Abstract
Diffuse astrocytoma (DA; WHO grade II) is a low-grade, primary brain neoplasm with high potential of recurrence as higher grade malignant form. We have analyzed differentially expressed membrane proteins from these tumors, using high-resolution mass spectrometry. A total of 2803 proteins were identified, 340 of them differentially expressed with minimum of 2 fold change and based on ≥2 unique peptides. Bioinformatics analysis of this dataset also revealed important molecular networks and pathways relevant to tumorigenesis, mTOR signaling pathway being a major pathway identified. Comparison of 340 differentially expressed proteins with the transcript data from Grade II diffuse astrocytomas reported earlier, revealed about 190 of the proteins correlate in their trends in expression. Considering progressive and recurrent nature of these tumors, we have mapped the differentially expressed proteins for their secretory potential, integrated the resulting list with similar list of proteins from anaplastic astrocytoma (WHO Grade III) tumors and provide a panel of proteins along with their proteotypic peptides, as a resource that would be useful for investigation as circulatory plasma markers for post-treatment surveillance of DA patients.
Similar content being viewed by others
Introduction
Diffuse astrocytoma (WHO grade II) is low-grade primary brain tumor of astrocytes. It is characterized by slow growth with low probability of infiltration into neighboring brain tissue. Though relatively rare1, it represents 10% of all astrocytic brain tumors with the mean survival time of 6–8 years2,3,4. It typically affects young adults, the standard method for diagnosis is based on histology and treatment includes surgery followed by radiotherapy. The tumors have an inherent potential of progression to malignant anaplastic astrocytoma (WHO Grade III) or secondary glioblastoma (GBM) over time5. The most common genetic alteration in diffuse astrocytoma is mutations of the TP53 and IDH1/2 genes in 32% cases, 1p/19q loss and IDH1/2 mutation in 37% cases and only IDH1/2 mutation in 17% cases6. Promoter hypermethylation of the DNA repair gene O-6-methylguanine-DNAmethyltransferase (MGMT) and the protocadherin-gamma subfamily A11 (PCDH-gamma-A11) are some of the epigenetic alterations7,8 reported for these tumors. Several differential gene expression studies have been carried out to understand pathogenesis or to distinguish primary and recurrent grade II tumors or to differentiate them from higher grade tumors9,10,11. Malzkorn et al. studied profiling of 157 microRNAs in four patients with grade II gliomas that spontaneously progressed to WHO grade IV secondary glioblastomas and showed possible role of 20 microRNAs (18-overexpressed and 2 repressed) in glioma progression12. Proteomics studies on these tumors have been, however, on the lower side. Earlier studies on differential protein expression of low grade and high grade gliomas were carried out using 2D-MS approach13,14. Iwadate et al. tried to classify the tumors for survival prediction based on expression patterns13. Recently, Gimenez et al. performed high throughput quantitative proteomic analysis of low grade and high grade astrocytomas and oligodendrogliomas15. They identified RNA binding protein NOVA-1 (NOVA1) to be a marker distinguishing astrocytoma with oligodendrogliomas and heat shock protein beta 1 (HSPB1) as a predictive marker for poor prognosis for GBM15. Using protein arrays, Jiang et al. studied the expression and phosphorylation status of 46 proteins involved in signaling pathways associated with cell proliferation, cell survival, apoptosis, angiogenesis, and cell invasion in lower grades of glioma16. The Cancer Genome Atlas (TCGA) group has recently carried out a large scale molecular profiling of diffuse gliomas using 1,122 samples. Some major pathways implicated include PI3K/mToR pathway along with Ras-Raf MEK-ERK, p53/apoptosis pathway and others. Similarly, they confirmed cohesin complex pathway, involved in cell division and telomere length regulation, to play a major role in gliomagenesis. Further, based on unsupervised clustering of protein profiles, TCGA analysis also revealed two macro clusters, one cluster (LGG cluster) with majorly lower grade (Gr II and Gr III) glioma samples while other cluster, GBM-like cluster, with mostly GBM samples. The LGG class showed increased activity of PKC, PTEN, BRAF, and phosphoP70S6K17.
In the present study, we have analyzed protein expression changes in the microsomal fraction of clinical tissue samples with diffuse astrocytoma in comparison to control, using iTRAQ and high-resolution mass spectrometry, followed by extensive bioinformatics analysis to get further insights into molecular changes in these tumors and to generate a resource which could be useful for developing circulatory biomarkers for clinical applications such as post-treatment surveillance.
Experimental procedures
Sample collection and processing
All the samples were collected at the time of surgery with informed consent from patients and approval of the Institutional Ethics Committee, Nizam’s Institute of Medical Sciences (NIMS), Hyderabad, India and all the experiments were performed in accordance with recommended guidelines and regulations. Tumor tissue specimens were snap frozen in liquid nitrogen and stored at −80 °C until use. Multiple sections from the temporal neocortex were studied and the tumor grade was assigned on the basis of clinical evaluation and histopathology as per WHO guidelines. Out of forty-five astrocytoma specimens collected over the period of 2 years, nine of them were grouped as diffuse astrocytomas. Six age matched samples (20–40 years) of either sex were selected for present study. Brain tissue obtained from temporal lobe epilepsy surgeries were collected as experimental controls. A large amount of temporal cortical tissue needs to be removed in these surgeries to reach hippocampus which is usually the most likely seizure focus. The temporal cortex used as control did not show any histological abnormalities by light microscopy. Further, immunohistochemistry (IHC) with antibodies directed against phosphorylated neurofilament and synaptophysin proteins did not reveal any abnormal neurons in the cortex. These control subjects were in the 20–30 year old age group.
