Abstract
Local inflammation in tissues is one of primary causes in development of metabolic disorder in obesity. The accumulation of macrophages in some tissues can induce inflammatory reactions in obesity. Gpr97 is highly expressed in some immunocytes, but its potential role in inflammatory regulation has not been revealed clearly. In our research, we investigated Gpr97 in regulating macrophage inflammation and metabolic dysfunction in the high-fat diet (HFD)-induced obese mice. The major metabolic phenotyping were not different after Gpr97 knockout in HFD-fed mice. Similar pathological alterations in adipose tissue, liver and kidney were observed in Gpr97−/− HFD mice compared with WT-HFD mice. In white adipose tissue, loss of Gpr97 reduced the ratio of M1-macrophages and increased the M2-macrophage ratio, which was opposite to that seen in the wild-type HFD mice. More macrophages invaded in the liver and kidney after Gpr97 knockout in HFD mice. Furthermore, the levels of TNF-α were higher in the liver and kidney of Gpr97−/− HFD mice compared to those in wild-type HFD mice. The data indicate that Gpr97 might be required for local inflammation development in obesity-relative tissues, but does not play a role in metabolic disorder in HFD-induced obesity.
Similar content being viewed by others
Introduction
In recent decades, the rate of obesity has increased, especially in Europe and other developed countries1,2. According to WHO statistics, the worldwide prevalence of obesity nearly doubled between 1980 and 2008 and obesity has become a serious public health problem3. Obesity is recognized as a severe metabolic disorder and is likely to be a risk factor for type 2 diabetes, fatty liver disease, chronic kidney diseases and some cancers4,5,6,7. However, there is evidence that obesity is also a kind of low-grade chronic inflammatory disease and there is a clear link between tissue inflammatory and metabolic responses in obesity8,9. Obese adipose tissue secretes more inflammatory cytokines, including TNF-α and IL-6, compared with lean tissue10,11. As in other inflammatory responses, obesity causes an increase in the infiltration of immune cells including macrophages, into key metabolic tissues, which would then be involved in the pathogenesis of metabolic syndrome12. In adipose tissue, liver, skeletal muscle and kidney, macrophage-associated local inflammatory response contributes to the development of obese metabolic dysfunction, such as insulin resistance and carbohydrate accumulation9,13. Furthermore, it is generally thought that obesity affects the balance of the M1/M2 macrophage ratio in these tissues. Furthermore, the imbalance of active macrophage phenotypes plays an important role in the pathogenesis of metabolic disorders. In adipose tissue, a high-fat diet (HFD) induces to enhance the population of M1-macrophages, which promotes the concentration of pro-inflammatory cytokines, while M2 anti-inflammatory macrophages are down-regulated8,14. During the process of HFD-induced obesity, the secreted inflammatory factors will attract multiple immune cells that will participate in this process, including T cells, B cells and mast cells. Then various activate inflammatory pathways were detected to find out the relationship between immune system and metabolic syndrome9.
Cytokines, immune cell activation and surface markers of immune cells have been key factors in studies of immune cell-mediated inflammation in HFD-induced metabolic dysfunction. Several G-protein-coupled receptors (GPCRs) have been identified as important regulators in obese metabolic disorder, such as GPR119 and GPR12015,16. GPR97, as well as GPR56 and GPR114, is a member of the adhesion GPCR family, whose encoding genes are all on the long arm of chromosome 1617. In mice, Gpr97 is only expressed in the hypothalamus, heart, kidney and especially highly on thymus, spleen and blood18. In immune cells, GPR97 is expressed on leukocytes, such as neutrophils, eosinophils and mast cells17. Recently, it was reported that the GPR97 plays a role in regulating B-cell fate19 and migration of lymphatic endothelial cells20. It was also reported that the expression of GPR97 was significantly down-regulated when using colony-stimulating factor-1 (CSF-1/M-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) to stimulate primary human macrophages differentiation from monocytes21, but its molecular mechanism in macrophage polarization and the related inflammatory response have not yet been reported. It seems that GPR97 might play an important role in immune system, but whether it also functions in inflammation during obesity has yet to be determined. Here we used Gpr97-knockout mice to elucidate whether Gpr97 has a role in macrophage-related inflammation and obesity-induced metabolic syndrome.
