Abstract
Tuberculosis caused by Mycobacterium tuberculosis is a global encumbrance and it is estimated that nearly one third population of the world acts as a reservoir for this pathogen without any symptoms. In this study, we attempted to characterise one of the genes of DosR regulon, Rv3131, a FMN binding nitroreductase domain containing protein, for its ability to alter cytokine profile, an essential feature of M. tuberculosis latency. Recombinant Rv3131 stimulated pro-inflammatory cytokines in THP-1 cells and human peripheral blood mononuclear cells in a time and dose dependent manner. In silico analyses using docking and simulations indicated that Rv3131 could strongly interact with TLR2 via a non-covalent bonding which was further confirmed using cell based colorimetric assay. In THP-1 cells treated with Rv3131 protein, a significant upsurge in the surface expression, overall induction and expression of mRNA of TLR2 was observed when analysed by flow cytometry, western blotting and real time PCR, respectively. Activation of TLR2 by Rv3131 resulted in the phosphorylation of NF- κβ. Results of this study indicate a strong immunogenic capability of Rv3131 elicited via the activation of TLR2 signalling pathway. Therefore, it can be surmised that cytokine secretion induced by Rv3131 might contribute to establishment of M. tuberculosis in the granulomas.
Similar content being viewed by others
Introduction
Tuberculosis remains a global burden and is accountable for elevated morbidity and mortality. Though individuals remain asymptomatic, in most cases M. tuberculosis remains in a latent stage. Then, the bacilli unleash their responses inside a specialised structure called granuloma; the microenvironment of the granulomas is characterized by hypoxia and elevated nitric oxide1. M. tuberculosis survives in this unreceptive milieu by up-regulating a set of 48 genes of the dormancy survival regulon (DosR), whose protein products regulate a variety of physiological processes of the bacterium. A hallmark of M. tuberculosis infection is the organism’s capability to rapidly acclimatise to altering environments since the cellular and biochemical dynamics of the granuloma are reliant on the oxygen tension2. Alternatively, during reactivation, M. tuberculosis adjusts to the aerobic conditions by switching on its metabolic activity. Under both these conditions, the DosR dependent regulation of DosR regulon member genes plays a critical role3. The protein products encoded by DosR genes can elicit humoral responses in the host, especially in patients with latent infection4. During latency, granuloma formation and its conservation requires production of pro-inflammatory cytokines and reactive oxygen intermediates5. These responses are instigated by the interaction of M. tuberculosis components with TLR1/TLR6 and TLR4 (Toll-like receptors) of macrophages6.
TLRs, also known as pattern recognition receptors are responsible for the detection of specific molecular components on the surface of the pathogens to induce immunologic responses. The cell wall components of M. tuberculosis, namely peptidoglycans, interact with TLR2 and influence expression of CD25, CD69, NKp44 and IFN-γ production in NK cells7. The lipid fractions of three different strains of M. tuberculosis elicited TLR2, cytokines and MHC class II expression in human macrophages8. Further, mycobacterial lipoprotein interaction results in TLR2 mediated apoptosis of T-cells9. Mycobacterial lipoarabinomannan mediated interaction between TLR1 and TLR2 has been reported10. Other TLRs on the macrophage surface were also found to be recognising mycobacterial proteins and lipids11. Such recognition results in the production of pro and anti-inflammatory cytokines, which in turn dictates macrophage effector functions12. A majority of the studies hitherto remained focused on the interaction of mycobacterial cell wall components and TLRs. However, such interactions between the proteins encoded by the DosR genes and TLRs are not very well studied.
Proteins encoded by the DosR regulon genes elicit immunomodulatory responses in the host. Some of the DosR antigens that were found to be immunodominant include alpha-cystallin 2 (Acr2; Rv0251)13, the alpha-crystallin homologue (also called 16-kDa protein; Rv2031c, HspX)14, Ag85A (Rv3804)15, Hsp65 (Rv0440)16, ESAT-6 (Rv3875)17 and CFP10 (Rv3874)18. Using mouse models and case controlled human studies in geographically diverse locations, the immunogenicity elicited by DosR regulon genes in latent infection was established4,19,20,21. Due to their immune-dominant nature they were considered to be potential candidates for the development of TB vaccines22. We previously reported and characterised the Dormancy Associated Translation Inhibitor (Rv0079/ DATIN) of M. tuberculosis, a protein coded by the DosR gene Rv0079 and which was shown to be potentially immunogenic and leading to secretion of cytokines in macrophages and PBMCs; the immunomodulatory activity of DATIN was due to its interaction with TLR223,27. Such interactions for other DosR antigens are not yet deciphered.
