms relevant to CF lung pathophysiology could be aspirated.
It is also likely that gastric juice Pa may derive from swallowed sputum containing Pa that is cleared from the lung following cough. We found one patient where identical Pa was found in sputum and gastric juice but where the sputum sample also contained Pa not isolated in gastric juice. Alongside our finding of Pa in the gastric juice of a control subject without lung disease, our data indicate that although Gastric juice Pa may be swallowed following mucocilliary clearance of a diseased airway, Pa could be derived through diverse routes and sources, including but not restricted to microaspiration for lung Pa.
It has long been recognised that as well as refluxing, even healthy adults may aspirate oropharyngeal secretions, especially during reclined sleep. An indium 111 chloride technique from 1978 showed that 45% of normal subjects aspirated during deep sleep and that in patients with “depressed consciousness”, aspiration was detectable in 70% of patients28. This study noted the potential for bacterial pneumonia as a result of failed clearance of aspirated bacteria. A further valuable quantitative study by Gleeson and colleagues employed infusion of 2 mL/h radioactive Tc-99m tracer into the nasopharynx to estimate the quantity of occult aspiration of nasopharyngeal secretions in normal humans. Aspiration was common, occurring in 50% of healthy young men during sleep and was variable within subjects studied on more than one occasion. The levels of aspiration measured were 0.01 to 0.2 mL; levels noted as being consistent with a potentially significant bacterial inoculum29.
Microbiological continuity of the aerodigestive tract, using culture independent methods has also previously been suggested in healthy adults indicating that microaspiration may be common in healthy individuals30. An association between GOR and lower respiratory tract Pa colonisation in children with CF is also consistent with potential gastric immigration31. More recently in children with chronic cough undergoing bronchoscopy and gastrointestinal endoscopy 8 of the most abundant bacteria in the gastric fluid were shown to be abundant in the lungs. The authors concluded that this represented evidence of microbiological exchange between the lung and the gastrointestinal tract, independent of the oropharyngeal microbiome32.
It is of interest that molecular epidemiology studies in CF lung transplant recipients show that lung allografts become recolonized with the same clone of Pa cultured before transplantation33. This recolonization is believed to originate from the upper airway and the sinuses34. It is also possible that the lungs of CF patients might be vulnerable to infection from aspiration of Pa colonising the gastrointestinal tract post transplantation however35 and that bi-directional transmission of pathogenic organisms, including Pa, occurs between the stomach and the oropharynx12. In lung transplantation, GOR has been substantially implicated as a potential non-alloimmune cause of Bronchiolitis Obliterans Syndrome (BOS) and anti-reflux fundoplication surgery has been assessed36 and associated with improved allograft function37.
Molecular methods have previously demonstrated that gastric microbiome diversity is normally high and similar to that described for the lower gastrointestinal tract22,38. Decreasing airway bacterial diversity in patients with CF is well described39 . This is potentially consistent with our study showing that the CF gastric microbiota showed lower diversity compared to non-CF in the molecular based approach. This loss of diversity was a common feature between sputum and CF gastric juice samples compared to non-CF gastric juices. Molecular identification and multivariate discriminant analysis showed that the CF gastric juice samples and sputum samples were clustered and distinct from the non-CF gastric juices. Moreover, sputum and CF gastric juice samples from the same patient also had comparable profiles. Overall this further suggests that a link may be present between microbes colonising the sputum and gastric juice in some CF patients and that this is worthy of further investigation. Such studies should investigate whether the same profile of micro-organisms in the sputum and the gastric juice we observed might be explained by a common origin in the sinus and/or oro-pharynx.
There are limitations to our study inherent to a real world clinical setting and our study design. We restricted our gastric juice sampling to PEG fed patients and sampled over 80% of all the PEG patients potentially available to us, but this limited our study and our reported data to a specific patient cohort. There was no exclusion based on severity of the disease or antibiotic exposure. It is relevant to consider that apart from PEG fed patients with CF we have previously isolated Pa from samples of gastric juice from non- PEG fed CF patients, where gastric juice was sampled by endoscopy35. Our previous study found strains of Pa identical at the molecular level in broncho alveolar lavage (BAL), endoscopically sampled gastric juice and sputa. This suggests that the findings of our present study, that the stomach constitutes a viable bacterial reservoir relevant to the overall pathophysiology of CF, is not restricted solely to PEG fed patients35.
We also recognise that long term antibiotic exposure and use of Proton Pump Inhibitors (PPIs) in our patients could impact on the gastric juice microbiology that we observed. Use of PPIs is increasingly recognised to be associated with alterations in the gastric, lung and oropharyngeal Microflora32. The importance of this is a source of current debate however, to which we have contributed40.
Gastric acid (GA) inhibition via proton pump inhibitors or histamine-2 receptor antagonists is prescribed to CF patients when fat absorption remains insufficient despite an adequate dosage of pancreatic enzyme replacement41. Gastroesophageal reflux disease is another reason to start drugs for GA inhibition in patients with CF and in North America it has been observed that the majority of patients with CF are on gastric acid suppressive treatments42. According to the clinical practice in our centre of study and that of others, all our CF patients were therefore on acid suppression therapy. Antibiotic therapy is also a current mainstay of therapy in CF. We feel that further studies regarding the effect of antibiotics and anti-acid therapy on the aerodigestive microbiome in CF, though challenging, could be important. We hope that such work may be aided and informed by our present study data.