Sub cellular fractionation for enrichment of microsomal proteins
Tissues from tumor patients (n = 6; 4 males and 2 females) or control subjects (n = 3; 2 males and 1 female) were pooled separately and microsomal fraction was prepared according to the procedure of Cox et al.18 and described by us earlier19 for microsomal protein enrichment. The procedure yields a preparation, which consists of membrane proteins of ER, golgi, intracellular vesicles and plasma membrane. Protein amount in the preparation was estimated using Bradford method.
Sample processing for iTRAQ labeling and SCX fractionation
Microsomal protein fraction from tumor or control tissues was subjected to trypsin digestion and the peptides were labelled with iTRAQ reagents according to the manufacturer’s instructions (iTRAQ Reagents Multiplex kit; Applied Biosystems/MDS Sciex, Foster City, CA) and as described previously19. Tumor tissue samples were labelled with 116 and 117 tags and control samples with 114 and 115 tags. All the four labelled peptide samples were pooled, vacuum-dried and subjected to strong cation exchange (SCX) chromatography as also described previously19. Peptides eluting from the column were collected and consecutive fractions were pooled to obtain a total of eight fractions. These fractions were desalted using C18 cartridge (Pierce, Rockford, USA) as per the manufacturer’s instructions for LC-MS/MS analysis.
LC-MS/MS analysis
Nanoflow electrospray ionization tandem mass spectrometric analysis was carried out using LTQ Orbitrap Velos (Thermo Scientific, Bremen, Germany) interfaced with Agilent’s 1200 Series nanoflow LC system. Peptides from each SCX fraction were enriched using a C18 trap column (75 μm × 2 cm) at a flow rate of 3 μl/min and fractionated on an analytical column (75 μm × 10 cm) at a flow rate of 350 nl/min using a linear gradient of 7–30% acetonitrile (ACN) over 65 min. Mass spectrometric analysis was performed in a data dependent manner using the Orbitrap mass analyzer at a mass resolution of 60,000 at m/z 400. For each MS cycle, twenty top most intense precursor ions were selected and subjected to MS/MS fragmentation and detected at a mass resolution of 15,000 at m/z 400. The fragmentation was carried out using higher-energy collision dissociation (HCD) mode. Collision energy (CE) between 39–42% was used for optimization and normalized CE of 40% was used to obtain release of reporter ions from all peptides detected in the full scan. The ions selected for fragmentation were excluded for next 30 sec. The automatic gain control for full FT MS and FT MS/MS was set to 1 million ions and 0.1 million ions respectively with a maximum time of accumulation of 500 ms. The lock mass option was enabled for accurate mass measurements.
Bioinformatics analysis
Protein identification, quantification and annotations of differentially expressed proteins were carried out as follows. The MS/MS data was analyzed using Proteome Discoverer (Thermo Fisher Scientific, version 1.4) in Sequest mode using NCBI RefSeq database (release 52) containing 33,985 proteins. Search parameters included trypsin as the enzyme with 1 missed cleavage allowed; precursor and fragment mass tolerance were set to 20 ppm (around 97% of the peptides detected conformed to <10 ppm mass error) and 0.1Da, respectively; Methionine oxidation was set as a dynamic modification while methylthio modification at cysteine and iTRAQ modification at N-terminus of the peptide and lysines were set as static modifications. The peptide and protein information were extracted using high peptide confidence and top one peptide rank filters. The FDR was calculated by enabling the peptide sequence analysis using a decoy database. High confidence peptide identifications were obtained by setting a target FDR threshold of 1% at the peptide level. Mass spectrometric analysis resulted in identification of a total of 20,783 peptides. After removing peptides not labelled with all the four labels (n = 212) and those (n = 1968) shared between multiple proteins, 18,603 peptides were considered for identification of proteins. The labelling efficiency was thus 99%.
Relative quantitation of proteins was carried out based on the intensities of reporter ions released during MS/MS fragmentation of peptides. The average relative intensities of the two reporter ions for each of the unique peptide identifiers for a protein were used to determine relative quantity of a protein and percentage variability. Appropriate filters at the level of peptides/peptide spectral matches (PSMs) and then at the protein level were applied to the quantification values as described in earlier publication20. In brief, Only PSMs that are ‘unique’ for a protein were included for fold change calculation. Next, PSMs with more than 30% co-efficient of variation (% CV) between the replicate label measurements (i.e., 114 and 115 for control) and (i.e., 116 and 117 for tumor) were removed programmatically. We then extracted PSMs corresponding to proteins with 1.5 fold change, applied 1.5 fold cut off to these subset of PSMs and recomputed fold change for proteins. Further filters were applied at protein level to select proteins with minimum 2 unique peptides and 2-fold expression change, with PSM quant ratio variability (% CV) of less than 40%. The median pair-wise quant ratio for 116/114, 116/115, 117/114, and 117/115 was used to compute the statistical significance (p-value < 0.05). The Benjamini Hochberg FDR corrected p-value is included in Supplementary Table S1 for proteins that were differential at 2-fold-change or above.