Results
Gpr97 is not involved in obesity and fasting blood glucose in HFD mice
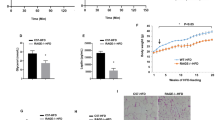
The mice were fed with 60% calories in fat for 16 weeks to induce the metabolic syndrome. Metabolic phenotype, including food intake, body weight and glucose tolerance, was examined in mice. The quantity of food intake and body weight was recorded once a month. According to the collected data, there was no obvious difference in food intake between WT and Gpr97−/− HFD-fed mice (Fig. 1a). Although HFD-fed mice were significantly heavier than chow-fed mice, especially after 12-weeks (P < 0.05), no alteration was shown in Gpr97-deficient HFD-fed mice (Fig. 1b). Moreover, we used an intraperitoneal glucose tolerance test (ipGTT) to examine glucose tolerance in our mice model. The fasting blood glucose of HFD mice was much higher than that of the chow-fed mice from 15 min (P < 0.01), but there was no change after loss of Gpr97 in HFD mice compared with WT HFD mice (Fig. 1c). These data indicate that Gpr97 deficiency does not influence the body weight or glucose tolerance of HFD mice.
The obese phenotype and fasting blood glucose of high-fat diet (HFD)-induced mice.
(a) Food intake. The average grams food intake in WT and Gpr97−/− mice each month. (n = 8) (b) Body weight. The growth of body weight in WT and Gpr97−/− mice by chow or HFD feeding. (n = 6–8) (c) Intraperitoneal glucose tolerance test (ipGTT). The levels of blood glucose were detected at 0, 15, 30, 45 and 60 minutes after intraperitoneal inject in chow or HFD fed mice (n = 8). Data are shown as means ± s.e.m. **0.01 < P < 0.05, ***P < 0.01.
Gpr97 does not play a role in HFD-induced metabolic syndrome
To determine whether Gpr97 impacts on HFD-induced metabolic syndrome, the levels of some metabolites in serum were measured in mice. The levels of cholesterol, glucose, high-density cholesterol lipoprotein (HDL) and low-density cholesterol lipoprotein (LDL) in serum were increased in both HFD-fed WT and Gpr97−/− mice. However, there was no significant alteration of these serum parameters after Gpr97 deficiency in HFD-fed mice (Fig. 2a–d). The levels of albumin, carbamide, creatinine, triglyceride and uric acid in serum were measured in mice. However, the HFD did not induce these indicators altering even in Gpr97−/− HFD mice (Fig. 2e–i). According to the metabolite analysis, Gpr97 is not involved in metabolic syndrome during HFD-induced obesity in mice.
Analysis of major metabolites in serum in mice.
(a) cholesterol, (b) glucose, (c) high-density lipoprotein (HDL), (d) low-density lipoprotein (LDL), (e) albumin, (f) carbamide, (g) creatinine, (h) triglyceride, (i) uric acid. Data are shown as means ± s.e.m. (n = 8). *P < 0.05, ** 0.01 < P < 0.05, ***P < 0.01.
Gpr97 is involved in the M1/M2 polarization of macrophages in white adipose tissue
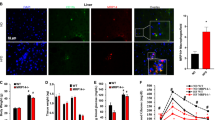
White adipose tissue (WAT), which is a critical regulator of adiposity and energy metabolism, has been found to undergo metabolic changes during a high-fat diet22. Adipocyte hypertrophy and hyperplasia occurs in HFD-induced obesity, including overgrowth of WAT23. In our results, hypertrophy of WAT was a typical change in HFD mice, but the morphology of WAT did not significantly alter between WT and Gpr97−/− HFD mice (Fig. 3a). It has been reported that the HFD will result in the accumulation of macrophage in WAT, which can lead to inflammation24. According to the alteration in the mRNA level of the macrophage marker F4/80, we found that Gpr97 deficiency can cause a reduction in macrophage invasion of WAT in HFD-fed mice (Fig. 3b). The general view of the inflammatory reactions in WAT is an imbalance in the ratio of M1/M2 macrophages24. We analyzed the relative mRNA expression of cytokines associated with M1- and M2-polarized macrophages to confirm the function of Gpr97 in M1/M2 polarization of WAT macrophages. The M1-polarized-related parameters TNF-α, IL-6, IL-1β and CD86 displayed a decrease in Gpr97−/− HFD mice compared to WT HFD mice (Fig. 3c–f). Meanwhile, the M2-polarized-related parameters, including CD68, CD163, CD206, Arg1 and IL-10 were increased in Gpr97−/− HFD mice (Fig. 3g–k). The accumulation of M1 “pro-inflammatory” macrophages and the reduction of M2 “anti-inflammatory” macrophages in white adipose tissue can lead to adipose tissue dysfunction24. We found that Gpr97 deficiency can reverse this imbalance of M1/M2 macrophages in WAT. In Gpr97−/− HFD mice, M2 macrophages were increased and M1 macrophages were decreased obviously, which indicate that Gpr97 might contribute to the local inflammatory reactions charged of M1-macrophages. In the obesity induced by HFD, loss of Gpr97 will cause opposite imbalance of M1/M2 and reduce levels of inflammatory cytokines in WAT of mice.
