Abstract
Phenological synchrony influences many ecological processes. Recent climate change has altered the synchrony of phenology, but little is known about the underlying mechanisms. Here using in situ phenological records from Europe, we found that the standard deviation (SD, as a measure of synchrony) of first leafing day (FLD) and the SD of first flowering day (FFD) among local plants were significantly smaller in the years and/or in the regions with a more rapid within-spring warming speed (WWS, the linear slope of the daily mean temperature against the days during spring, in oC/day) with correlation coefficients of −0.75 and −0.48 for FLD and −0.55 and −0.23 for FFD. We further found that the SDs of temperature sensitivity of local plants were smaller under the rapid WWS conditions with correlation coefficients of −0.46 and −0.33 for FLD and FFD respectively. This study provides the first evidence that the within-season rate of change of the temperature but not the magnitude determines plant phenological synchrony. It implies that temporally, the asymmetric seasonal climatic warming may decrease the synchrony via increasing WWS, especially in arctic regions; spatially, plants in coastal and low latitude areas with low WWS would have more diverse spring phenological traits.
Similar content being viewed by others
Introduction
Phenological synchrony, which is referred to here as the temporal convergence of phenological events, can occur within individuals, among individuals or among species1,2,3. The synchrony of phenology has various ecological consequences for individual survival, species fitness and ecosystem stability3,4,5,6,7. For example, decreasing synchrony in flowering date can lead to assortative mating since early bloomers are more likely pollinated by other early plants and late plants by late plants8. Besides, the synchrony among plants could augment the bee visitation rates by attracting more pollinators9 or decrease the rates by competing for pollinators10. There is increasing evidence that recent climate change has altered the phenological synchrony of various organisms11,12,13,14, but the underlying mechanisms are unclear, particularly in plants.
Both biotic and abiotic factors are expected to be involved in the phenological synchrony of plant15,16,17,18. Biotic factors, such as pollinator phenology and activity, may result in either high flowering synchrony9 or asynchrony19. Of the abiotic factors, temperature is the primary factor determining plant phenology20,21,22,23. Both the magnitude of the environmental temperature and the rate of the temperature change (speed) could affect phenological development, but to our knowledge, there is no evidence to date on whether or how the rate of change in environmental temperature affects plant phenological synchrony.
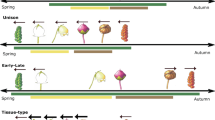
For a phenological event presumably determined only by temperature, there should be a range of environmental temperatures necessary or suitable for the event. Outside of this range, the plants would suffer from heat or cold stress. Flowering too early in the spring season may result in frost damage24,25, whereas flowering too late may decrease seed production as a result of high temperature stress26. The rate of seasonal change in environmental temperature, e.g., the speed of within-spring warming or within-autumn cooling, can differ between years and/or locations. A rapid within-spring warming period means a short period for any particular spring-season temperature assuming that the speed and direction of temperature change are monotonous and consistent (Fig. 1). We therefore hypothesized that under rapid spring warming conditions, plant phenology will have strong synchrony, thereby allowing a phenological event to align with the appropriate temperature within the short period available.
Conceptual diagram showing how the relative abundance of a phenological event is distributed temporally.
The upper panel shows two typical time series for temperature elevation within the spring season. If a phenological event requires a range of necessary or suitable temperatures and that range does not change, a rapid temperature elevation would correspond to a short period, as indicated on the right in the bottom panel and a slow temperature elevation would correspond to a long period. In the bottom panel, the letters a, b, c can represent three individuals or three species within a habitat or within a phenological period, such as the spring season.
Here, using field observations of two spring events, the first leafing day (FLD) and the first flowering day (FFD) in Europe, we demonstrate that the synchrony of the timing of FLD and FFD among local plants are highly correlated with the within-spring warming speed (WWS, °C/day) both temporally and spatially. In addition, we show that the synchrony of phenological sensitivity of FLD and FFD to temperature as well are associated with WWS spatially.