Gene Ontology annotations of the proteins identified were carried out based on Human Protein Reference Database (HPRD, http://www.hprd.org)21. Mapping of molecular functions and pathways was done using the Ingenuity Pathway Knowledge Base (Ingenuity Systems, Redwood City, CA) tool. Proteins containing signal peptide and transmembrane domains were identified using SignalP 4.1 and TMHMM 2.0 software tools. Exocarta database was used to map the human exosomal proteins22.
Immunohistochemistry (IHC)
The expression level of four of the select proteins, epidermal growth factor receptor (EGFR), brevican core protein (BCAN), ectonucleotide pyrophosphatase/phosphodiesterase family member 6 (ENPP6) and heterogeneous nuclear ribonucleoprotein (HNRNP) K were studied by immunohistochemistry using commercially available Tissue microarray containing 13 Diffuse Astrocytomas cases and 4 control tissue cores (US BioMax). In brief, after deparaffinization and rehydration of formalin-fixed paraffin-embedded tumor tissue sections, antigen retrieval was performed by immersing the slide in antigen retrieval buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at 95 °C for 5 min. Endogenous peroxidases were blocked with 0.03% hydrogen peroxide, and nonspecific binding was blocked with 2% fetal calf serum in Tris-buffered saline with 0.1% Triton X-100 (TBST, pH 7.6). Sections were then incubated for 1 h at RT with EGFR (dilution 1:100; Cat No. HPA018530), BCAN (dilution-1:200; Cat No. HPA007865), ENPP6 (dilution-1:10; Cat No. HPA042740) and HNRNP K (dilution-1:250; Cat No. HPA007644) primary antibodies (Atlas Antibodies, Sigma) followed by peroxidase-labelled polymer conjugate to anti-rabbit or anti-mouse immunoglobulins compatible with the primary antibody, for 1 h and developed with DAB system (DAKO, Denmark). Sections were counter stained with the Mayer’s hematoxylin, dehydrated and images were taken under microscope.
Results and Discussion
Identification of differentially expressed proteins
DAs are low incidence tumors, yet important as they mostly occur in younger age group individuals with a high chance of recurrence and significantly long median survival time. Presently the general treatment modality is surgery followed by radiation, with mixed outcome. Better treatment strategies as well as post treatment surveillance are important unmet clinical needs. With this focus, we have studied differentially regulated proteins from the microsomal fraction from clinical tissues to understand molecular changes underlying DA and to identify proteins that may have strong secretory potential for application as post treatment surveillance markers. Considering low incidence of these tumors and sample paucity, our experimental approach has been to carry out quantitative LC-MS/MS analysis using iTRAQ, on microsomal fraction purified from pooled tissue biopsies from patients diagnosed with DA, followed by cross-comparison with transcript data from individual patient samples and/or verification of the functionally significant members by immunohistochemistry on tissue microarrays with individual samples. We also screened the proteins from the dataset applying bioinformatics for their secretory potential and identified a set of proteins that may serve as candidates for investigation towards application for post-treatment surveillance. Thus the study represents discovery-stage findings that could be used by us and others for clinical validations.
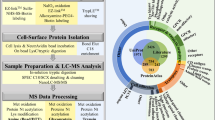
A pool of biopsies from six male and female patients between 20–40 years of age group was used to prepare the microsomal fraction containing endoplasmic reticulum, golgi, intracellular vesicles, and plasma membrane proteins. This was analyzed to identify differentially expressed proteins using iTRAQ labeling of tryptic peptides followed by LC-MS/MS analysis using LTQ Orbitrap Velos mass spectrometer. Microsomal fraction from a pool of temporal lobe epilepsy surgery specimens was used as control. The workflow of the analysis is given in Fig. 1A.
(A) Overall workflow for quantitative proteomic analysis of the tumor samples. Details of preparation of microsomal membrane proteins, iTRAQ labeling, LC-MS/MS analysis and protein identifications are provided under Methods. (B) Subcellular classification of differentially expressed proteins. Subcellular classification of differentially expressed proteins (n = 340) was carried out using Human Protein Reference Database and shows the enrichment of the membrane proteins.
A total of 18,603 iTRAQ labelled peptides was identified which mapped to 2803 proteins, majority of them with multiple peptides. A total of 340 proteins were found to be differentially expressed with at least 2-fold change (218 upregulated and 122 down regulated proteins). The altered levels of each of the identified proteins were based on at least two peptides with two reporter ions for each peptide. We have identified and quantified 84 proteins with 2 peptides, 73 with 3 peptides and remaining 183 proteins with 4 or more peptides. For averaging the quantities of the proteins, we used only unique peptides identifying a protein with variability of less than 40% in the peptide ratio. Subcellular classification of the 340 differentially expressed proteins using Gene Ontology information from Human Protein Reference Database (HPRD) revealed majority (53%) of them as proteins known to be associated with the endoplasmic reticulum and plasma membrane (Fig. 1B). Supplementary Table S1 provides the list of these proteins along with their peptide information, quantitative levels, molecular or biological functions and cellular localizations.