Detection of macrophages polarization in white adipose tissue (WAT) in HFD mice.
(a) Hematoxylin and eosin staining of WAT sections of WT and Gpr97−/− mice. Pictures were captured at 40× under optical microscope. (b) The expression of the relative factor associated with total macrophages F4/80 in mRNA levels in WAT. (c–f) The expression of relative factors in mRNA levels associated with M1-polarized macrophages in WAT. (g–k) The expression of relative factors associated with M2-polarized macrophages in WAT. Data are shown as means ± s.e.m. (n = 8). 0.01 < P < 0.05, ***P < 0.01.
Gpr97 enhances hepatic inflammation in HFD mice
The liver plays an important role in lipid metabolism and hepatic steatosis was measured in an obese animal model8. HFD not only introduced obesity, but was also involved in glycometabolism and lipid metabolism in the liver. In our HFD mice, we used the Oil Red O stain to visualize hepatic neutral lipid accumulation in liver sections. The accumulation of lipid was severe in the livers of mice fed the HFD, but this accumulation was not significantly different between WT and Gpr97−/− HFD mice (Fig. 4a). Next, we measured the metabolism of carbohydrate in the liver by periodic acid-Schiff (PAS) staining and found that there was more glycogen storage in the liver in HFD-fed mice. However, there was no difference between WT and Gpr97−/− mice (Fig. 4b). The loss of Gpr97, therefore, did not affect lipid or glycogen storage, or metabolic disorder, in the HFD-induced liver.
To determine the function of Gpr97 in the liver of HFD mice, we measured the mRNA expression of metabolic factors, including those involved in transcriptional regulators which influence pathways of fatty acid oxidation (FoxO1, Pparα, Pgc1α, Pgc1β, Errα, Prc and Atp5s) (Fig. 5a), mitochondrially encoded genes (ND1, ND2, ND4, ATP6, ATP8 and COX2) (Fig. 5b), fatty acid oxidation (Acox1, Fgf21), mitochondrial function (Cs, Atp5g1), lipogenesis (SREBP2, Fasn), glucose metabolism (PEPCK, G6Pase) (Fig. 5c) and inflammation (F4/80, CD68, TNF-α, IL-6 and IL-1β) (Fig. 5d) 25. These genes, which are involved in the major metabolic pathways and inflammatory reactions in the liver, showed slightly higher expression in Gpr97−/− mice than in WT mice under a HFD-fed. Gpr97 deficiency, therefore, will aggravate some key gene expression which are involved in hepatic metabolism such as transcriptional regulation in fatty acid oxidation and mitochondrial function. Moreover, the inflammatory factor TNF-α, IL-6 and IL-1β were increased after Gpr97 knockout in HFD-fed mice. The higher expression of F4/80 and CD68 indicates that more macrophages gathered in the liver of Gpr97−/− HFD mice and that the loss of Gpr97 may cause more serious inflammation and metabolic stress in the liver of obese mice.
Analysis of metabolite, transcriptional, inflammatory factors in mRNA levels in the livers of HFD-fed mice.
The expression of genes in mRNA levels which are associated with transcriptional regulators (a), mitochondrially encoded genes (b), fatty acid oxidation, hepatic mitochondrial function, lipogenesis, glucose metabolism (c) and inflammatory factors (d) in HFD mice. Data are shown as means ± s.e.m. (n = 8). *P < 0.05, ** 0.01 < P < 0.05.
