Abstract
A rich fossil record chronicles the distant origins of mammals, but the evolution of defining soft tissue characters of extant mammals, such as mammary glands and hairs is difficult to interpret because soft tissue does not readily fossilize. As many soft tissue features are derived from dermic structures, their evolution is linked to that of the nervous syutem and palaeoneurology offers opportunities to find bony correlates of these soft tissue features. Here, a CT scan study of 29 fossil skulls shows that non-mammaliaform Prozostrodontia display a retracted, fully ossified and non-ramified infraorbital canal for the infraorbital nerve, unlike more basal therapsids. The presence of a true infraorbital canal in Prozostrodontia suggests that a motile rhinarium and maxillary vibrissae were present. Also the complete ossification of the parietal fontanelle (resulting in the loss of the parietal foramen) and the development of the cerebellum in Probainognathia may be pleiotropically linked to the appearance of mammary glands and having body hair coverage since these traits are all controlled by the same homeogene, Msx2, in mice. These suggest that defining soft tissue characters of mammals were already present in their forerunners some 240 to 246 mya.
Similar content being viewed by others
Introduction
Understanding the origin and evolution of mammary glands and hairs in mammals is one of the most fascinating and intensive fields of biological research (e.g.1, reviewed in2,3). Both features are signature traits of extant mammals and their evolution is tightly intermeshed genetically, functionally and morphologically, as modern lactation might have evolved from a specialization of hairs and dermal glands as exemplified in the monotremes2,3,4. Molecular evidence point out that hair and mammary glands might be older than 200 Ma3. As hair and mammary glands are soft tissue, they are not readily fossilized. A fossil record for mammary glands does not exist2 and the oldest known fossilized hairs date to the Late Jurassic5. Hair-like structures discovered inside coprolites dating from the Late Permian suggest that fur coverage might have existed as early as 252 to 254 mya, provided that these structures are not artifacts, remains of plants, worms or fungal material6,7.
Facial and body tactile sensibility is often directly related to pilosity and given that stem mammaliaforms and a number of non-mammaliaform therapsids (including cynodonts) were apparently nocturnal and may not have strongly relied on vision to monitor their environment8,9,10, the evolution of hair must have dramatically influenced the survival of the lineage leading to mammaliaforms, i.e. the Late Paleozoic and early Mesozoic non-mammaliaform therapsids commonly known as the ‘mammal-like reptiles’11,12. Because mammary glands and hairs are derived from dermic structures, their evolution is closely linked to that of the nervous system (including the central nervous system) and skin sensitivity2,3,11,12. Here we report the paleoneurology of a variety of non-mammaliaform therapsids (NMT) using in silico studies and X-ray microtomography (μCT). This study documents the evolution of these key soft tissue mammalian characters and demonstrates that both direct and indirect evidence for the evolution of such soft tissues can be addressed using the fossil record.
Comparative description
The maxillary canal is a bony tube which runs parallel to the tooth row in the maxilla and premaxilla. In extant non-avian sauropsids, it carries the maxillary branch of the trigeminal nerve (CNV2), as well as a branch of the facial nerve (CNVII) and some blood vessels13,14,15. The maxillary canal communicates with tooth roots in therapsids16,17. As such, not all ramifications of the canal are relevant to the evolution of facial sensitivity and motility. In order to focus our study on the bony structures that carried tissues which actively played a role in the innervation, sensitivity and nutrition of the face, only the parts of the canal that directly communicate with the external surface were segmented and are described herein. In accordance with the direct phylogenetic relationship unifying Mammaliaformes and NMT and despite similarities with the anatomy of the maxillary canal in extant reptiles (Fig. 1A), the identification of the rami of the maxillary canal are based on the name of the corresponding ramus of the CNV2 in therian mammals. The aim is to maximize primary hypotheses of homology. However, the reader should keep in mind that these are bony structures that may have housed more than the CNV2. Comparisons with extant species are restricted to therian mammals as monotremes do not display the primitive condition for mammals since: (i) their rostrum (and incidentally the anatomy of their maxillary canal) is closely associated with their peculiar foraging strategies (i.e. aquatic invertebrate hunting for platypus and ant-eating for echidna); and (ii) in both the echidna and platypus the maxilla and mandible are covered with unique electroreceptors and mechanoreceptors innervated by an hypertrophied trigeminal nerve, leading to a dramatically derived condition18,19,20. As the maxillary canal is similar in most specimens examined, a detailed description is provided only for early cynodonts, the best documented taxon in our sample and only diagnostic differences are listed for other taxa.
Lateral view, with the skull transparent, of the maxillary canal (in green) and maxillary antrum (in purple) in (A) the non-avian sauropsid Varanus, (B) the early cynodont Thrinaxodon, (D) the cynognathian Trirachodon, (E) the early probainognathian Ecteninion, (F) the probainognathian Tritylodon, (G) the therocephalian Olivierosuchus, (H) an indeterminated gorgonopsian and (I) the biarmosuchian Herpetoskylax; and (C) the canal for the ophthalmic nerve in the early cynodont Thrinaxodon in dorsal view with the skull transparent. Rostral is to the left. Scale bar = 10 mm. The condition in Homo (top) illustrates the nomenclatural terms in Mammalia (after 20). Dotted line indicates the caudal extremity of the infraorbital nerve, which is marked by its bifurcation from the rostral alveolar nerve. The position of this dotted line is homologous to the infraorbital foramen in Tritylodon and mammaliaforms. Videos of the CT images of the specimens are available in the Supplementary Information. Abbreviations: Alv.Can., alveolar canal; Caud.Al., caudal alveolar rami; Ext.Nas., external nasal rami of the infraorbital nerve; For., foramina for the maxillary branch of the trigeminal nerve; Int.Nas., internal nasal rami of the infraorbital nerve; Intraorb.For., intraorbital foramen for the infraorbital nerve; I.O.For, infraorbital foramen; I.O.N., infraorbital nerve; Med.Al., medium alveolar nerve; Mx.Ant., maxillary antrum; Mx.Can., maxillary canal; Oph.Fr., frontal ramus of the ophtalmic nerve; Oph.Nas., nasal ramus of the ophtalmic nerve; Rost.Al., rostral alveolar nerve; Sph.P.For., sphenopalatine foramen; Sup.Lab., supralabial ramus of the infraorbital nerve.