In summary, we have shown a novel association between the sputum and gastric juice microbiome of CF patients. This study demonstrates that the stomach may represent an under recognised foci of bacterial infection and potential reservoir of Pa in CF. Gastric microbiology could be contributed to by cough, expectoration and swallowing of organisms from the lung. Patients with CF are also known to reflux and aspirate however, indicating that in principle gastric microorganisms could contribute to the lung compartment. We conclude that the ‘aerodigestive microbiome’ may have potential relevance in the pathophysiology of CF and may have therapeutic implications, since Pa eradication does not consider a gastric niche of organisms.
Additional Information
How to cite this article: Al-momani, H. et al. Microbiological profiles of sputum and gastric juice aspirates in Cystic Fibrosis patients. Sci. Rep. 6, 26985; doi: 10.1038/srep26985 (2016).
References
Gibson, R. L., Burns, J. L. & Ramsey, B. W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168, 918–951 (2003).
Andersen, D. H. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child 56, 344–399 (1938).
Davis, P. B. Cystic fibrosis since 1938. Am J Respir Crit Care Med 173, 475–482 (2006).
Govan, J. R. & Deretic, V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60, 539–574 (1996).
Blondeau, K. et al. Gastro-oesophageal reflux and aspiration of gastric contents in adult patients with cystic fibrosis. Gut 57, 1049–1055 (2008).
Pauwels, A. et al. Gastric emptying and different types of reflux in adult patients with cystic fibrosis. Aliment Pharmacol Ther 34, 799–807 (2011).
Navarro, J. et al. Factors associated with poor pulmonary function: cross-sectional analysis of data from the ERCF. Eur Respir J 18, 298–305 (2001).
Sheikh, S. I., Ryan‐Wenger, N. A. & McCoy, K. S. Outcomes of surgical management of severe GERD in patients with cystic fibrosis. Pediatr Pulmonol 48, 556–562 (2013).
DiMango, E. et al. Effect of esomeprazole versus placebo on pulmonary exacerbations in cystic fibrosis. BMC Pulm Med 14, 21 (2014).
Du Moulin, G., Hedley-Whyte, J., Paterson, D. & Lisbon, A. Aspiration of gastric bacteria in antacid-treated patients: a frequent cause of postoperative colonisation of the airway. The Lancet 319, 242–245 (1982).
Madan, J. C. et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio 3, e00251–00212 (2012).
Segal, R., Dan, M., Pogoreliuk, I. & Leibovitz, A. Pathogenic colonization of the stomach in enterally fed elderly patients: comparing percutaneous endoscopic gastrostomy with nasogastric tube. J Am Geriatr Soc 54, 1905–1908 (2006).
Belafsky, P. C., Postma, G. N. & Koufman, J. A. Validity and reliability of the reflux symptom index (RSI). J Voice 16, 274–277 (2002).
Blauwendraat, C., Dixon, G. L. J., Hartley, J. C., Foweraker, J. & Harris, K. A. The use of a two-gene sequencing approach to accurately distinguish between the species within the Mycobacterium abscessus complex and Mycobacterium chelonae. Eur J Clin Microbiol Infect Dis 31, 1847–1853 (2012).
Harris, K. A. et al. Molecular fingerprinting of Mycobacterium abscessus strains in a cohort of paediatric Cystic Fibrosis patients. J Clin Microbiol, JCM. 00155–00112 (2012).
Turton, J. F. et al. Evaluation of a nine‐locus variable‐number tandem‐repeat scheme for typing of Pseudomonas aeruginosa. Clin Microbiol Infect 16, 1111–1116 (2010).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79, 5112–5120 (2013).
Schloss, P. D., Gevers, D. & Westcott, S. L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLos One 6, e27310 (2011).
Eriksson, L., Kettaneh-Wold, N., Trygg, J., Wikström, C. & Wold, S. Multi-and megavariate data analysis: Part I: basic principles and applications. PLS (Umetrics, Umea), pp 63–101 (2006).
Dickson, R. P., Martinez, F. J. & Huffnagle, G. B. The role of the microbiome in exacerbations of chronic lung diseases. The Lancet 384, 691–702 (2014).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nature Reviews Genetics 13, 260–270 (2012).
Bik, E. M. et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA 103, 732–737 (2006).
Jelsbak, L. et al. Molecular epidemiology and dynamics of Pseudomonas aeruginosa populations in lungs of cystic fibrosis patients. Infect Immun 75, 2214–2224 (2007).
Burns, J. L. et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 183, 444–452 (2001).
Johansson, E., Welinder-Olsson, C., Gilljam, M., Pourcel, C. & Lindblad, A. Genotyping of Pseudomonas aeruginosa reveals high diversity, stability over time and good outcome of eradication. Journal of Cystic Fibrosis 14, 353–360 (2015).