Effect of Gpr97 on HFD-induced renal inflammation
HFD can also cause renal inflammation and hypofunction26. Renal structural changes were evaluated by histochemistry with the PAS stain after HFD. Clear and unbroken structures of renal sections were shown in both WT and Gpr97−/− chow-fed mice. There was also no interstitial fibrosis in the renal tubules or glomeruli. Mice fed the HFD exhibited renal injury, such as renal tubular epithelial cell cytoplasm vacuole degeneration, mesangial matrix expansion and increased renal interstitial cells (Fig. 6a). However, the absence of Gpr97 did not impact upon the HFD-induced renal injury. In HFD-fed mice, a high inflammation status would increase renal injury and lipid metabolic disorder and some inflammatory markers, such as TNF-α, IL-6 and MCP-1, were induced by the HFD27,28. Next, we measured the alterations in renal inflammatory cytokines in HFD mice after Gpr97 knockout. In Gpr97-deficient HFD mice, there were more macrophages invading the kidney according to the increase in F4/80 mRNA levels (Fig. 6b). Meanwhile, the inflammatory factor TNF-α was increased in Gpr97−/− HFD mice compared with that in WT HFD mice (Fig. 6c), but there was no significant change in IL-1β and CD86 after Gpr97 knockout in HFD mice (Fig. 6d,e). So Gpr97 did not affect ratio of M1-polarized macrophages in kidney of HFD-fed mice. Further, we detected markers of M2- polarized macrophages including CD68, CD163, CD206, Arg1 and IL-10 in kidney and found increasing of those in kidney after Gpr97 deficiency in HFD-mice (Fig. 6f–j). According to our results, Gpr97 might be involved in the renal macrophage-inflammatory reaction and macrophage invasion in HFD mice, but not in the imbalance in the ratio of M1/M2 macrophages.
The HFD-induced inflammation analysis in kidney in the WT and Gpr97−/− mice.
(a) The morphological evaluation of kidney by PAS staining after HFD fed in mice. (b) Detecting of macrophage makers F4/80 in mRNA level to determine invasion of macrophage into kidney in HFD mice. (c–e) The expression of relative markers in mRNA levels associated with M1-polarized macrophages in kidney of HFD-fed mice. (f–j) The analysis of markers of M2-polarized macrophages in mRNA level in kidney of HFD-fed mice. Data are shown as means ± s.e.m. (n = 8). *P < 0.05.
Discussion
Obesity is a chronic low-grade inflammatory disease29. Inflammation plays an important role in the pathological process of obesity. For obese rodents and humans, activated macrophages infiltrate metabolic tissues, including adipose tissue10,12, liver30,31 and kidney32 and such a large number of macrophages can cause serious local inflammation. Furthermore, they can impair the balance of the resident macrophage population and can affect the metabolic regulation function in these tissues9,33. However, how specific genes involved in the regulation of tissue-specific inflammation and metabolic function are induced by a HFD has rarely been reported. A family of GPCRs were found that have important roles in various physiological and pathological processes, such as inflammatory regulation34,35. The potential functions of Gpr97, which is an adhesion GPCR, are little known. Gpr97 has shown effects on B-lymphocyte development19 and migration of lymphatic endothelial cells20. Here we focused on the role of Gpr97 on tissue-specific inflammation in HFD-induced obese mice.
Firstly, we determined whether Gpr97 had a function in HFD-induced metabolic abnormalities. The general physiological parameters, the intraperitoneal glucose tolerance and metabolic characterization were examined in both WT and Gpr97−/− mice fed a chow diet or the HFD. Our results showed that the Gpr97−/− mice had almost equal levels of physiological parameters and serum parameters after the HFD. In the ipGTT test, there was also no significant difference between the wild-type and Gpr97-knockout obese mice (Figs 1 and 2). Therefore, Gpr97 might not protect from or sensitize to the development of HFD-induced obesity, glucose tolerance and systemic metabolic regulation in mice. Next, we focused on the function of Gpr97 in some important tissues involved in HFD-induced obesity.