Early cynodonts display several small foramina for the external opening of the maxillary canal on their rostrum. The main axis of the maxillary canal can be divided into a caudal and a rostral part in early cynodonts. The caudal half extends from the maxillary antrum (or maxillary sinus) to the bifurcation of the rostral alveolar nerve. The diameter of the caudal part is relatively large as it may have carried the main trunk of the infraorbital nerve (Fig. 1B). It bears only two ramifications oriented ventrally toward two foramina for the innervation and nutrition of the upper lip. The caudal and rostral parts are supplemented by a third canal located more caudally which does not originate from the maxillary canal. These three canals may have borne the three alveolar rami of the CNV2 (Fig. 1B). The canal has a larger diameter at the level of the root of the rostral alveolar ramus, perhaps as a consequence of the anastomoses of the branches of the alveolar nerves, infraorbital nerve and the palatine nerve as is the case in extant reptiles13,14. In extant mammals the infraorbital nerve (ION) is the branch of the CNV2 that diverges from the rostral alveolar nerve and passes through the infraorbital foramen21. In the absence of an infraorbital foramen in early cynodonts, the rostral-most part of the maxillary canal, that diverges from the rostral alveolar nerve, can be reasonably identified as the homologue of the tube for the ION (Fig. 1B, marked by the dotted line). This part of the maxillary canal houses the same ramifications of the ION as seen in extant mammals. Proximally, depending on the specimen, two to four ramifications are sent dorsally in dorso-caudal, dorsal and dorso-rostral directions. These are identified as the external nasal (or palpebral) rami of the ION, even though they may have also innervated the skin covering the maxillary and frontal bones. The main branch of the maxillary canal tapers rostrally as it ramifies into numerous branches dorsally (internal nasal rami of the ION) and ventrally (superior labial rami of the ION).
The root of the maxillary canal is not ossified, such that the maxillary canal disappears caudally into the maxillary antrum, an air sinus. However, there is evidence for an early divergence of the caudal alveolar nerve because it does not originate from the maxillary canal, as in Varanus (Fig. 1A,B). This branch may have remained independent or may have joined the maxillary canal rostrally, because the bony tubes for the medial and rostral alveolar nerves originate from the maxillary canal (Fig. 1B).
The tube for the ophthalmic nerve is not ossified in Cynosaurus and Galesaurus. Only marks of the branches of the ophthalmic nerve are represented by some short bony channels that pierce the nasal and frontal bones. This is the condition in all NMT we have investigated, except Thrinaxodon. Thrinaxodon displays a complete ossification of the ducts for the ophthalmic nerve on the rostrum (Fig. 1C). This canal is divided into two main branches which are highly ramified themselves. There is a rostral branch to innervate the skin of the nasal bone (nasal ramus) and a caudal one to innervate the skin of the frontal bone (fontal ramus).
The cynognathians differ from the early cynodonts only by the less marked differences between the diameters of the stem of the maxillary canal and the ION. Also, the alveolar nerves do not originate from a side branch of the maxillary canal, which possibly means that they might have passed through the maxillary antrum (Fig. 1D).
Probainognathians are distinguished by the small number of external openings of the maxillary canal. Ecteninion, the basal-most genus of Probainognathia examined here, has only five visible foramina (Fig. 1E). Three foramina open on the extremity of the rostrum and the ION appears trifurcated. The dorsal and medial branches may have housed the internal nasal nerve, whereas the ventral-most branch might have housed the superior labial nerve. There is no evidence of an external nasal ramus of the ION. Caudally, only two of the three foramina for the alveolar rami are present. The caudal-most one is here identified as the foramen for the caudal alveolar ramus based on its independent origin in the maxillary antrum (Fig. 1E). The rostral foramen is connected to the maxillary canal, but it is not clear whether it is for the rostral or medial alveolar ramus (Fig. 1E). Unlike the more derived probainognathians, the course of the trigeminal nerve inside the maxillary antrum is not ossified, but a distinct foramen for the CNV2 is nevertheless present inside the orbit (Fig. 1E).
Pachygenelus and Tritylodon show the typical mammalian condition of the presence of a short infraorbital canal for the ION. As a consequence there is only one single and caudally retracted infraorbital foramen (Fig. 1F). In contrast to other NMT, the infraorbital canal is fully ossified, as in mammals. Three foramina are present rostral to the orbit, but only the infraorbital foramen transmitted the infraorbital nerve (Fig. 1F). The remaining two foramina, located more rostrally, originate through an independent canal from what Sues22 termed the sphenopalatine foramen (Fig. 1F). Given the pattern observed in the closely related Ecteninion, we choose here to homologize these foramina with those for the alveolar rami of the CNV2 (which implies that these canals would be homologous to the alveolar canals of extant mammals). However, they could also provide passage for the posterior superior lateral nasal rami or the nasopalatine rami of the CNV2. In any case, they do not appear to be related to the passage of the the infraorbital nerve.