Comparison of 340 differentially expressed proteins with the differentially expressed transcript data (≥1.5 fold change) by Sun et al.11 and accessed using Oncomine data resource (www.oncomine.org) in DA tumors revealed a total of 195 proteins (57.4%) to be common (Supplementary Table S2). Of these, 189 proteins showed positive correlation in expression supporting our observations and the proteomic data. The comparative differential protein and transcript expression in fold changes are shown in Fig. 2.
The total number of differentially expressed proteins observed in the present study was compared with differentially expressed transcript data available in Oncomine resource (www.oncomine.org, ref. 11). (A) shows profiles of upregulated entities and (B) represents downregulated entities. The fold change values of these entities are given in Supplementary Table S2.
Changes at the chromosome levels such as mutations, copy number variations are important factors that may affect downstream events relevant to tumor development. We also mapped differentially expressed proteins to the chromosome 12 which is implicated in glial tumors23, and found that three of the over expressed proteins, CNPY2, MYL6, LIMA1, mapped to the regions on the chromosome that have been described as amplicons24,25. This provides a rationale and biological basis for their overexpression and confirms mass spectrometry results. To further confirm the quantitative differences observed by iTRAQ analysis, verification of the expression levels of EGFR, BCAN, ENPP6 and HNRNPK was carried out using immunohistochemistry (IHC) in tissue microarrays with DA tumor tissue sections. EGFR is well known for its involvement in tumorigenesis in general, BCAN is a brain-specific protein involved in brain development, ENPP6 is a protein implicated in the development of myelin sheath and HNRNP K is an important protein involved in post transcriptional regulation of gene expression. EGFR and BCAN are found to be over expressed at both protein and transcript level whereas over expression of the other two was observed only at protein level and not supported at the transcript level. MS/MS spectra of the peptide of representative overexpressed proteins, BCAN, EGFR, ENPP6, and HNRNP K and the corresponding IHC images are given in Fig. 3. We found that EGFR protein was overexpressed in 85% of DAs and BCAN showed overexpression in 77% of DAs in consistence with earlier observations26,27. ENPP6 was observed to be overexpressed in 30% cases of DA, while HNRNPK showed strong overexpression in all the DA cases (Fig. 3, Supplementary Table S3).
(A) shows MS/MS spectra of peptides with their reporter ion intensities for representative differentially expressed proteins - BCAN, EGFR, ENPP6 and HNRNP K. (B) immunohistochemistry (IHC) images acquired for the above proteins. IHC protocol is described under Methods and the staining and scoring details for each protein are shown in Supplementary Table S3. For BCAN, normal brain tissue shows low staining with pyramidal cells negative (a), Grade II tumor cells show strong cytoplasmic positivity (b). For EGFR, normal brain tissue shows negative staining (c) and Grade II tumor cells show medium intensity cytoplasmic staining (d). ENPP6 shows medium intensity staining of neurophils in normal brain with no staining of normal glial and neuronal cells (e), while Grade II tumor cells show low to medium intensity staining for ENPP6 in neurophil as well as in tumor cells (f). For HNRNP K, normal brain tissue scored negative (g) whereas Grade II tumor cells showed strong positivity (h).
Altered processes, enriched pathways and key molecular entities
Ingenuity Pathway Knowledge Base classification of the protein differentials observed by us indicated following molecular and cellular functions, networks and canonical pathways. The top network identified includes molecules associated with cell-to-cell signaling and interactions, tissue development and cellular movement. Major molecular and cellular functions and canonical pathways enriched in the dataset are shown in Fig. 4. Protein synthesis, cell-to-cell signaling and interactions, RNA post transcriptional modification are the molecular and cellular functions identified. In a recent study by TCGA group, genomic alterations including mutation, copy number variations and fusion transcript profiles, showed PI3K/AKT/mTOR signaling to be one of the major drivers for diffuse glioma17. It is interesting to note that among the canonical signaling pathways, we observed mTOR signaling and the downstream pathways i.e. eIF2, eIF4 and p70S6K signaling as most enriched pathways. The protein IDs and P-values associated with these molecular and cellular functions, networks and canonical pathways are shown in Supplementary Table S4A–C.
The differentially expressed proteins (n = 340) as listed in Supplementary Table S1 were used for these analyses. Top 5 molecular and cellular functions and canonical pathways are shown in the figure. Threshold criteria considered for the analysis are -log p-value > 1.3 or p-value < 0.05. The list of proteins under each category is provided in Supplementary Table S4.
PI3K/AKT/mTOR signaling is known to play important role in cell proliferation and cell growth and mTOR is a master regulator of cell growth through its ability to stimulate ribosome biogenesis and mRNA translation28. DA are low grade tumors which represent an early stage of uncontrolled cell proliferation and growth with higher demands on increased protein synthesis. Consistent with this, protein synthesis is the major cellular process enriched in these tumors. The dataset also showed over expression of 43 ribosomal proteins of both small and large subunit (Supplementary Table S4C) suggesting increased ribosome biogenesis. Thus the increase in ribosome biogenesis which may be linked to mTOR activation is reflected by the enrichment of eIF2 pathway to provide the machinery required to promote cell growth and proliferation. Some of the ribosomal proteins include those with extra ribosomal functions which include tumor suppressor and proto-oncogene regulation (RPL5, RPL11, RPL23, RPL7A)29. mTOR is also implicated in early stage tumors of other tissues as well as low grade pediatric gliomas and is considered to be a potential therapeutic target30,31. However, inhibitors of mTOR have not been as successful, presumably because mTOR has multifunctional roles. Targeting multiples kinases or other molecules may be one possibility. On the other hand, it may be useful to view and integrate the mutational or the fusion transcript profiles discussed in the context of deregulated mToR cascades downstream17 and explore other possible targets.