WAT is regarded as an active metabolic tissue that can produce adipokines, as well as pro- or anti-inflammatory cytokines capable of regulating carbohydrate, lipid metabolism and local inflammation36. To respond to excess nutrients, WAT showed hypertrophy and hyperplasia in HFD-fed mice (Fig. 3a). It was reported that adipocyte size is related to insulin sensitivity and human with smaller adipocytes have a lower degree of inflammation37. Moreover, obesity can also induce macrophage invasion into WAT and can lead to WAT inflammation. The accumulated macrophages in WAT secrete pro-inflammatory factors to enhance metabolic dysfunctions38. Our results indicate that Gpr97 deficiency reduces macrophage invasion into WAT (Fig. 3b). The balance in the ratio of the macrophage population in WAT plays a key role in the development of HFD-induced obesity. In lean rodents, there are more M2-macrophges in adipose tissue, which are known to secrete anti-inflammatory cytokines39. In contrast, in obese animals, more activated macrophage and monocytes were recruited to the WAT, which turn into M1-macrophages. During this process, pro-inflammatory cytokines, including TNF-α and IL-1β, produced by M1-macrophages are capable of limiting glucose uptake in peripheral tissue36,40,41. Interestingly, we found that less macrophages invasion in the WAT and there was a reversed imbalance of ratio of M1/M2 macrophages in the WAT of obese Gpr97-knockout HFD-fed mice. This indicates that loss of Gpr97 will cause an M2-macrophage-related anti-inflammatory response to modulate healing repair process in WAT. Such evidence suggests that Gpr97 could modulate macrophage polarization during metabolic disorder and local inflammation in adipose tissue. However, further studies should be carried out to determine the potential mechanisms for macrophage polarization and the inflammatory reaction regulated by Gpr97 in the HFD-induced WAT.
Furthermore, for metabolic organs, the liver has a central position in the endocrine and metabolic system. For example, it plays a vital role in the metabolism of sugar, sugar storage and decomposition and regulation of blood glucose. Excessive lipogenesis contributes to the development of hyperglycemia and hepatic steatosis9,42. Lipid and glycogen accumulation occurred after 16 weeks of the HFD in both WT and Gpr97-knockout mice. Similar to previous results in white adipose tissue, Gpr97 may not be important enough to affect the hepatic phenotypic alterations seen during HFD-induced obesity. However, Gpr97 deficiency led to a slight increase in the expression of major metabolic genes involved in different physiological process of the liver. It was known that HFD causes hepatic lipid accumulation and that the complex and coordinated metabolic processes are also aggravated, including lipogenesis, fatty acid oxidation, glucose metabolism, mitochondrial function and metabolic transcriptional regulation in the liver25,43,44,45,46. According to our results, loss of Gpr97 might slightly increase these metabolic programs in mRNA levels. Meanwhile, inflammatory cytokines including TNF-α, IL-6 and IL-1β and number of macrophages were increased sharply in the liver after Gpr97 knockout in HFD mice. In summary, Gpr97 may participate in the regulation of expression of metabolic factors and the macrophage-related inflammatory response in liver and the loss of Gpr97 may cause more serious hepatic dysfunction and inflammation. But how Gpr97 regulates altering of these factors in HFD-induced obesity should be further investigated.
Moreover, obesity is a potential risk factor for kidney disease in which fibrosis and inflammation play important roles. In HFD-fed mice, renal interstitial fibrosis and inflammatory cells were increased, which may develop into diabetes even diabetic nephropathy26. Whether Gpr97 contributes to the process is being considered. Our results indicate that Gpr97 did not affect the structural changes seen in the kidney during a HFD in mice (Fig. 6a). However, Gpr97 seems to play an underlying role in the regulation of macrophage infiltration in HFD-induced renal inflammation. It was reported that macrophages can also be activated by a HFD and can infiltrated into the kidney47. After the loss of Gpr97, more macrophages invaded into the kidney and the pro-inflammatory factor TNF-α was slightly increased in HFD-fed mice. Then we detected the altering of ratio of M1 and M2-related macrophages in kidney. It was found that more number of M2 macrophages and no change of ratio of M1 macrophages in kidney after Gpr97 knockout in HFD-fed mice. Therefore, we propose that Gpr97 is also involved in macrophage-related inflammation but not involved in macrophage polarization in kidney. How Gpr97 affects renal macrophage-related inflammatory response should be investigated further to reveal the potential regulatory mechanisms of Gpr97 in HFD-induced obesity.
In summary, we report the potential role of Gpr97 in metabolic syndrome and inflammation in HFD-fed mice. No phenotypic change was observed between wild-type and Gpr97−/− HFD-fed mice, which indicates that Gpr97 does not play a role in the phenotypic development and metabolic homeostasis under such extreme conditions. However, further studies demonstrated that the absence of Gpr97 could recruit more macrophages into metabolic organs, including the liver and kidney. Then it would lead to initiation of tissue-specific macrophage-inflammation and promotion of metabolic regulation. Further, there was consistent evidence to suggest that Gpr97 was involved in macrophage polarization in WAT in HFD-induced obesity in mice. However, more evidence should be found to explain the mechanism of inflammation regulated by Gpr97 in the process of obesity induced by HFD.