In contrast to what is observed in early cynodonts, in therocephalians the caudal part of the maxillary canal for the main trunk of the infraorbital nerve appears shorter because it is not ossified (Fig. 1G). In Bauria, there appear to be five external nasal rami, all oriented dorso-rostrally (see Fig. 2).
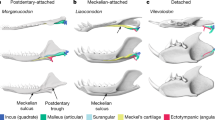
The evolution of the bony structures associated with the infraorbital nerve in Therapsida.
Digital reconstruction of the maxillary canal (in green) and maxillary antrum (in purple) based on CT scan images (see Material and Methods). Scale bars = 10 mm. Phylogeny after references Rubidge and Sidor31 and Liu and Olsen33. Lateral views of the skulls with bones transparent. Rostral is to the left. Videos of the CT images of the specimens are available in the Supplementary Information. Abbreviations : Cyn, Cynodontia clade; Prb, Probainognathia clade; Prz, Prozonstrodontia clade; Thr, Therapsida clade.
In gorgonopsians, the lack of ossification of the caudal part of the maxillary canal is even more pronounced than in therocephalians. The maxillary canal is limited to the part corresponding to the infraorbital nerve. The caudal half of the canal is not ossified and is merged with the extensive maxillary antrum. There are only one or two distinct alveolar rami that open on the maxilla above the large canines (Fig. 1H), possibly as a result of the reduction of the post-canine dentition. In contrast, biarmosuchians show a condition similar to that in early cynodonts, except that all three alveolar rami originate from the maxillary canal (Fig. 1I). The caudal alveolar nerve is nevertheless connected to a side branch, which is reminiscent of the condition in early cynodonts (Fig. 1I).
Discussion
The passage of the infraorbital nerve and presence of maxillary vibrissae
In extant mammals and reptiles (non-avian sauropsids), facial sensitivity is relayed to the brain mainly by the maxillary branch of the trigeminal nerve, the so-called maxillary nerve (CNV2)14,23,24,25. This nerve (mainly its infraorbital branch, termed the maxillary nerve in non-mammals) passes, together with some small blood vessels and the palatine nerve (facial nerve), through the maxillary canal in the upper jaw14,23. In mammals, the maxillary canal is homologous to the infraorbital canal as it carries the infraorbital nerve and vessels24,25. The structure - sometimes termed the “maxillary canal” in mammals (but alveolar canal is usually preferred) - that carries nutrient vessels and the alveolar nerves to innervate teeth roots21 therefore does not correspond to the entire maxillary canal in therapsids, but only to a side branch related to the alveolar nerves (Fig. 1). Generally the infraorbital canal is short in mammals and extends rostrally under the orbit to the infraorbital foramen (Fig. 2), although the infraorbital canal can be secondarily quite long in monotremes18,19. In reptiles (non-avian sauropsids), the maxillary canal for the CNV2 is longer than in most mammals, ramifies and then opens more rostrally into a number of foramina often aligned above the tooth row (Fig. 1A)14,15,23. Thus, the maxillary and infraorbital canals assume the same critical role in the innervation and nutrition of facial skin and they can be used to provide direct evidence for the evolution of facial sensitivity in fossil taxa25,26,27. Based on extant phylogenetic braketing, CNV2 and other tissues supplying the facial skin of NMT likely passed through the maxillary canal. Accordingly, it has been proposed that some foramina located on the rostrum of NMT may have innervated the snout with large sensory maxillary vibrissae16,27,28,29. Such foramina and a ramified maxillary canal characterize most NMT26,27, which would imply a wide occurrence of maxillary vibrissae among mammaliaform progenitors (Fig. 2). However, because similar foramina are present in a number of lizards, snakes and archosaurs15,23,26,28,30 (Fig. 1A), the only argument that remains in favour of the presence of maxillary vibrissae in NMT is their apparent close relationship to mammaliaforms16,30,31.
In mammals, the CNV2, after exiting the skull and traversing the ventral orbit, passes through the infraorbital canal and exits the canal by the infraorbital foramen at a position ventral to the rostral margin of the orbit. This part of the CNV2, the infraorbital nerve (ION), is the one responsible for facial and vibrissae sensitivity24,25. The mammalian face and cheeks are flexible, highly sensitive and sometimes motile structures for whisking, implying that the branches of the ION must also be flexible to prevent damage during flexion to maintain innervation to the facial vibrissae. This facial flexibility is incompatible with the complete enclosure of CNV2 in a bony tube as seen in NMT27,30. As such, the infraorbital nerve no longer branches inside the maxilla in mammals, but rather in soft tissues of the face, which reflects the shorter and unbranched infraorbital canal observed in mammals compared to NMT (Figs 1 and 2).
The use of CT on some of the best preserved therapsid specimens from the extensive fossil record of the South African Karoo Supergroup enables us to reconstruct the transformation from a long, ramified, but not fully ossified maxillary canal to a short and retracted infraorbital canal for the ION in therapsids (Fig. 2). Non-mammaliaform Prozostrodontia (NMPZ) are considered the stem group of mammaliaforms32,33,34. In non-prozostrodontian therapsids, the maxillary canal is separated from the orbit by the maxillary antrum (or maxillary sinus)16,17,35, a condition not seen in Prozostrodontia (Fig. 2). As such, the pathway of the CNV2 from this antrum to the orbit might not have been ossified (Figs 1 and 2). Ecteninion and Probainognathus36, two basal probainognathians, appear intermediate between non-prozostrodontian therapsids and prozostrodontians for this condition as they display a partial ossification of the canal resulting in the presence of a foramen for the CNV2 inside the orbit (Fig. 1E).