RTK signalling is one of the most frequently observed pathway in human cancers and EGFR is one of the best known oncogenic RTK for several cancers including gliomas32. It is linked to the malignant transformation of these tumours through mutations and copy number variations as well as overexpression at RNA and protein level33,34. EGFR is often used to evaluate primary GBMs35. EGFRvIII being the most common mutation observed33, is viewed for targeted therapy for gliomas. TERT promoter mutation along with wildtype IDH status has been associated with glioma prognosis and in some conditions it is implicated with alterations in chromosome 7, which harbours EGFR gene17. A survey through literature until year 201233, indicated several reports of overexpression of EGFR in Gr II tumors as has been also observed in our dataset. EGFR, and its mutant, EGFRvIII, have been also shown in exosomes and micro vesicles isolated from sera of patients with brain tumors36. Our observations thus further support EGFR having some potentially interesting features in the context of DA.
Brevican core protein (BCAN) is a brain-specific chondroitin sulfate proteoglycan has been observed to be highly expressed during development, in response to injury and in primary brain tumors37. This protein is reported to be overexpressed at gene and protein level in astrocytomas, including DAs. Functional studies showed that BCAN is upregulated during glial cell adhesion, motility and tumor growth37,38,39. We also observed BCAN to be overexpressed in our study. In view of being a brain-specific protein and its functional relevance to cancer progression, we believe BCAN may be considered as a candidate with significant biological and clinical implication. In addition, it should be noted that BCAN occurs both as soluble isoforms secreted into the extracellular space and membrane-bound isoforms which are anchored to the cell surface, raising its circulatory potential.
Ectonucleotide pyrophosphatase/phosphodiesterase family member 6 (ENPP6) was observed to be overexpressed in proteomic data. It is a glycosylphosphatidylinositol (GPI)-anchored alkaline lysophospholipase C predominantly expressed in brain myelin and kidney40,41. Other ENPP family proteins, ENPP 1, has been reported to be associated with maintenance of stem cell characteristics in glioblastoma, ENPP3 has been shown to have a role in cell invasion in human colon cancer42, however, the role of ENPP6 is not yet shown in cancer. Heterogeneous nuclear ribonucleoprotein (HNRNP) are important regulatory proteins involved in post-transcriptional regulation of gene expression43. We have earlier reported a large group of HNRNPs were found to be elevated in Gr III tumors. In the present analysis we identified 7 HNRNPs which include an important member HNRNP K. It was observed to be overexpressed in DA in our proteomic data. HNRNPs are generally localised in the nuclei or cytoplasm of the cell, interact with different classes of proteins or mRNAs to form complexes and regulate post transcriptional events such as splicing, stability or translation of mRNA in the gene expression cascade. Over expression of HNRNP K even in early stage, low grade tumors suggest the possible significance of alterations in these regulatory mechanisms as initial events. Interestingly, the localization of HNRNP K on the cell surface and their role in cell adhesion44 has also been been demonstrated.
Differential proteins as potential surveillance markers for targeted investigation for recurrence
DAs have a long median survival but invariably recur. After treatment, they may recur as Gr II or higher grade anaplastic astrocytomas (WHO grade III) or secondary glioblastomas (WHO Grade IV)5,45. Some of the grade II tumor progress rapidly while others take more indolent course5. Histology at times helps in prediction of progression. For example, gemistocytic Grade II astrocytomas have higher chance of progression into malignant astrocytoma than fibrillary or protoplasmic type. IHC for proliferating antigen (Ki67/PCNA) or p53 and micro vessel density are sometimes used46,47, but they are not definitive for distinguishing the two tumor types. Alternative methods to monitor the recurrence post-treatment would therefore be useful48. Thus, we looked at the circulatory potential of the differentially expressed proteins in Gr II and III to explore them as circulatory protein markers for predicting recurrence. Although blood brain barrier may be a factor challenging appearance of tumor proteins in the plasma of DA patients, it is to be noted that the barrier is usually breached significantly as the tumor progresses to higher grades - a frequent feature of these tumors49,50.
On mapping of the differentially expressed protein dataset (n = 340) to SignalP 4.1 and TMHMM 2.0 software tools, or Exocarta database, we identified 84 signal sequence containing proteins, 106 with transmembrane domain containing proteins and 157 as exosomal proteins (Supplementary Table S5). Taking proteins that meet at least two of the above criteria namely, Signal Sequence/transmembrane domain/presence in exosomes, we arrived at a subset of 81 proteins which may be considered to have strong secretory potential. Comparison of this subset (n = 81) with the proteins experimentally detected in blood plasma or cerebrospinal fluid leaves a filtered list of 43 proteins with secretory character51,52. We generated a similar list of proteins (n = 40) from our dataset on anaplastic astrocytoma (WHO grade III)20 using the same criteria as above. Integration of the two resulted in a non-redundant list of 64 potential secretory proteins representing both grade II and III tumors (Supplementary Table S6). In view of their potential for clinical applications, their validation in specific sample cohorts is required. For this purpose, we have extracted their proteotypic peptides using GPMdb’s MRM database (Supplementary Table S6) for targeted analysis by mass spectrometry. Representative members under this category are shown in Table 1 which include EGFR, BCAN (discussed above) and others such as PDIA4, SPARC, ITGB1, SERPINA1 which are known to play important role in cancer. PDIA4 is reported to be associated with chemo resistance53 SPARC is reported to have role in cancer progression;54 Protein S100A10 is reported to have role in cell proliferation;55 SERPINA1 and ITGB1 are reported to play role in invasion and migration56,57. We believe this high confidence list of proteins with their proteotypic peptides would serve as a protein/peptide resource for further investigation by us and others in the community for tumor recurrence in a follow-up study cohort of patients diagnosed and treated for grade II tumors.