Results
Temporal correlation
We firstly examined the degree of synchrony of FLD and FFD, in relation to the WWS temporally. The phenological data were selected from the Pan European Phenological Database (PEP725)27. The daily gridded climate data, with a spatial resolution of 0.25°, were obtained from the European Climate Assessment & Dataset project (E-OBS)28. The degree of phenological synchrony, i.e., the degree of timing-convergence, was assessed by the standard deviation (SD) of the FLD and FFD among all individual plants within an area coincident with the geographical grids defined in the climate data1. A low SD indicates a high degree of synchrony. The WWS for the FLD and FFD was calculated as the linear slope of the daily mean temperature against the days of the year during spring (see Method).
During the period from 1951 to 2011, the average FLD and FFD for all individual plants in the PEP725 dataset advanced significantly and the long-term advancement was closely associated with a distinct increase in the long-term temperature in the spring season (supplementary Figure 1). This is consistent with many previous claims that recent global warming has resulted in significant phenological shifts worldwide11,13,29,30,31. The SDs of the FLD and FFD among individual plants within each grid, however, exhibited large year-to-year fluctuations without a distinct long-term trend (Fig. 2a,c), indicating that the long-term temperature increase, i.e., the change in the temperature magnitude alone had no significant effect on the annual synchrony of the FLD or FFD.
The standard deviation (SD) of spring phenological dates in relation to the within-spring warming speed (WWS, °C/day) during the period 1951–2011.
(a,c) show the annual variations in the SD of the first leafing day (FLD) and the first flowering day (FFD). (b,d) show the WWS during spring for FLD and FFD. For each year, the SDs of the FLD or FFD were obtained for all the individual plants located within each grid. The bottom and the top of the box denote the 25th and 75th percentiles, respectively and the line within the box represents the 50th percentile (the median). The whiskers extends to the maximum and the minimum SD excluding the outliers, which are the SD >(q3 + 1.5(q3 − q1)) or SD< (q1 − 1.5(q3 − q1)), where q1 and q3 are the 25th and 75th percentiles, respectively. (e,f) are scatterplots of the annual mean SD in relation to the annual mean WWS for the FLD (r = −0.75, P < 0.01) and FFD (r = −0.55, P < 0.01). The shaded region represents the 95% confidence interval of the regression line. All of the regressions have P-values < 0.01.
We then examined the relationship between the annual SDs of the two spring events in relation to the within-spring warming speed. Visually, year-to-year fluctuations of WWS presented a rough inverse pattern with that of SDs (Fig. 2b,d). Decrease in SD was associated with increase in WWS. In addition, a simple linear regression analysis showed that the annual SDs of the FLD and FFD were both significantly higher in the years with lower annual WWSs (Fig. 2e,f). To reduce the bias caused by a small number of observational data in a grid, we also conducted the regression analysis after excluding grids containing fewer than 10 individuals and the results were consistent (supplementary Figure 2). Further partial correlation analysis confirmed that the annual SDs showed no significant partial correlation with the annual averaged spring temperature and total spring precipitation (Supplementary Figure 3). These results indicate that the rate of temperature increase but not the magnitude of temperature within the spring season is probably responsible for the annual fluctuation in the synchrony of the spring phenological events.
Spatial correlation
The within-spring warming rate is also expected to change spatially. The WWS calculated from the multi-year mean daily temperature for each geographical grid was higher in inland areas than in coastal areas (Fig. 3). We investigated whether spatial changes in the WWS affected the synchrony of local plants. The results showed that the SDs of the multi-year mean FLD and FFD were lower in the inland grids than in the coastal grids, especially for the FLD (Fig. 4a,b). As a result, the SDs decreased significantly with the increase of the local WWS, with a correlation coefficient of −0.48 for the FLD and −0.23 for the FFD (Fig. 4c,d). Moreover, this correlation pattern was consistent for both individual plants grouped by each species and for species averages per grid (supplementary Figures 4 and 5).
The geographical pattern of the within-spring warming speed (WWS, °C/day).
The map was created using MATLAB 8.0 (http://cn.mathworks.com/).