In contrast, in the more derived Prozostrodontia (including mammaliaforms), the maxillary antrum no longer merges with the maxillary canal which is fully ossified (Figs 1 and 2). Serial grinding and CT scan analyses of the NMPZ Oligokyphus37, Kayentatherium22, Brasilitherium38, Tritylodon and Pachygenelus (Figs 1 and 2) confirm that (i) the maxillary canal is fully ossified and forms a complete infraorbital canal and (ii) that the infraorbital canal is retracted in NMPZ, as in mammals. The infraorbital foramen is accompanied by a variable number of lesser foramina in NMPZ, as in extant mammals and most Mesozoic mammaliaforms39,40, but the CT scan survey conducted here reveals that only the most proximal of these foramina may have carried the infraorbital nerve (Fig. 1). Similarly, CT data of Brasilitherium, the closest relative of mammaliaforms among NMT, show that the infraorbital foramen diverge immediatly under the rostral margin of the orbit while the other, more rostral foramina are supplied by a distinct canal (presumably for the alveolar nerves) that originate more caudally38, like in Tritylodon (Fig. 1). The poorly preserved maxillae attributed to the stem mammaliaform Morganucodon seems to display more than one large foramen41, but the CT scan of a weathered skull of Morganucodon available on Digimorph.org12,36 shows compelling evidence that the infraorbital canal is separated from the canal leading to these large foramina. This strongly suggests that the structures identified here as the infraorbital canal and foramen in NMPZ are truly homologous to the mammalian infraorbital canal and foramen. Only Lumkuia has numerous large foramina above the tooth row, suggesting branching of the proper maxillary canal and matching its position as the basal-most Probainognathia32,34. Ecteninion and Probainognathus display a reduced number of openings with respect to Lumkuia32,42, which is more similar to the condition in NMPZ but still implies a ramified maxillary canal (Fig. 1E). In this respect, the presence of a short, unbranched infraorbital canal, similar in every way to the one providing passage to the ION for the vibrissae in therian mammals, strongly supports the existence of a flexible and sensitive face bearing tactile hairs in NMPZ (Fig. 2), some 240 mya34. This implies that NMPZ may already have possessed the genetic toolkit to produce hairs, including whiskers and by extension a fur coverage. This is consistent with Eckhart et al.43 who have already suggested that some genes for secreting the mammalian hard α-keratine may have been present in the last common ancestor of amniotes. Hence, the base material for hair was already available to NMPZ. The size of the infraorbital foramen in Tritylodon and Pachygenelus (Table 1) is similar to that in small mammals such as rodents which, given the strong correlation between IOF size and the vibrissae count25, strongly suggests that they may have displayed a similar number of macro- and microvibrissae. The presence in NMPZ of such facial tactile hairs would have aided orientation in the dark if these animals were nocturnal8,9,10.
The parietal foramen, hair coverage and mammary glands
Indirect evidence supporting the hypothesis of the evolution of hairs in Probainognathia comes from the fossil record of the parietal foramen, a midline opening located between the parietal bones (Fig. 3A). This foramen houses the third eye, or pineal eye, a photoreceptive organ that acts like a biological clock detecting daylight variation, for the modulation of biological rhythms and body temperature44,45. The parietal foramen was widespread in early vertebrates before being lost in most lineages, including synapsids44,45. Most NMT have a parietal foramen31,40,44,45, but occasional closure of the parietal foramen is documented, mostly in non-mammaliaform cynodonts (NMC) and in some therocephalians and dicynodonts33,44,45,46. The only therapsid group in which the parietal foramen is consistently absent is the Probainognathia and it is now well established that the absence of a parietal foramen is a synapomorphy of the clade Probainognathia, including Mammaliaformes31,39,40,45.
The skull roof of juvenile trirachodontid (A), BPI/1/4534 and juvenile Pachygenelus (B), BPI/1/5691 with the parietal bone (top, in yellow) and the cranial endocast reconstructed (below, in purple) based on CT scan images (see material and methods).
Abbreviations: Cer, cerebellum; Hem: cerebral hemisphere; Par, parietal bone; Par f, parietal foramen.
In lizards, a parietal foramen forms during ontogeny when ossification of the fronto-parietal fontanel is interrupted in the region of the third eye44,47,48. Complete ossification of the fronto-parietal fontanel in these species is the result of the failure of the third eye to reach the skull roof44,47. In extant mammals, in which a parietal foramen is absent, the genetic pathway controlling the ossification of the fronto-parietal fontanel has been linked to the activity of the homeogene Msx24,49,50,51. Indeed, Msx2 mutant humans and mice display a lack of ossification of the cranial roof. In Msx2 mutant humans this sometimes results in the persistence of a large circular opening on the midline between both parietal bones, reminiscent of the parietal foramen in NMT52. The activity of Msx2 has been linked to a number of developmental processes associated with the differentiation and proliferation of osteogenic cells, such as the morphogenesis and growth of the limbs53,54. In the skull, its expression is complemented by that of several other genes (e.g. Fgfr1, Twist) in the midsutural mesenchyme of the frontoparietal suture50 and is associated with the ossification of the posterior part of the cranial vault51. In a noteworthy manner, Msx2 has also been observed to have pleiotropic effects on the retina55 and the double knockout of Msx1 and Msx2 affects the ossification of the middle ear ossicles56. Despite this, it has been shown that the same mutation of Msx2, which leads to the persistence of the fronto-parietal foramen in mice, also causes deficiencies in hair follicle maintenance (except on the snout and around the orbits) and in the development of mammary glands4,57. These traits are prominent defining characteristics of extant mammals31,33,40. This coincidence suggests that the loss of the parietal foramen might be related to a mutation or changes in the expression or function of the Msx2 homeogene at the root of the Probainognathia clade, which would be consistent with the origin of hair in this group as hypothesized above (Fig. 2).