Rv3131, a hitherto uncharacterised member of the DosR regulon codes for a nitroreductase that might be involved in detoxification of nitrogen by-products in the host24. Nitroreductase genes, acg (Rv2032), Rv3131 and Rv3127 were predicted to guard against the nitrogen stress25. Rv3131, expressed robustly under stress, was found to contain two DevR/DosR binding sites24. The transcription of Rv3131 encompasses binding of the DosR to the promoter in a cooperative manner24. Besides induction under hypoxic conditions, Rv3131 was also found to be antigenic26. In an earlier study comprising an African population, Rv3131 was found to be one of the most potent inducers of cytokine production among a set of seven classical and fifty one DosR regulon-encoded M. tuberculosis recombinant protein antigens19.
Though the immunogenic property of most of the proteins encoded by the DosR genes was characterised extensively, the mechanisms by which they elicit this response are not yet fully understood. It is possible that the modulation of immune responses may be different for each of them or certain set of DosR antigens may use a common mechanism of action. Thus, it is essential to understand the molecular mechanisms involved in the immunogenic properties of the DosR regulon antigens. Except for the induction under stress conditions and the antigenic property, information on the role of Rv3131 in mycobacterial latency, manipulation of host responses and the molecular machinery used for its action was not yet deciphered. Characterising the antigenic nature of this protein and its contribution to the survival of M. tuberculosis under latency might facilitate novel strategies that can be directed in boosting the host’s immune system or attenuation of survival of M. tuberculosis in the granulomas, respectively. In this study, we endeavoured to analyse the ability of recombinant Rv3131 to elicit cytokine response and its interaction with TLR2/4 and the ensuing downstream signalling.
Methodology
Molecular cloning, expression and purification of Rv3131 gene
Rv3131 was cloned as described previously with slight modifications27. Briefly, Rv3131 gene was amplified (supplementary information: Figure S1) using H37Rv DNA as a template and cloned into pET28a plasmid between BamHI and Hind III restriction enzyme sites. The plasmid was then transformed in to E.coli BL21 (DE3) and the recombinant protein was induced with 0.5 mM IPTG (Sigma Aldrich, USA) for 4 hours in LB broth containing Kanamycin 50 μg/ml. Protein extraction was carried out as described earlier27. The purity of recombinant Rv3131 (rRv3131) was checked by electrophoresing different fractions of the eluates on SDS gels. Rv3131 resolved as a single band and the purity of the protein as visualised was more than 95%. Endotoxin contamination was removed by incubating the protein with polymyxin-B agarose (Sigma, MO, USA). Limulus amebocyte lysate assay (Pierce™ LAL Chromogenic Endotoxin Quantitation Kit, ThermoFisher, USA) was used to measure the endotoxin content of the recombinant protein. Endotoxin contamination was undetectable in the protein fractions incubated with Polymyxin-B agarose.
Quantification of cytokines from culture supernatants of THP-1 cells and PBMCs
Isolation of peripheral blood mononuclear cells (PBMCs) from human blood was performed using Ficoll (GE Healthcare, USA) gradient method and 1 million PBMCs were seeded into each well suspended in 1 ml of RPMI (Invitrogen Life technologies, CA, USA) with 10% Fetal Bovine Serum (FBS) (Invitrogen Life technologies, CA, USA) and incubated overnight. Blood samples for isolation of PBMCs were collected from volunteers who appeared healthy and reported no history of tuberculosis. The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The study was approved by Ethics Committee of University of Hyderabad. An educated and written informed consent was obtained from all the subjects before obtaining the blood samples. THP-1 cells obtained from National Centre for Cell Science, Pune, India, were seeded (approximately 0.2 million per well) in 24 well plate and treated overnight with 10 ng/ml Phorbol 12-myristate 13-acetate (PMA) (Sigma, MO, USA) to allow differentiation into macrophage-like phenotype. THP-1 cells / PBMCs were treated with various concentrations of recombinant Rv3131 protein (rRv3131) (10, 100 and 1000 ng/ml) or LPS (100 ng/ml; positive control) (Sigma, USA). Cells treated with Proteinase K digested rRv3131 were used as negative control. After the treatment, supernatants were collected at 24 and 48 hrs time points and stored in −80 °C. Quantification of pro-inflammatory cytokines such as IL-1β, IL-8, TNF-α and IFN-γ were performed using Elisa- Set Go eBiosciences (CA, USA) kit following manufacturer’s instructions.