Methods
Animals and diet
Six-week-old male C57BL/6 mice (Shanghai Laboratory Animal Company, China) and Gpr97−/− C57BL/6 mice were housed in the Laboratory Animal Center of East China Normal University. Gpr97−/− mice were donated by the Dr. Zhugang Wang Laboratory (Shanghai Research Center for Model Organisms, Shanghai, China). Mice were raised in a regulated temperature (22 ± 2 °C) with a 12-h light/12-h dark cycle and free access to food and water. Animals were fed a standard chow diet or a high-fat diet for 16 weeks. The blood glucose concentration, body weight, food intake and water intake were measured once a week to monitor the general changes during the whole feeding process. Before experiments, the overnight fasted mice were sacrificed by dislocation of the vertebrae to obtain tissues and blood samples. Liver and kidney tissues were weighed and kept at −80 °C for subsequent analysis. All animal experiments conformed to the regulations drafted by the Association for Assessment and Accreditation of Laboratory Animal Care in Shanghai and were approved by the East China Normal University Center for Animal Research.
Intraperitoneal glucose tolerance test
At the end of the feeding phase, an intraperitoneal glucose tolerance test (ipGTT) was performed on mice that had been fasted for 12 hours. Mice were intraperitoneally injected with glucose (1 g/kg) and then blood glucose concentration from vein blood were measured at 0, 15, 30, 45 and 60 minutes using ACCU-CHEK Active (Roche, Switzerland).
Blood biochemical indicator detection
Mice were fed with the high-fat diet or chow diet for 16 weeks to detect blood biochemical indicators. Samples were collected from the 16–18 h fasted mice after sacrifice and then serum was obtained by centrifuging at 3000 g for 10 min at 4 °C. The serum was used to determine the levels of some major biochemical indicators, such as triglyceride (TG), cholesterol (CHO) and the serum lipid profile. The test of blood metabolites was conducted by ADICON Clinical Laboratory, Inc. (Shanghai, China).
Tissue staining
Tissues, including epididymal fat tissue, liver and kidney, from all sacrificed mice were collected and fixed in 4% paraformaldehyde overnight. Samples were incubated in graded ethanol, followed by embedding in paraffin. Six-μm sections, which were obtained by using a slicer (Leica, Germany), were stained with periodic acid-Schiff (PAS) stain for pathological examination48. The slices were incubated in periodic acid alcohol before PAS staining and then were washed with sulfurous acid, followed by nucleolus staining using hematoxylin and eosin and mounting in glycerol.
Alternatively, fixed tissues were dehydrated in gradient sucrose solution, frozen in optimal cutting temperature (OCT) compound (SAKURA Tissue-Tek, USA). Ten-μm sections were obtained with frozen section (Leica, Germany) at −10 °C and stained with Oil Red O (Sigma, USA) to observe the lipid accumulation.
RNA isolation and reverse transcription
Total RNA was isolated from murine liver, adipose tissues and kidney using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. cDNA was reverse transcribed from RNA using the PrimeScript RT Master Mix Kit (TaKaRa, Japan), which was used as the template for the following real-time PCR reaction.
Real-time PCR
Quantitative real-time PCR was performed on a Stratagene Mx3005P Real-time PCR System (Agilent Technologies, USA), using SYBR Premix Ex Taq™ (TaKaRa, Japan) and following the manufacturer’s instructions. Transcript levels of the housekeeping gene β-actin in the each sample were used for normalization. The gene-specific primer sequences are listed in Table 1.
Statistical analysis
All the experimental data are presented as means ± s.e.m. Statistical significance was assessed using a two-tailed unpaired Student’s t test or one-way analysis of variance (ANOVA) using SPSS 20.0 software (IBM). Statistical significance was considered to be at P < 0.05.
Additional Information
How to cite this article: Shi, J. et al. Gpr97 is dispensable for metabolic syndrome but is involved in macrophage inflammation in high-fat diet-induced obesity in mice. Sci. Rep. 6, 24649; 10.1038/srep24649 (2016).
References
Malik, V. S., Willett, W. C. & Hu, F. B. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 9, 13–27, 10.1038/nrendo.2012.199 (2013).
Berghofer, A. et al. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 8, 200, 10.1186/1471-2458-8-200 (2008).
World Health Organization. Global Health Observatory data. Obestiy: situation and trends (2008) Available at: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/. (Accessed: 8th October 2015).