Msx2 mutant mice also fail to develop a large cerebellum, which demonstrates that this gene is also involved in the cerebellar development4. In contrast to other soft tissues, the fossil record of cerebellar size can be assessed using endocranial casts (physical or digital internal casts of the braincase). Among NMC, Probainognathia are the only species that display a clear enlargement of the cerebellum with distinct laterally expanded cerebellar hemispheres, as demonstrated previously39,58 (Fig. 3B), a feature not seen in other NMC. Taken together, these observations support the hypothesis that a mutation of the Msx2 homeogene may have occurred at the root of the Probainognathia clade in the Early Triassic, some 246 mya34. Many genes are involved in the mineralization of the bones of the cranial vault and the development of the cerebellum, hairs and mammary glands, but given that Msx2 plays a role in all these phenomena, it would be an extraordinary coincidence if all these characters did change at about the same time in the phylogenetic history of synapsids but were not accompanied by a mutation of Msx2. Finally, it must be noted that Msx2 plays a role in follicle maintenance, not in the development of hair4,57. Therefore, the existence of fur coverage could have predated this mutation at the root of the probainognathian clade, as suggested by the discovery of hair-like structures inside coprolites as early as the Late Permian6,7.
Conclusion
In silico paleoneurology suggests that the evolution of hair occurred prior to the evolution of mammaliamorphs. First, fossilized evidence for the evolution of tactile facial hairs suggests that they might not have appeared prior to the reduction in the number of openings for the CNV2 and the appearance of a retracted infraorbital foramen in the NMPZ, at the root of the probainognathian clade at the very end of the Early Triassic, around 240–246 Ma (Fig. 2). Second, the complete disappearance of the parietal foramen in Probainognathia correlates with the definitive ossification of the fronto-parietal fontanelle in adult individuals, which, together with the enlargement of the cerebellum, suggests that the Msx2 gene mutated at the root of this clade (Fig. 2). This gene also pleiotropically controls hair follicle maintenance and the development of mammary glands, which implies that this mutation might have played a significant role in the evolution of both complete body hair coverage and lactation. Since the development of body hairs is believed to have increased body insulation59 and sensitivity11, this single mutation event might have been the root source of some of the most prominent characters defining mammals, such as hair coverage, lactation, lateral cerebellar expansion and endothermy. These typical mammalian characters might thus have first evolved in NMC.
Material and Methods
Institutional abbreviations: AM: Albany Museum (Grahamstown, South Africa), BPI/1: Evolutionary Studies Institute (Johannesburg, South Africa); MS: School of Anatomical Science (Johannesburg, South Africa) SAM-PK: Iziko Museum (Cape Town, South Africa); PVSJ: Museo de Ciencias Naturales, Universidad Nacional de San Juan, Argentina; RC: Rubidge collection (Graaff-Reinet, South Africa); TM: Distsong (Transvaal) Museum (Pretoria, South Africa).
List of the scanned material:
Sauropsida
MS53: Varanus sp., voxel size: 0.0666 mm, dry skull of an extant species.
Biarmosuchia
BPI/1/816: Biarmosuchia, Burnetiamorph, Lemurosaurus pricei, voxel size: 0.05 mm, Cistecephalus AZ, Wuchiapigian, Late Permian.
BPI/1/3924: Biarmosuchia, Burnetiamorph, Herpetoskylax hopsoni, voxel size: 0.0689 mm, Cistecephalus AZ, Wuchiapigian, Late Permian.
Gorgonopsia
BPI/1/216: Gorgonopsia, Scylacocephalus watermeyeri, voxel size: 0.0589 mm, Cistecephalus assemblage zone (AZ), Wuchiapigian, Late Permian.
BPI/1/155: Gorgonopsia, indet. Gorgonopsia, voxel size: 0.0799 mm, Cistecephalus AZ, Wuchiapigian, Late Permian.
Therocephalia
BPI/1/100: Therocephalia, Whaitsiidae, Theriognathus microps, voxel size: 0.0801 mm, Daptocephalus AZ, Changhsingian, Late Permian.
BPI/1/512: Therocephalia, Whaitsiidae, Theriognathus microps, voxel size: 0.0756 mm, Daptocephalus AZ, Changhsingian, Late Permian.
BPI/1/1180: Therocephalia, Baurioidea, Bauria cynops, voxel size: 0.0668 mm, Cynognathus AZ, Anisian, Early Triassic.
BPI/1/3770: Therocephalia, Baurioidea, Bauria cynops, voxel size: 0.0728 mm, Cynognathus AZ, Anisian, Early Triassic.
BPI/1/3849: Therocephalia, Akidognathiidae, Olivierosuchus parringtoni, voxel size: 0.0655 mm, Lystrosaurus AZ, Induan, Early Triassic.
BPI/1/4401: Therocephalia, Hofmeyriidae, Hofmeyria sp., voxel size: 0.0555 mm, Cistecephalus AZ, Wuchiapigian, Late Permian.
Cynodontia
Early Cynodontia
BPI/1/3926: Cynodontia, Cynosaurus suppostus, voxel size: 0.0708 mm, Daptocephalus AZ, Changhsingian, Late Permian.
BPI/1/4469: Cynodontia, Cynosaurus suppostus, voxel size: 0.0342 mm, Daptocephalus AZ, Changhsingian, Late Permian.
BPI/1/1563: Cynodontia, Cynosaurus suppostus, voxel size: 0.0291 mm, Daptocephalus AZ, Changhsingian, Late Permian.