The spatial patterns of the standard deviation (SD) of spring phenological dates, the temperature sensitivity and their association with the within-spring warming speed (WWS, °C/day).
(a,b) show the SDs of the FLD and FFD; (e,f) show the SDs of the temperature sensitivity of the FLD and FFD, respectively. Each coloured point indicates the SD of the multi-year averaged phenological dates or their temperature sensitivity for all plants within a grid. The scatterplots show the SDs in relation to the WWS, with each data point denoting one grid. c and d show the SDs of the FLD and FFD associated with their WWS; (g,h) show the SDs of the FLD and FFD temperature sensitivity associated with their WWS, respectively. Grids with individual plant numbers <=10 are indicated by white colour and were not included in the correlation analysis. The line is the linear regression line and the shaded region represents the 95% confidence interval. All of the regressions have P-values < 0.01. (a,b,e,f) were created using MATLAB 8.0 (http://cn.mathworks.com/).
Some factors may affect the correlations between the SDs and WWS. To evaluate whether any of the data processes introduced bias into the results obtained above, we performed the following procedures. First, to reduce the bias caused by a small number of observational data in a grid, we excluded grids containing fewer than 20 individuals (supplementary Figure 6). Second, to cope better with the phenological data, we examined synchrony by deeming each phenology station as the minimum unit instead of using the grid (supplementary Figure 7). Third, we examined the interquartile range (i.e., the upper quartile minus the lower quartile) instead of the SD to quantify synchrony (supplementary Figure 8). After the above procedures, we found that all of the results were consistent with our original findings. Finally, the results were also consistent with the initial results after controlling for other potential factors including the altitudinal variance, the spatial aggregation of the phenology stations, species diversity of the collected data within a grid as well as the mean spring temperature and total spring precipitation (supplementary method, supplementary Figure 9).
Temperature sensitivity
The phenological trait, temperature sensitivity which characters the response of phenology to temperature, receives a lot of concerns in the context of global warming18,19,26. The synchrony of the temperature sensitivity as well has important ecological and evolutionary consequences27. We thus further examined whether the synchrony of the temperature sensitivity is also correlated to the within-spring warming speed. Herein, the temperature sensitivity was evaluated as a linear slope of the event dates with respect to an effective temperature (see method, days/°C). We conducted all the analysis performed above and found that in the areas with a slower WWS, the FLD and FFD show larger SDs of temperature sensitivity among individual plants, among individual plants grouped by each species or for species averages per grid (Fig. 4g,h; supplementary Figures 4–10). It is in line with the synchrony of the timing of phenological events.
Discussion
Phenological synchrony among plants has long been a focus of ecologists and plant physiologists1,3,4,28. We find that temporally the degrees of synchrony in the first leaf unfolding day and the first flowering day among local plant individuals are highly dependent on the within-spring warming speed but not the magnitude of spring tempertaure. A previous research however showed that the synchrony of spring events was related with monthly mean temperature32. This difference may arise from the difference of the spatial scales in calculating the synchrony. A national standard deviation were calculated to represent synchrony on regional level in Menzel et al.32. While in the current study, the synchrony is defined at the grid or site level. On the basis of this finding, we assume that the rate of temperature increase within each spring season may act as a signal triggering the synchrony among individuals of different species. Physiologically, plants are able to sense either the magnitude of the temperature itself or the rate of temperate change33. Abrupt and gradual changes in temperature of the same magnitude may have different physiological consequences33,34,35. However, there is currently no evidence from either physiological or ecological studies to elucidate how the rate of temperature change induces yearly differences in phenological synchrony in plants.