Conclusions
We have identified 340 high confidence differentially expressed proteins with high resolution mass spectrometry, from the microsomal fraction of low-grade (Grade II) glioma - diffuse astrocytoma. mTOR activation, increase in ribosome biogenesis and protein translation were found to be major processes altered in these tumors; PI3Kc/mTOR pathway is also implicated in the large study carried out by TCGA. Differentially expressed proteins in this early stage, low-grade gliomas, include important regulatory proteins such as EGFR, HNRNP K and BCAN. Though not specific to DA they may be promising candidates for confirmatory diagnosis of these early stage tumors. We also provide a catalogue of differentially expressed proteins in Gr II and Gr III with secretory potential along with their proteotypic peptides which may be a useful resource for targeted investigation as surveillance markers for tumor recurrence - an important unmet clinical need.
Additional Information
How to cite this article: Polisetty, R. V. et al. Microsomal membrane proteome of low grade diffuse astrocytomas: Differentially expressed proteins and candidate surveillance biomarkers. Sci. Rep. 6, 26882; doi: 10.1038/srep26882 (2016).
References
Bourne, T. D. & Schiff, D. Update on molecular findings, management and outcome in low-grade gliomas. Nat Rev Neurol. 6, 695–701, doi: 10.1038/nrneurol.2010.159 (2010).
Kitange, G. J., Templeton, K. L. & Jenkins, R. B. Recent advances in the molecular genetics of primary gliomas. Curr Opin Oncol. 15, 197–203 (2003).
Kleihues, P. et al. Diffuse astrocytoma Kleihues, P., Cavenee, W. K. eds Pathology and Genetics of Tumours of the Nervous System. Int Agency for Res on Cancer., Press Lyon, France, 22–26 (2000).
Jones, D. T. et al. Adult grade II diffuse astrocytomas are genetically distinct from and more aggressive than their paediatric counterparts. Acta Neuropathol. 121, 753–761, doi: 10.1007/s00401-011-0810-6 (2011).
Khan, O. H., Mason, W., Kongkham, P. N., Bernstein, M. & Zadeh, G. Neurosurgical management of adult diffuse low grade gliomas in Canada: a multi-center survey. J Neurooncol. doi: 10.1007/s11060-015-1949-0 (2015).
Kim, Y. H. et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 177, 2708–2714, doi: 10.2353/ajpath.2010.100680 (2010).
Riemenschneider, M. J., Jeuken, J. W., Wesseling, P. & Reifenberger, G. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 120, 567–584, doi: 10.1007/s00401-010-0736-4 (2010).
Waha, A. et al. Epigenetic silencing of the protocadherin family member PCDH-gamma-A11 in astrocytomas. Neoplasia. 7, 193–199, doi: 10.1593/neo.04490 (2005).
Huang, H. et al. Gene expression profiling of low-grade diffuse astrocytomas by cDNA arrays. Cancer Res. 60, 6868–6874 (2000).
van den Boom, J. et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. Am J Pathol. 163, 1033–1043, doi: 10.1016/S0002-9440(10)63463-3 (2003).
Sun, L. et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer cell. 9, 287–300, doi: 10.1016/j.ccr.2006.03.003 (2006).
Malzkorn, B. et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 20, 539–550, doi: 10.1111/j.1750-3639.2009.00328.x (2010).
Iwadate, Y. et al. Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res. 64, 2496–2501 (2004).
Chumbalkar, V. C. et al. Differential protein expression in human gliomas and molecular insights. Proteomics. 5, 1167–1177, doi: 10.1002/pmic.200401202 (2005).
Gimenez, M. et al. Quantitative proteomic analysis shows differentially expressed HSPB1 in glioblastoma as a discriminating short from long survival factor and NOVA1 as a differentiation factor between low-grade astrocytoma and oligodendroglioma. BMC Cancer. 15, 481, doi: 10.1186/s12885-015-1473-9 (2015).
Jiang, R. et al. Pathway alterations during glioma progression revealed by reverse phase protein lysate arrays. Proteomics. 6, 2964–2971, doi: 10.1002/pmic.200500555 (2006).
Ceccarelli, M. et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 164, 550–563, doi: 10.1016/j.cell.2015.12.028 (2016).
Cox, B. & Emili, A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc. 1, 1872–1878, doi: 10.1038/nprot.2006.273 (2006).