AM4947: Cynodontia, Cynosaurus suppostus, voxel size: 0.0476 mm, Daptocephalus AZ, Changhsingian, Late Permian.
BPI/1/7199: Cynodontia, Thrinaxodon liorhinus, voxel size: 0.03 mm, Lystrosaurus AZ, Induan, Early Triassic60.
SAM-PK-K3781: Cynodontia, Thrinaxodon liorhinus, voxel size: 0.0513 mm, Lystrosaurus AZ, Induan, Early Triassic.
BPI/1/4623: Cynodontia, Thrinaxodon liorhinus, voxel size: 0.050 mm, Lystrosaurus AZ, Induan, Early Triassic.
RC845: Cynodontia, Galesaurus platyceps, voxel size: 0.050 mm, Lystrosaurus AZ, Induan, Early Triassic.
TM80: Cynodontia, Galesaurus platyceps, voxel size: 0.050 mm, Lystrosaurus AZ, Induan, Early Triassic.
Cynodontia
Cynognathia
BPI/1/4534: Cynodontia, Cynognathia, indet.Trirachodontidae, voxel size: 0.0556 mm, Cynognathus AZ, Anisian, Early Triassic.
BPI/1/4658: Cynodontia, Cynognathia, Trirachodon berryi, voxel size: 0.0667 mm, Cynognathus AZ, Anisian, Early Triassic.
AM461: Cynodontia, Cynognathia, Trirachodon berryi, voxel size: 0.0668 mm, Cynognathus AZ, Anisian, Early Triassic.
BPI/1/5362: Cynodontia, Cynognathia, Langbergia modisei, voxel size: 0.0796 mm, Cynognathus AZ, Anisian, Early Triassic.
BPI/1/3776: Cynodontia, Cynognathia, Diademodon tetragonus, voxel size: 0.0801 mm, Cynognathus AZ, Anisian, Early Triassic.
Cynodontia
Probainognathia
BPI/1/5691: Cynodontia, Probainognathia, Pachygenelus monus, voxel size: 0.025 mm, Massospondylus range zone (RZ), Hettangian, Sinnemurian and Pliensbachian, Early Jurassic.
BPI/1/4778: Cynodontia, Probainognathia, Tritylodon longaevus, voxel size: 0.0930 mm, Massospondylus RZ, Hettangian, Sinnemurian and Pliensbachian, Early Jurassic.
BPI/1/5088: Cynodontia, Probainognathia, Tritylodon longaevus, voxel size: 0.0908 mm, Massospondylus RZ, Hettangian, Sinnemurian and Pliensbachian, Early Jurassic.
BPI/1/5288: Cynodontia, Probainognathia, Tritylodon sp., voxel size: 0.0509 mm, Massospondylus RZ, Hettangian, Sinnemurian and Pliensbachian, Early Jurassic.
PVSJ 422: Ecteninion lunensis after Digimorph.org, voxel size: 0.1624 mm36. Courtesy of Timothy Rowe of University of Texas, Department of Geological Sciences.
CT scanning: twenty nine therapsid skulls were scanned for this study. All specimens were scanned at the Evolutionary Studies Institute (ESI) scanning facility using Nikon Metrology XTH 225/320 LC except Thrinaxodon BPI/1/7199 that was scanned at the European Synchrotron Radiation Facility, Grenoble (see 60 for details) and Ecteninion which was scanned at the University of Texas (see the list of the scanned specimens for details). A list of specimens examined, with taxonomic assignment, voxel size and stratigraphic position is provided above. Three-dimensional renderings of the internal structure of the maxillary canal, parietal bone and cranial endocasts were obtained using manual segmentation under Avizo 8 (VSG). In order to focus our study on the bony structures that carried tissues actively playing a role in the innervation and nutrition of the face and thus are relevant to the evolution of facial sensitivity and motility, only the parts of the canal that directly communicate with the external surface were segmented. For request concerning access to CT scan data, please contact K. Carlson (CT facility manager) : Kristian.Carlson@wits.ac.za. Area of the infraorbital foramen was obtained using direct measurement with a caliper.
Additional Information
Data availability: For request concerning access to CT scan data, please contact K. Carlson (CT facility manager): Kristian.Carlson@wits.ac.za.
How to cite this article: Benoit, J. et al. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Sci. Rep. 6, 25604; doi: 10.1038/srep25604 (2016).
References
Martin, T. et al. A Cretaceous eutriconodont and integument evolution in early mammals. Nature 526, 380–384 (2015).
Oftedal, O. T. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia 7, 225–252 (2002).
Lefèvre, C. M., Sharp, J. A. & Nicholas, K. R. Evolution of Lactation: Ancient Origin and Extreme Adaptations of the Lactation System. Annu Rev Genomics Hum Genet 11, 219–38 (2010).
Satokata, I. et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24, 391–395 (2000).
Ji, Q., Luo, Z.-X., Yuan, C.-X. & Tabrum, A. R. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311(5764), 1123–1127 (2006).
Smith, R. M. H. & Botha-Brink, J. Morphology and composition of bone-bearing coprolites from the Late Permian Beaufort Group, Karoo Basin, South Africa. Palaeogeogr Palaeoclimatol Palaeoecol 312, 40–53 (2011).
Bajdek, P. et al. Microbiota and food residues including possible evidence of pre-mammalian hair in Upper Permian coprolites from Russia. Lethaia 10.1111/let.12156 (2015).
Jerison, H. J. Evolution of the brain and intelligence (Academic Press, New York, 1973).
Gerkema, M. P. et al. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc R Soc B 280, 20130508 (2013).