The plant phenological traits are important determinants of fitness and the results of long-term adaptation to the local environment36,37. The close spatial correlation between the SDs of the multi-year mean phenological date or temperature sensitivity and the WWS among different grids indicates that the local rate of temperature increase within the spring season has strongly affected the adaption of the synchrony of the spring phenology. The potential role of the rate of temperature increase in influencing the adaption of phenological traits has been suggested in several previous literatures. B. VEEN (1954) hypothesised that early flushing trees would be selected out because of frequently frost damage if the climate has a gradually increasing mean temperature in spring38. A later research supported his hypothesis by growing seedlings from different locations under a uniform environment and finding that sources from north and inland area where daily mean temperature increase rapidly usually flushed early39. A recent research using satellite based vegetation index demonstrated that plants in areas with rapid within-spring warming were more sensitive to temperature in the Northern Hemisphere40. These studies jointly with the current research call for the caution that the within-season rate of change of the environmental temperature should be involved in the investigation of phenological adaption to local environment.
In temperate and boreal regions, temperature is usually the dominant factor determining the plant phenology21,41. However, in tropical and arid regions, the seasonal pattern of moisture availability may play more important role in regulating plant phenology42,43,44,45. Accordingly, the importance of the change rate of other environmental factors should be emphasized in these regions. Domínguez & Dirzo42 conducted two experiment designs, one mimicked a sudden increase in soil humidity and the other simulated a gradual one. They found a high synchrony of the FFD when moisture increase rapidly which is consistent with our speculation. However, to date there is still very little knowledge available for exploring the relation between the environment change rate and phenological synchronization. Further studies are needed.
The implications of our findings are multiple. Most importantly, the current study greatly improves our ability to predict the phenological synchrony both temporally and spatially. Recent climate change and climate projections all indicate a faster warming in the winter than in the summer, particularly in arctic regions46. This should result in a smaller range of variation in seasonal temperature in the spring and thus slower within-spring warming speed. The implication is that future climate change may increase phenological divergence if we assume that the phenological response to temperature change remains consistent. Spatially, low latitudes and coastal areas usually have gradual temperature increase in spring40. We thus predict that plants in these areas would present high levels of diversity in the phenological traits. We also analyzed the synchrony of temperature sensitivity in this research. The synchrony in sensitivity indicates the diversity in plants response to temperature. It could determine the species’ or communities’ ability to buffer and adapt to the changing climate47. In addition, this diversity can complicate efforts to predict response to climate change. On the basis of our result, when forecast future phenology pattern on large scales such as using satellite images, researchers should be aware that the estimates are more reliable in areas with high WWS because the responses of the plants are more synchronous in theses grids. Accordingly, incorporating this rate of change in analyses could help predict better plant phenology.
Method
Climate data
We used the E-OBS daily gridded dataset, with a spatial resolution of 0.25° for temperature and precipitation (http://www.ecad.eu). The E-OBS was derived from the interpolation of data from over 2,000 stations throughout Europe from ECA&D (European Climate Assessment and Data) and some additional data sets28. A prior study showed a very strong correlation between this dataset and the high-resolution regional gridded datasets in Europe48.
Phenological data
The first leaf unfolding date (FLD) and the first flowering date (FFD) were obtained from the Pan European Phenology Database (PEP725; http://www.pep725.eu). In the dataset, there are 9 million records for 139 plants and 33 growth stages over approximately 20,000 locations across Europe (mainly in Germany, Austria and Switzerland) starting in the year 1868. These locations cover a region with a mean annual temperature ranging from 5 to 12 °C and an annual precipitation ranging from 550 to 1200 mm49. The dataset has been widely used in plant phenological studies37,49.
Subsets of records from PEP725 were selected for temporal and spatial analysis to maximize the data set size and conduct robust analyses. To select the data for temporal analysis, we (1) excluded data from before 1951 because of limited record numbers or/and overly spatially distributed records, (2) focused on individuals with a multi-year mean phenological date from 60 DOY to 150 DOY and (3) focused on grids containing at least 3 records for any year during the study period (1951–2011). As a result, a total of approximately 20,000 time series within 3,000 observation stations over 13 plant species were included for the FLD analysis and approximately 56,000 time series within 3,500 observation stations over 35 plant species were included for the FFD analysis.