Polisetty, R. V. et al. LC-MS/MS analysis of differentially expressed glioblastoma membrane proteome reveals altered calcium signaling and other protein groups of regulatory functions. Mol Cell Proteomics. 11, M111 013565, doi: 10.1074/mcp.M111.013565 (2012).
Polisetty, R. V. et al. Heterogeneous nuclear ribonucleoproteins and their interactors are a major class of deregulated proteins in anaplastic astrocytoma: a grade III malignant glioma. J Proteome Res. 12, 3128–3138, doi: 10.1021/pr400339h (2013).
Keshava Prasad, T. S. et al. Human Protein Reference Database–2009 update. Nucleic Acids Res. 37, D767–772, doi: 10.1093/nar/gkn892 (2009).
Mathivanan, S., Fahner, C. J., Reid, G. E. & Simpson, R. J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 40, D1241–1244, doi: 10.1093/nar/gkr828 (2012).
Ruano, Y. et al. Identification of novel candidate target genes in amplicons of Glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer. 5, 39, doi: 10.1186/1476-4598-5-39 (2006).
Gupta, M. K. et al. Chromosome-centric human proteome project: deciphering proteins associated with glioma and neurodegenerative disorders on chromosome 12. J Proteome Res. 13, 3178–3190, doi: 10.1021/pr500023p (2014).
Jayaram, S. et al. Insights from Chromosome-Centric Mapping of Disease-Associated Genes: Chromosome 12 Perspective. J Proteome Res. 14, 3432–3440, doi: 10.1021/acs.jproteome.5b00488 (2015).
Hu, X. et al. Expression of p53, epidermal growth factor receptor, Ki-67 and O-methylguanine-DNA methyltransferase in human gliomas. Oncol Lett. 6, 130–134, doi: 10.3892/ol.2013.1317 (2013).
Viapiano, M. S., Matthews, R. T. & Hockfield, S. A novel membrane-associated glycovariant of BEHAB/brevican is up-regulated during rat brain development and in a rat model of invasive glioma. J Biol Chem. 278, 33239–33247, doi: 10.1074/jbc.M303480200 (2003).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell. 149, 274–293, doi: 10.1016/j.cell.2012.03.017 (2012).
Lai, M. D. & Xu, J. Ribosomal proteins and colorectal cancer. Curr Genomics. 8, 43–49 (2007).
Hutt-Cabezas, M. et al. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro Oncol. 15, 1604–1614, doi: 10.1093/neuonc/not132 (2013).
Porta, C., Paglino, C. & Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 4, 64, doi: 10.3389/fonc.2014.00064 (2014).
Nakada, M. et al. Aberrant signaling pathways in glioma. Cancers. 3, 3242–3278, doi: 10.3390/cancers3033242 (2011).
Hagen, K. W. & Torp, S. H. Prognostic Significance of EGFR Gene Amplification and Overexpression in Diffuse Astrocytomas—A Literature Study. Open J Pathol. 2, 71–80, doi: 10.4236/ojpathology.2012.23014 (2012).
Paul, I., Bhattacharya, S., Chatterjee, A. & Ghosh, M. K. Current Understanding on EGFR and Wnt/beta-Catenin Signaling in Glioma and Their Possible Crosstalk. Genes Cancer. 4, 427–446, doi: 10.1177/1947601913503341 (2013).
Maire, C. L. & Ligon, K. L. Molecular pathologic diagnosis of epidermal growth factor receptor. Neuro Oncol. 16 Suppl 8, viii1-6, doi: 10.1093/neuonc/nou294 (2014).
Graner, M. W. et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 23, 1541–1557, doi: 10.1096/fj.08-122184 (2009).
Gary, S. C., Kelly, G. M. & Hockfield, S. BEHAB/brevican: a brain-specific lectican implicated in gliomas and glial cell motility. Curr Opin Neurobiol. 8, 576–581 (1998).
Lu, R. et al. The role of brevican in glioma: promoting tumor cell motility in vitro and in vivo . BMC Cancer. 12, 607, doi: 10.1186/1471-2407-12-607 (2012).
Varga, I. et al. Brevican, neurocan, tenascin-C and versican are mainly responsible for the invasiveness of low-grade astrocytoma. Pathol. Oncol. Res. 18, 413–420, doi: 10.1007/s12253-011-9461-0 (2012).
Greiner-Tollersrud, L., Berg, T., Stensland, H. M., Evjen, G. & Greiner-Tollersrud, O. K. Bovine brain myelin glycerophosphocholine choline phosphodiesterase is an alkaline lysosphingomyelinase of the eNPP-family, regulated by lysosomal sorting. Neurochem Res. 38, 300–310, doi: 10.1007/s11064-012-0921-z (2013).
Greiner-Tollersrud, O. K. The non-classical N-glycan processing pathway of bovine brain ecto-nucleotide phosphodiesterase/pyrophosphatase 6 (eNPP6) is brain specific and not due to mannose-6-phosphorylation. Neurochem Res. 39, 2025–2029, doi: 10.1007/s11064-014-1412-1 (2014).
Yano, Y. et al. Expression and localization of ecto-nucleotide pyrophosphatase/phosphodiesterase I-3 (E-NPP3/CD203c/PD-I beta/B10/gp130RB13-6) in human colon carcinoma. Int J Mol Med. 12, 763–766 (2003).