Danaei, G. et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle and metabolic risk factors. Plos Med 6, e1000058, 10.1371/journal.pmed.1000058 (2009).
Yilmaz, Y. & Younossi, Z. M. Obesity-associated nonalcoholic fatty liver disease. Clin Liver Dis 18, 19–31, 10.1016/j.cld.2013.09.018 (2014).
Khandekar, M. J., Cohen, P. & Spiegelman, B. M. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 11, 886–895 (2011).
Mathew, A. V., Okada, S. & Sharma, K. Obesity related kidney disease. Curr Diabetes Rev 7, 41–49 (2011).
Kraakman, M. J., Murphy, A. J., Jandeleit-Dahm, K. & Kammoun, H. L. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol 5, 470, 10.3389/fimmu.2014.00470 (2014).
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 18, 363–374, 10.1038/nm.2627 (2012).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112, 1821–1830, 10.1172/jci19451 (2003).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808, 10.1172/JCI19246 (2003).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation and insulin resistance. Annu Rev Physiol 72, 219–246, 10.1146/annurev-physiol-021909-135846 (2010).
Fujisaka, S. et al. Regulatory Mechanisms for Adipose Tissue M1 and M2 Macrophages in Diet-Induced Obese Mice. Diabetes 58, 2574–2582, 10.2337/db08-1475 (2009).
Ichimura, A. et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354, 10.1038/nature10798 (2012).
Zhang, S. et al. Molecular matchmaking between the popular weight-loss herb Hoodia gordonii and GPR119, a potential drug target for metabolic disorder. Proc Natl Acad Sci USA 111, 14571–14576, 10.1073/pnas.1324130111 (2014).
Peng, Y. M. et al. Specific expression of GPR56 by human cytotoxic lymphocytes. J Leukoc Biol 90, 735–740, 10.1189/jlb.0211092 (2011).
Haitina, T. et al. Expression profile of the entire family of Adhesion G protein-coupled receptors in mouse and rat. BMC Neurosci 9, 43, 10.1186/1471-2202-9-43 (2008).
Wang, J. J. et al. Gpr97 is essential for the follicular versus marginal zone B-lymphocyte fate decision. Cell Death Dis 4, e853, 10.1038/cddis.2013.346 (2013).
Valtcheva, N., Primorac, A., Jurisic, G., Hollmen, M. & Detmar, M. The orphan adhesion G protein-coupled receptor GPR97 regulates migration of lymphatic endothelial cells via the small GTPases RhoA and Cdc42. J Biol Chem 288, 35736–35748, 10.1074/jbc.M113.512954 (2013).
Hohenhaus, D. M. et al. An mRNA atlas of G protein-coupled receptor expression during primary human monocyte/macrophage differentiation and lipopolysaccharide-mediated activation identifies targetable candidate regulators of inflammation. Immunobiology 218, 1345–1353, 10.1016/j.imbio.2013.07.001 (2013).
Amengual-Cladera, E., Llado, I., Proenza, A. M. & Gianotti, M. High-fat diet feeding induces a depot-dependent response on the pro-inflammatory state and mitochondrial function of gonadal white adipose tissue. Br J Nutr 109, 413–424, 10.1017/S0007114512001171 (2013).
Daquinag, A. C. et al. Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death Differ 22, 351–363, 10.1038/cdd.2014.148 (2015).
Surmi, B. K., Webb, C. D., Ristau, A. C. & Hasty, A. H. Absence of macrophage inflammatory protein-1{alpha} does not impact macrophage accumulation in adipose tissue of diet-induced obese mice. Am J Physiol Endocrinol Metab 299, E437–445, 10.1152/ajpendo.00050.2010 (2010).
Yu, J. et al. Metabolic characterization of a Sirt5 deficient mouse model. Sci Rep 3, 2806, 10.1038/srep02806 (2013).
Wang, Y., Lin, C., Ren, Q., Liu, Y. & Yang, X. Astragaloside effect on TGF-beta1, SMAD2/3 and alpha-SMA expression in the kidney tissues of diabetic KKAy mice. Int J Clin Exp Pathol 8, 6828–6834 (2015).
Ahad, A., Ganai, A. A., Mujeeb, M. & Siddiqui, W. A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol 279, 1–7, 10.1016/j.taap.2014.05.007 (2014).