The subset of records for spatial analysis was selected by performing steps (1) and (2) as with the temporal subset and (3) focusing on individuals with a time series longer than 15 years. As a result, a total of approximately 23,000 time series from 4,300 observation stations covering 25 plant species were included for the FLD analysis and approximately 52,000 time series from 4,600 observation stations covering 39 plant species were included for the FFD analysis.
Calculating the temperature sensitivity of spring phenology
The temperature sensitivity for a particular phenological event can be quantified as a linear slope of the event dates with respect to an effective temperature (days/°C)21,40,50. The effective temperature is often derived from the mean temperature of a period before the phenological event20,40. To obtain the effective temperature, we conducted a stepwise regression using the FLD and FFD as dependent variables against the independent variable, i.e., the monthly mean temperature, for each month from November of the previous year to June of the current year. We then performed a stepwise regression for each individual plant. The effective-temperature month was selected by the model with the input P-value of 0.05 and the output P-value of 0.1. To find the most effective period for a phenological event, we then obtained the percentage of the total number of individuals with the effective temperature out of the total number of individuals for each month:

where pi is the percentage for the month i, Nt is the total number of individual plants and Ne is the number of individual plants for which the temperature of month i is effective. As a result, the effective temperature of the FLD for most individuals occurred in March and April and that of the FFD for most individuals occurred in February, March and April (supplementary Figure 11). Within the months spanning the effective temperature, the majority of individuals, 98.4% for the FLD and 98.7% for the FFD, had a negative linear correlation with the monthly mean temperature, with 80.2% and 85.0% of the individuals yielding a P-value <0.05 for the FLD and FFD, respectively. We therefore used the above months for further analysis.
We also examined the results for temperature sensitivity for the following four cases: temperature sensitivity was recalculated after the effective temperature and phenological date were detrended, or the effective temperature was redefined as the mean temperature of the 30, 60 or 90 days before the multi-year mean phenological date for each individual. All the results showed results consistent with our original findings (supplementary Figure 10).
Analysis
To examine the temporal correlation between the synchrony of phenological events and the within-spring warming speed (WWS), we obtained the standard deviation (SD) of the FLD and the FFD. For each year within each grid (0.25 × 0.25 degrees), the SDs were calculated for the phenological date of all plant individuals. The WWS was defined as the linear slope of the daily mean temperature against the days during spring (DOY 60–DOY 150). We then conducted a correlation analysis between the spatially averaged SDs and the WWS across years.
To conduct the spatial analysis, for each grid, the SDs were calculated for the yearly averaged phenological date and the temperature sensitivity of all plant individuals. The WWS was calculated using the yearly averaged daily mean temperature (1951–2011). We also calculated the SDs of all individuals by species and the species averages within each grid. Three species for the FLD and five species for the FFD with a large number of observations were used for the analysis at the species level (supplementary Table 1). We then conducted a correlation analysis between the SDs and the WWS across grids.
For the case of all of the individuals within a grid, we also performed a partial correlation analysis to exclude the potential influence of other factors (see supplementary method).
Additional Information
How to cite this article: Wang, C. et al. Plant phenological synchrony increases under rapid within-spring warming. Sci. Rep. 6, 25460; doi: 10.1038/srep25460 (2016).
References
Augspurger, C. K. Phenology, flowering synchrony and fruit set of six neotropical shrubs. Biotropica 15, 257–267 (1983).
Rathcke, B. & Lacey, E. P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Evol. Syst 16, 179–214 (1985).
Ims, R. A. The ecology and evolution of reproductive synchrony. Trends Ecol. Evol. 5, 135–140 (1990).
Hay, R. & Kirby, E. Convergence and synchrony-a review of the coordination of development in wheat. Crop Pasture Sci. 42, 661–700 (1991).
English-Loeb, G. M. & Karban, R. Consequences of variation in flowering phenology for seed head herbivory and reproductive success in Erigeron glaucus (Compositae). Oecologia 89, 588–595 (1992).
Ovaskainen, O. et al. Community-level phenological response to climate change. Proc. Natl Acad. Sci. 110, 13434–13439 (2013).