Angielczyk, K. D. & Schmitz, L. Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proc R Soc B 281, 20141642 (2014).
Rowe, T. B. Coevolution of the mammalian middle ear and neocortex. Science 273, 651 (1996).
Rowe, T. B., Macrini, T. E. & Luo, Z.-X. Fossil evidence on origin of the mammalian brain. Science 332, 955–957 (2011).
Abdel-Kader, T. G., Ali, R. S. & Ibrahim, N. M., The Cranial Nerves of Mabuya quinquetaeniata III: Nervus Trigeminus. Life Sci J 8, 650–669 (2011).
Bellairs, A. D’. A. Observations on the snout of Varanus and a comparison with that of other lizards and snakes. J Anat 83, 116–146 (1949).
Leitch, D. B. & Catania, K. C. Structure, innervation and response properties of integumentary sensory organs in crocodilians. J Exp Biol 215, 4217–4230 (2012).
Fourie, S. The cranial morphology of Thrinaxodon liorhinus Seeley. Ann S Af Mus 65, 337–400 (1974).
Sigurdsen, T. New features of the snout and orbit of a therocephalian therapsid from South Africa. Acta Palaeontol Pol 51, 63–75 (2006).
Andres, K. H., von During, M., Iggo, A. & Proske, U. The anatomy and fine structure of the echidna Tachyglossus aculeatus snout with respect to its different trigeminal sensory receptors including the electroreceptors. Anat Embryol 184, 371–393 (1991).
Manger, P. R. & Pettigrew, J. D. Ultrastructure, number, distribution and innervation of electroreceptors and mechanoreceptors in the bill skin of the platypus. Brain Behav Evol 48, 27–54 (1996).
Rowe, T. et al. The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proc Natl Acad Sci USA 105(4), 1238–1242 (2008).
Rodella, L. F., Buffoli, B., Labanca, M. & Rezzani, R. A review of the mandibular and maxillary nerve supplies and their clinical relevance. Arch Oral Biol 57(4), 323–334 (2012).
Sues, H.-D. The skull and dentition of two tritylodontid synapsids from the Lower Jurassic of westem North America. Bull Mus Comp Zool 151, 217–268 (1986).
Düring von, M. & Miller, M. R. in Biology of the Reptilia Vol. 9 (eds Gans, C., Northcutt, R. G. & Ulinski, P. ), 407–411. (Academic Press, New York, 1979).
Muchlinski, M. N. The relationship between the infraorbital foramen, infraorbital nerve and maxillary mechanoreception: implications for interpreting the paleoecology of fossil mammals based on infraorbital foramen size. Anat Rec 291, 1221–1226 (2008).
Muchlinski, M. N. A comparative analysis of vibrissa count and infraorbital foramen area in primates and other mammals. J Hum Evol 58, 447–473 (2010).
Tatarinov, L. P. Morphological evolution of the Theriodonts and the general problems of Phylogenetics (NAUKA, Moscow, 1976).
Lingham-Soliar, T. The Vertebrate Integument Volume 1 (Springer-Verlag: Berlin, Heidelberg,, 2014).
Van Valen, L. Therapsids as mammals. Evolution 14, 304–313 (1960).
Watson, D. M. S. On the skeleton of a bauriamorph reptile. J Zool 1931, 1163–1205 (1931).
Estes, R. Cranial anatomy of the cynodont reptile Thrinaxodon liorhinus. Bull Mus Comp Zool 125, 165–180 (1961).
Rubidge, B. S. & Sidor, C. A. Evolutionary patterns among Permo-Triassic Therapsids. Annu Rev Ecol Syst 32, 449–480 (2001).
Hopson, J. A. & Kitching, J. W. A probainognathian cynodont from South Africa and the phylogeny of nonmammalian cynodonts. Bull Mus Comp Zool 156, 5–35 (2001).
Liu, J. & Olsen, P. The phylogenetic relationships of Eucynodontia (Amniota: Synapsida). J Mammal Evol 17, 151–176 (2010).
Ruta, M., Botha-Brink, J., Mitchell, S. A. & Benton, M. J. The radiation of cynodonts and the ground plan of mammalian morphological diversity. Proc Biol Sci 280, 20131865 (2014).
Kemp, T. S. The Primitive Cynodont Procynosuchus: Functional Anatomy of the Skull and Relationships. Philos Trans R Soc Lond B Biol Sci 285, 73–122 (1979).
Rowe, T. (Dir.)., Digimorph, Digital Morphology library (online), University of Texas Digital Morphology Group, http://digimorph.org (2002–2005) Date of access:06/09/2015.
Kuhne, W. G. The Liassic therapsid Oligokyphus. (British Museum Natural History, London, 1956).
Ruf, I., Maier, W., Rodrigues, P. G. & Schultz, C. L. Nasal Anatomy of the Non-mammaliaform Cynodont Brasilitherium riograndensis (Eucynodontia, Therapsida) Reveals New Insight into Mammalian Evolution. Anat Rec 297, 2018–2030 (2014).
Kielan-Jaworowska, Z., Cifelli, R. L. & Luo, Z.-X. Mammals from the Age of Dinosaurs Origins, Evolution and Structure (Columbia University Press, New York, 2004).
Kemp, T. S. The origin and evolution of mammals (Oxford University Press, Oxford, 2005).
Kermack, K. A., Mussett, F. & Rigney, H. W. The skull of Morganucodon. Zool J Linn Soc 71, 1–158 (1981).
Martinez, R. N., May, C. L. & Forster, C. A. A new carnivorous cynodont from the Ichigualasto Formation (Late Triassic, Argentina), with comments on eucynodont phylogeny. J Vert Paleontol 16, 271–284 (1996).