Zheng, Y. et al. Anti-Inflammatory Effects of Ang-(1-7) in Ameliorating HFD-Induced Renal Injury through LDLr-SREBP2-SCAP Pathway. Plos One 10, e0136187, 10.1371/journal.pone.0136187 (2015).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17, 179–188, 10.1038/nm.2279 (2011).
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 7, 496–507, 10.1016/j.cmet.2008.04.003 (2008).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11, 183–190, 10.1038/nm1166 (2005).
Navarro-Gonzalez, J. F., Mora-Fernandez, C., de Fuentes, M. M. & Garcia-Perez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7, 327–340 (2011).
Qatanani, M. & Lazar, M. A. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 21, 1443–1455, 10.1101/gad.1550907 (2007).
Zalewska, M., Siara, M. & Sajewicz, W. G protein-coupled receptors: abnormalities in signal transmission, disease states and pharmacotherapy. Acta Pol Pharm 71, 229–243 (2014).
Alexander, S. P. H., Mathie, A. & Peters, J. A. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol 164, S1–S2, 10.1111/j.1476-5381.2011.01649_1.x (2011).
Grant, R. W. & Dixit, V. D. Adipose tissue as an immunological organ. Obesity (Silver Spring), 10.1002/oby.21003 (2015).
Arner, E. et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59, 105–109, 10.2337/db09-0942 (2010).
Ruggiero, C., Ehrenshaft, M., Cleland, E. & Stadler, K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab 300, E1047–1058, 10.1152/ajpendo.00666.2010 (2011).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117, 175–184, 10.1172/jci29881 (2007).
Liu, L. et al. LRP130 protein remodels mitochondria and stimulates fatty acid oxidation. J Biol Chem 286, 41253–41264, 10.1074/jbc.M111.276121 (2011).
Akie, T. E., Liu, L., Nam, M., Lei, S. & Cooper, M. P. OXPHOS-Mediated Induction of NAD+ Promotes Complete Oxidation of Fatty Acids and Interdicts Non-Alcoholic Fatty Liver Disease. Plos One 10, e0125617, 10.1371/journal.pone.0125617 (2015).
Obstfeld, A. E. et al. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59, 916–925, 10.2337/db09-1403 (2010).
Zhang, Y. L. et al. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia and hepatic steatosis. J Biol Chem 281, 37603–37615, 10.1074/jbc.M604709200 (2006).
Matsumoto, M., Han, S., Kitamura, T. & Accili, D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest 116, 2464–2472, 10.1172/JCI27047 (2006).
Bonzo, J. A. et al. Hepatic sirtuin 1 is dispensable for fibrate-induced peroxisome proliferator-activated receptor-alpha function in vivo. Am J Physiol Endocrinol Metab 306, E824–837, 10.1152/ajpendo.00175.2013 (2014).
Xu, F. et al. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/− mice: a role of lipid mobilization and inflammation. Endocrinology 151, 2504–2514, 10.1210/en.2009-1013 (2010).
Decleves, A. E. et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int 85, 611–623, 10.1038/ki.2013.462 (2014).
Sangeetha, M. K., Balaji Raghavendran, H. R., Gayathri, V. & Vasanthi, H. R. Tinospora cordifolia attenuates oxidative stress and distorted carbohydrate metabolism in experimentally induced type 2 diabetes in rats. J Nat Med 65, 544–550, 10.1007/s11418-011-0538-6 (2011).
Acknowledgements
This work was supported by National Basic Research Program of China (2012CB910404); National Natural Science Foundation of China (grants 81272369, 81172816, 31470040, 31000346); Doctoral Fund of Ministry of Education of China (20130076110013); the Shanghai Committee of Science and Technology, China (15JC1401500, 14140904200, 15ZR1411100).
Author information
Authors and Affiliations
Contributions
H.R. and M.Q. directed this research work. J.S. and X.Z. performed most experiments. S.W. measured the serum biochemical indicators. J.W. and Z.W. prepared the Gpr97 deficient mouse. W.J. determined the construction of diet-induced mouse model. J.S. and H.R. wrote the manuscript. B.D. and M.L. revised and finalized this manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shi, J., Zhang, X., Wang, S. et al. Gpr97 is dispensable for metabolic syndrome but is involved in macrophage inflammation in high-fat diet-induced obesity in mice. Sci Rep 6, 24649 (2016). https://doi.org/10.1038/srep24649
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24649
This article is cited by
-
The Roles of Orphan G Protein-Coupled Receptors in Autoimmune Diseases
Clinical Reviews in Allergy & Immunology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.