Bolmgren, K. & Eriksson, O. Are mismatches the norm? Timing of flowering, fruiting, dispersal and germination and their fitness effects in Frangula alnus (Rhamnaceae). Oikos 124, 639–648, 10.1111/oik.01491 (2014).
Weis, A. E. & Kossler, T. M. Genetic variation in flowering time induces phenological assortative mating: quantitative genetic methods applied to Brassica rapa. Am. J. Bot. 91, 825–836 (2004).
Augspurger, C. K. Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators in Hybanthus prunifolius (Violaceae). Ecology 62, 775–788 (1981).
Brown, B. J., Mitchell, R. J. & Graham, S. A. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83, 2328–2336 (2002).
Walther, G.-R. et al. Ecological responses to recent climate change. Nature 416, 389–395 (2002).
Edwards, M. & Richardson, A. J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (2004).
Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst 37, 637–669 (2006).
Yang, L. H. & Rudolf, V. H. W. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett. 13, 1–10, 10.1111/j.1461-0248.2009.01402.x (2010).
Sakai, S. Asynchronous leaf expansion and shedding in a seasonal environment: result of a competitive game. J. Theor. Biol. 154, 77–90 (1992).
Elzinga, J. A. et al. Time after time: flowering phenology and biotic interactions. Trends Ecol. Evol. 22, 432–439 (2007).
Bustamante, E. & Búrquez, A. Effects of plant size and weather on the flowering phenology of the organ pipe cactus (Stenocereus thurberi). Ann. Bot. 102, 1019–1030 (2008).
Forrest, J., Inouye, D. W. & Thomson, J. D. Flowering phenology in subalpine meadows: Does climate variation influence community co-flowering patterns? Ecology 91, 431–440, 10.1890/09-0099.1 (2010).
Waser, N. M. Interspecific pollen transfer and competition between co-occurring plant species. Oecologia 36, 223–236 (1978).
Menzel, A. et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (2006).
Wolkovich E. M. et al. Warming experiments underpredict plant phenological responses to climate change. nature 485, 292–297 (2012).
Shen, M. Spring phenology was not consistently related to winter warming on the Tibetan Plateau. Proc. Natl Acad. Sci. 108, E91–E92 (2011).
Shen, M. et al. Specification of thermal growing season in temperate China from 1960 to 2009. Climatic Change 114, 783–798 (2012).
Inouye, D. W. Effects of climate change on phenology, frost damage and floral abundance of montane wildflowers. Ecology 89, 353–362 (2008).
Augspurger, C. K. Spring 2007 warmth and frost: phenology, damage and refoliation in a temperate deciduous forest. Funct. Ecol. 23, 1031–1039 (2009).
Young, L. W., Wilen, R. W. & Bonham-Smith, P. C. High temperature stress of Brassica napus during flowering reduces micro-and megagametophyte fertility, induces fruit abortion and disrupts seed production. J. Exp. Bot. 55, 485–495 (2004).
Koch, E., Adler, S., Lipa, W., Ungersböck, M. & Zach-Hermann, S. The Pan European phenological database PEP725. Ber. Meteor. Inst. Univ. Freiburg Nr. 20, 331–335 (2010).
Haylock, M. et al. A European daily high‐resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. 113, D20119, 10.1029/2008JD010201 (2008).
Schwartz, M. D., Ahas, R. & Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 12, 343–351 (2006).
Fu, Y. H. et al. Recent spring phenology shifts in western Central Europe based on multiscale observations. Global Ecol. Biogeogr. 23, 1255–1263 (2014).
Fu, Y. H. et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 526, 104–107 (2015).
Menzel, A., Sparks, T., Estrella, N. & Roy, D. Altered geographic and temporal variability in phenology in response to climate change. Global Ecol. Biogeogr. 15, 498–504 (2006).
Ruelland, E. & Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 69, 225–232 (2010).
Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 74, 9–16 (2011).
Mittler, R., Finka, A. & Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci 37, 118–125 (2012).