Eckhart, L. et al. Identifcation of reptilian genes encoding hair keratin-like proteins suggests a new scenario for the evolutionary origin of hair. Proc Natl Acad Sci USA 105, 18419–18423 (2008).
Quay, W. B. In Biology of the Reptilia Vol. 9 (eds Gans, C., Northcutt, R. G. & Ulinski, P. ), 245–406 (Academic Press, New York, 1979).
Benoit, J., Abdala, F., Manger, P. R. & Rubidge, B. S. The sixth sense in mammals forerunners: variability of the parietal foramen in South African Therapsida suggests convergent evolution of their physiology. Acta Paleontol Pol, doi: 10.4202/app.00219.2015 (2016).
Benoit, J. et al. Physiological implications of the abnormal absence of the parietal foramen in a late Permian cynodont (Therapsida). Naturwissenschaften 102(11), 69, doi: 10.1007/s00114-015-1321-4 (2015).
Roth, J. J., Roth, E. C. & Hotton, N. N. In Ecology and Biology of Mammal-like Reptiles (eds Hotton, N., MacLean, P. D., Roth, J. J., Roth, E. D. ), 173–184 (Smithsonian Institution Press, Washington, 1986).
de Beer, G. R. The Development of the Vertebrate Skull (Oxford University Press, 1937).
Garcia-Miñaur, S. et al., Parietal foramina with cleidocranial dysplasia is caused by mutation in MSX2. Eur J Hum Genet 11, 892–895 (2003).
Morriss-Kay, G. M. Derivation of the mammalian skull vault. J Anat 199, 143–151 (2001).
Roybal, P. G. et al. Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev Biol. 343, 28–39 (2010).
Agarwal, P., Pandey, M., Baranwal, S. & Roy, K., Large midline persistent parietal foramina with occipital encephalocele and abnormal venous drainage. J Cleft Lip Palate Craniofac Anomal 2, 66–9 (2015).
Akimenko, M. A., Johnson, S. L., Westerfield, M. & Ekker, M. Differential induction of four msx homeobox genes during fin development and regeneration in zebrafish. Development 121, 347–357 (1995).
Tokita, M., Chaeychomsri, W. & Siruntawineti, J. Skeletal gene expression in the temporal region of the reptilian embryos: implications for the evolution of reptilian skull morphology. SpringerPlus 2, 336–356 (2013).
Lamba, D. A. & Reh, T. A. Microarray Characterization of Human Embryonic Stem Cell–Derived Retinal Cultures. Invest Ophthalmol Vis Sci 52, 4897–4906 (2011).
Zhang, Z. et al. Malleal processus brevis is dispensable for normal hearing in mice. Dev Dyn 227, 69–77 (2003).
Ferguson, M. W. J. A hole in the head. Nat Genet 24, 330–331 (2000).
Hopson, J. A. In Biology of the Reptilia (eds Glans, C., Northcutt, R. G., Ulinski, P. ), 39–146 (Academic Press, New York, 1979).
Hillenius, W. J. & Ruben, J. A. The Evolution of Endothermy in Terrestrial Vertebrates: Who? When? Why? Physiol Biochem Zool 77, 1019–1042 (2014).
Fernandez, V. et al. Synchrotron Reveals Early Triassic Odd Couple:Injured Amphibian and Aestivating Therapsid Share Burrow. PLoS One 8(6), e64978 (2013).
Acknowledgements
F. Abdala (ESI, Johannesburg), B. Billings (School of Anatomical Sciences, Johannesburg), E. Butler (NM, Bloemfontein), K. Carlson (ESI, Johannesburg), Z. Erasmus (SAM, Cape Town), V. Fernandez (ESRF), H. Fourie (DM, Pretoria), K. Jakata (ESI, Johannesburg), S. Jasinoski (ESI, Johannesburg), S. Jirah (ESI, Johannesburg), A. Kruger (ESI, Johannesburg), R. and M. Rubidge (Graaff-Reinet), R. Smith (SAM, Cape Town), M. Van Der Brandt (ESI, Johannesburg) for access to specimens and scans. This research was kconducted with financial support from PAST and its scatterlings projects; the NRF African Origins Platform; and DST/NRF Centre of Excellence in Paleosciences. We greatly thank DigiMorph.org, particularly Prof. T.B. Rowe, Dr. M. Colbert and Dr. J. Maisano for providing the CT images of Ecteninion.
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: J.B. Acquisition of data: J.B. Analysis and interpretation of data: J.B., P.R.M., B.S.R. Drafting of the manuscript: J.B. Critical revision of the manuscript for important intellectual content: P.R.M. and B.S.R. Obtained funding: J.B., P.R.M. and B.S.R. Administrative, technical and material support: P.R.M. and B.S.R. Study supervision: P.R.M. and B.S.R. All authors gave final approval for publication.
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Benoit, J., Manger, P. & Rubidge, B. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Sci Rep 6, 25604 (2016). https://doi.org/10.1038/srep25604
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25604
This article is cited by
-
Ancient lineages of the keratin-associated protein (KRTAP) genes and their co-option in the evolution of the hair follicle
BMC Ecology and Evolution (2023)
-
The maxillary canal of the titanosuchid Jonkeria (Synapsida, Dinocephalia)
The Science of Nature (2023)
-
A new cynodont from the Upper Triassic Los Colorados Formation (Argentina, South America) reveals a novel paleobiogeographic context for mammalian ancestors
Scientific Reports (2022)
-
Inner ear biomechanics reveals a Late Triassic origin for mammalian endothermy
Nature (2022)
-
Revisiting life history and morphological proxies for early mammaliaform metabolic rates
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.