Chaudhury, A., Chander, P. & Howe, P. H. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1’s multifunctional regulatory roles. RNA. 16, 1449–1462, doi: 10.1261/rna.2254110 (2010).
de Hoog, C. L., Foster, L. J. & Mann, M. RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. Cell. 117, 649–662 (2004).
Soffietti, R. et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 17, 1124–1133, doi: 10.1111/j.1468-1331.2010.03151.x (2010).
Chaloob, M. K., Ali, H. H., Qasim, B. J. & Mohammed, A. S. Immunohistochemical Expression of Ki-67, PCNA and CD34 in Astrocytomas: A Clinicopathological Study. Oman Med J. 27, 368–374, doi: 10.5001/omj.2012.93 (2012).
Malhan, P., Husain, N., Bhalla, S., Gupta, R. K. & Husain, M. Proliferating cell nuclear antigen, p53 and micro vessel density: Grade II vs. Grade III astrocytoma. Indian J Pathol Microbiol. 53, 20–23, doi: 10.4103/0377-4929.59177 (2010).
Jayaram, S., Gupta, M. K., Polisetty, R. V., Cho, W. C. & Sirdeshmukh, R. Towards developing biomarkers for glioblastoma multiforme: a proteomics view. Expert Rev Proteomics. 11, 621–639, doi: 10.1586/14789450.2014.939634 (2014).
Syed, P. et al. Autoantibody Profiling of Glioma Serum Samples to Identify Biomarkers Using Human Proteome Arrays. Sci Rep. 5, 13895, doi: 10.1038/srep13895 (2015).
Dubois, L. G. et al. Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci. 8, 418, doi: 10.3389/fncel.2014.00418 (2014).
Farrah, T. et al. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics. 10, M110 006353, doi: 10.1074/mcp.M110.006353 (2011).
Zougman, A. et al. Integrated analysis of the cerebrospinal fluid peptidome and proteome. J Proteome Res. 7, 386–399, doi: 10.1021/pr070501k (2008).
Tufo, G. et al. The protein disulfide isomerases PDIA4 and PDIA6 mediate resistance to cisplatin-induced cell death in lung adenocarcinoma. Cell Death Differ. 21, 685–695, doi: 10.1038/cdd.2013.193 (2014).
Tai, I. T. & Tang, M. J. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat. 11, 231–246, doi: 10.1016/j.drup.2008.08.005 (2008).
Shang, J. et al. S100A10 as a novel biomarker in colorectal cancer. Tumour Biol. 34, 3785–3790, doi: 10.1007/s13277-013-0962-1 (2013).
Kwon, C. H. et al. Serpin peptidase inhibitor clade A member 1 is a biomarker of poor prognosis in gastric cancer. Br J Cancer. 111, 1993–2002, doi: 10.1038/bjc.2014.490 (2014).
Kwon, J. et al. Integrin alpha 6: a novel therapeutic target in esophageal squamous cell carcinoma. Int J Oncol. 43, 1523–1530, doi: 10.3892/ijo.2013.2097 (2013).
Acknowledgements
The work reported here was carried out under the Network Project IAP001 to Ravi Sirdeshmukh with financial support from the Council of Scientific and Industrial Research, Govt. of India (CSIR) at the Centre for Cellular and Molecular Biology (CCMB), Hyderabad. The mass spectrometric analysis was carried out at the MS facility at Institute of Bioinformatics, Bangalore, with financial support from the Department of Biotechnology (DBT), Govt. of India, New Delhi. Rakesh Sharma was a Research Associate under this program. Ravi Sirdeshmukh’s lab also receives financial support from DBT and Indian Council of Medical Research (ICMR), Govt. of India, New Delhi. MKG is a Ph.D. student registered under Manipal University, Manipal and a recipient of Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), Govt. of India. HCH is a Wellcome Trust/DBT India Alliance Early Career Fellow. SRISTEK Hyderabad was involved in specimen collections, coordination, and clinical documentations.
Author information
Authors and Affiliations
Contributions
Concept and overall implementation of the study- R.S.; Clinical samples and data management- A.K.P., C.S., M.S.U., P.A. and R.S.; Experimental design and experimental work including sample preparation for proteomic analysis- R.S., R.V.P. and P.G.; Mass spectrometry and bioinformatics analysis- R.Sh., H.G. and M.K.G.; Statistical analysis- D.R. and M.K.G.; Immunohistochemistry and image analysis- B.M.S., A.L., K.M. and M.S.U.; Data interpretation- R.S., R.V.P., P.G., M.K.G. and B.M.S.; Manuscript writing- R.S., R.V.P., P.G., M.K.G. and B.M.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Polisetty, R., Gautam, P., Gupta, M. et al. Microsomal membrane proteome of low grade diffuse astrocytomas: Differentially expressed proteins and candidate surveillance biomarkers. Sci Rep 6, 26882 (2016). https://doi.org/10.1038/srep26882
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26882
This article is cited by
-
Quantitative proteomic analysis of GnRH agonist treated GBM cell line LN229 revealed regulatory proteins inhibiting cancer cell proliferation
BMC Cancer (2022)
-
Plasma-derived candidate biomarkers for detection of gallbladder carcinoma
Scientific Reports (2021)
-
Altered transcriptional regulatory proteins in glioblastoma and YBX1 as a potential regulator of tumor invasion
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.