Chuine, I. Why does phenology drive species distribution? Philos. Trans. R. Soc. London, Ser. B 365, 3149–3160 (2010).
Duputié, A., Rutschmann, A., Ronce, O. & Chuine, I. Phenological plasticity will not help all species adapt to climate change. Glob. Change Biol. 21, 3062–3073, 10.1111/gcb.12914 (2015).
Veen, B. General remarks on provenance research in forestry. Euphytica 3, 89–96 (1954).
Burley, J. Genetic Variation in Seedling Development of Sitka Spruce, Picea sitchensis (Bong). Carr. Forestry 39, 68–94 (1966).
Wang, C., Cao, R., Chen, J., Rao, Y. & Tang, Y. Temperature sensitivity of spring vegetation phenology correlates to within-spring warming speed over the Northern Hemisphere. Ecol. Indic. 50, 62–68 (2015).
Shen, M., Cong, N. & Cao, R. Temperature sensitivity as an explanation of the latitudinal pattern of green‐up date trend in Northern Hemisphere vegetation during 1982–2008. Int. J. Climatol. 35, 3707–3712 (2015).
Domínguez, C. A. & Dirzo, R. Rainfall and flowering synchrony in a tropical shrub: Variable selection on the flowering time of Erythroxylum havanense. Evol. Ecol. 9, 204–216 (1995).
Mduma, S. A., Sinclair, A. & Turkington, R. The role of rainfall and predators in determining synchrony in reproduction of savanna trees in Serengeti National Park, Tanzania. J. Ecol. 95, 184–196 (2007).
Shen, M., Tang, Y., Chen, J., Zhu, X. & Zheng, Y. Influences of temperature and precipitation before the growing season on spring phenology in grasslands of the central and eastern Qinghai-Tibetan Plateau. Agr Forest Meteorol 151, 1711–1722, 10.1016/j.agrformet.2011.07.003 (2011).
Shen, M., Piao, S., Cong, N., Zhang, G. & Jassens, I. A. Precipitation impacts on vegetation spring phenology on the Tibetan Plateau. Glob. Change Biol. 21, 3647–3656 (2015).
Kirtman, B. et al. Near-term Climate Change: Projections and Predictability. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [eds Stocker, T. F., D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex & P. M. Midgley ], pp. 984 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013).
Diez, J. M. et al. Forecasting phenology: from species variability to community patterns. Ecol. Lett. 15, 545–553, 10.1111/j.1461-0248.2012.01765.x (2012).
Hofstra, N., Haylock, M., New, M. & Jones, P. D. Testing E‐OBS European high‐resolution gridded data set of daily precipitation and surface temperature. J. Geophys. Res. 114, D21101, 10.1029/2009JD011799 (2009).
Wang, T. et al. The influence of local spring temperature variance on temperature sensitivity of spring phenology. Glob. Change Biol. 20, 1473–1480 (2014).
Shen, M. et al. Earlier-season vegetation has greater temperature sensitivity of spring phenology in Northern Hemisphere. PloS one 9, e88178 (2014).
Acknowledgements
This study is supported by 1) the Fund for Creative Research Groups of National Natural Science Foundation of China (No. 41321001), 2) the project of “early detection and prediction of climate warming based on the long-term monitoring of fragile ecosystems in the East Asia” funded by the Ministry of Environment, Japan and 3) State Key Laboratory of Earth Surface Processes and Resource Ecology under Grant 2015-TDZD-011.
Author information
Authors and Affiliations
Contributions
C.W., Y.T. and J.C. conceived and designed the study. C.W. performed the analysis. Y.T., C.W. and J.C. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wang, C., Tang, Y. & Chen, J. Plant phenological synchrony increases under rapid within-spring warming. Sci Rep 6, 25460 (2016). https://doi.org/10.1038/srep25460
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25460
This article is cited by
-
Pan European Phenological database (PEP725): a single point of access for European data
International Journal of Biometeorology (2018)
-
A high-density consensus linkage map of white lupin highlights synteny with narrow-leafed lupin and provides markers tagging key agronomic traits
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.