Mogayzel, P. J. Jr. et al. Cystic Fibrosis Foundation Pulmonary Guideline. Pharmacologic Approaches to Prevention and Eradication of Initial Pseudomonas aeruginosa Infection. Annals of the American Thoracic Society 11, 1640–1650 (2014).
Brodzicki, J., Trawinska-Bartnicka, M. & Korzon, M. Frequency, consequences and pharmacological treatment of gastroesophageal reflux in children with cystic fibrosis. Med Sci Monit 8, 537 (2002).
Huxley, E. J., Viroslav, J., Gray, W. R. & Pierce, A. K. Pharyngeal aspiration in normal adults and patients with depressed consciousness. The American journal of medicine 64, 564–568 (1978).
Gleeson, K., Eggli, D. F. & Maxwell, S. L. Quantitative aspiration during sleep in normal subjects. CHEST Journal 111, 1266–1272 (1997).
Bassis, C. M. et al. Analysis of the Upper Respiratory Tract Microbiotas as the Source of the Lung and Gastric Microbiotas in Healthy Individuals. mBio 6, e00037–00015 (2015).
Palm, K., Sawicki, G. & Rosen, R. The impact of reflux burden on Pseudomonas positivity in children with cystic fibrosis. Pediatr Pulmonol 47, 582–587 (2012).
Rosen, R. et al. 16S Community Profiling Identifies Proton Pump Inhibitor Related Differences in Gastric, Lung and Oropharyngeal Microflora. The Journal of pediatrics 166, 917–923 (2015).
Walter, S. et al. Epidemiology of chronic Pseudomonas aeruginosa infections in the airways of lung transplant recipients with cystic fibrosis. Thorax 52, 318–321 (1997).
Nunley, D. R. et al. Allograft colonization and infections with pseudomonas in cystic fibrosis lung transplant recipients. CHEST Journal 113, 1235–1243 (1998).
Krishnan, A. et al. Identical Biofilm Forming Strains of Pseudomonas aeruginosa Occur in Lung Allograft BAL and Gastric Juice from CF Patients with Gastro Oesophageal Reflux. The Journal of Heart and Lung Transplantation 32, S28 (2013).
Griffin, S. M. et al. Aspiration and Allograft Injury Secondary to Gastroesophageal Reflux Occur in the Immediate Post–Lung Transplantation Period (Prospective Clinical Trial). Ann Surg 258, 705–712 (2013).
Cantu Iii, E. et al. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. The Annals of thoracic surgery 78, 1142–1151 (2004).
Andersson, A. F. et al. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLos one 3, e2836 (2008).
Zhao, J. et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proceedings of the National Academy of Sciences 109, 5809–5814 (2012).
Jones, R., Pearson, J. & Ward, C. Functional Dyspepsia. The New England journal of medicine 374, 895 (2016).
Littlewood, J. M., Wolfe, S. P. & Conway, S. P. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol 41, 35–49 (2006).
Com, G., Cetin, N. & O’Brien, C. E. Complicated Clostridium difficile colitis in children with cystic fibrosis: Association with gastric acid suppression? Journal of Cystic Fibrosis 13, 37–42 (2014).
Acknowledgements
H Al-momani received financial support from the faculty of medicine at The Jordanian Hashemite University. C Ward, J Pearson, SM Griffin and Rhys Jones were supported by a UK Government Technology Strategy Board Knowledge Transfer Partnership (Certificate No. KTP008821), between Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust M Brodlie supported by a Medical Research Council Clinician Scientist fellowship.
Author information
Authors and Affiliations
Contributions
H.A. was responsible for the patient sampling. He analysed data and co-wrote the manuscript. A.P. was the Microbiology Biomedical Scientist for the study and co-wrote the manuscript. C.S. and S.C. were responsible for the NGS methodology, data analysis and co-wrote the manuscript. R.J., A.K., A.R. and M.G. were clinical gastrointestinal investigators. They recruited the non C.F. patients and performed endoscopy. They reviewed and contributed to manuscript drafts. S.B., S.D., A.A. and T.F. were responsible for the recruitment and clinical phenotyping of the CF patients. They co-wrote and/or reviewed the manuscript. M.B. co-wrote and reviewed the manuscript. J.P. and C.W. were lead investigators for the study, responsible for the study design. Pearson co-wrote the manuscript. Ward wrote the manuscript drafts.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Al-momani, H., Perry, A., Stewart, C. et al. Microbiological profiles of sputum and gastric juice aspirates in Cystic Fibrosis patients. Sci Rep 6, 26985 (2016). https://doi.org/10.1038/srep26985
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26985
This article is cited by
-
A prospective study of extraesophageal reflux and potential microaspiration in patients hospitalized with COVID-19 in Jordan
BMC Pulmonary Medicine (2023)
-
Exposure to bile and gastric juice can impact the aerodigestive microbiome in people with cystic fibrosis
Scientific Reports (2022)
-
Nontuberculous mycobacteria in gastrostomy fed patients with cystic fibrosis
Scientific Reports (2017)
-
Microbiome effects on immunity, health and disease in the lung
Clinical & Translational Immunology (2017)
-
The Microbiome and the Pathophysiology of Asthma
Respiratory Research (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.