Gene expression by reverse transcription PCR analysis
Differentiated THP-1 cells were treated with or without rRv3131 protein and total RNA was extracted using Trizol (Sigma, USA). 2 μg of DNase (sigma) treated RNA was converted into cDNA by SuperScriptIII reverse transcriptase (Invitrogen, USA) following recommendations of the manufacturer. Real-time PCR was carried out on a realplex machine (Eppendorf, Hamburg, Germany), using SYBR Green SuperMix (Clontech, USA) as described earlier28. Gene specific primers used in this study are mentioned in table 1. Mean fold changes in various cytokines were analysed using the ΔΔCT method. Peptidylprolyl isomerase B (PPIB) and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) were used as housekeeping genes for cytokines and TLR2 / TLR4 respectively.
Protein sequence analysis of Rv3131 and docking study with TLR2
Amino acid sequence of Rv3131 (332 residues) was retrieved from Mycobacterium tuberculosis Database (http://tuberculist.epfl.ch/) and subjected to Pfam29 and TOPCONS30 for sequence analysis. The program BLAST-P has been used to detect similar crystallographic protein structures of Rv3131 and the template structures were formerly downloaded from RCSB Protein Data Bank (PDB). Nitroreductase proteins from Mycobacterium smegmatis (PDB ID: 2YMV) and Streptococcus pneumonia (PDB ID: 2B67) were used as templates to perform homology modelling using Modeller9.1531. Further, the geometry of generated model was checked and analysed using PROCHECK32. PyMOL program was used for molecular visualization and MetaPPISP33 program was employed to predict the protein–protein interaction site. Simultaneously, for protein–protein docking studies, the web version of PatchDock34 was executed and further improvement and ranking was done using FireDock35. Docking studies were done under default complex-type settings using Rv3131 model (ligand) and the crystal structure of TLR2 (PDB ID: 2Z82) was obtained from Protein Data Bank (receptor).
TLR2/TLR4 interaction assay
Interaction of Rv3131 with TLR2/TLR4 was analysed using HEK-Blue 293 hTLR-2 and hTLR-4 engineered cell lines (HEK-Blue™-hTLR-2/hTLR-4, InvivoGen, San Diego, US) which are co-transfected with TLR2/TLR4 and SEAP reporter gene (secreted embryonic alkaline phosphatase). Stimulation of TLR2/TLR4 ligands activates NF-κB and AP-1 which in turn induces the production of SEAP that can be readily assessed with HEK-Blue™ detection medium (with colour change from pink to purple) by measuring spectrophotometrically36. Different concentrations of rRv3131 (10–200 ng) was added to approximately 50,000 HEK-Blue 293 hTLR2 and 25,000 HEK-Blue 293 hTLR4 cells respectively. Another His-tagged recombinant protein, Rab5 was used as negative control and Mycoplasma salivarium derived synthetic lipoprotein FSL-1 (10 ng) (Sigma, USA) and LPS (100 ng) were used as positive controls. The intensity of colour formation was measured in an ELISA plate reader (Infinite® 200 PRO - Tecan) at 620 nm.
Flow Cytometry analysis
Surface expression of TLR2/TLR4 was determined by using differentiated THP-1 cells treated with various concentrations of rRv3131. Cells were harvested after a period of 24 hrs and incubated with goat anti-human TLR2 (2.5 μg/million cells) and goat anti-human TLR4 (R&D, MN, USA) or isotype-matched anti-goat mouse IgG antibodies for 60 min at 4 °C followed by incubation with donkey anti-goat IgG conjugated with FITC (0.5 μg/million cells) (Santa cruz, CA, USA) at 4 °C for another 45 min. Finally, cells were washed and re-suspended in 1× PBS containing 2% FBS. Fluorescence intensity was measured using BD FACS Canto II flow cytometer (BD Biosciences, NJ, USA). Untreated and stained cells were taken as negative control in both the assays. The data were analysed by Flow Jo software (Tree Star Inc., USA).
Western blot analysis
The total protein was extracted from rRv3131 (1000 ng/ml) treated and untreated THP-1 cells. Trichostatin A (TSA) (250 ng) and LPS (100 ng/ml) were included as positive controls for checking TLR2 and NF-κβ activities, respectively. Untreated cells served as negative control. In brief, 1 × 106 (TLR2) or 6 × 106 (NF-κβ) cells were washed with cold 1× PBS and pelleted by centrifugation at 400 g for 5 min. Cell pellets were treated with ice cold lysis buffer (0.1% NP-40 in 2 mM EDTA, 200 mM Tris–HCl, pH, 7.5, 250 mM NaCl, 100 mM PMSF, 1 M DTT and protease inhibitor cocktail (Sigma, USA) by repeated pipetting at 4 °C. Supernatants, were collected after centrifugation at 13,000 g for 5 min and protein concentrations were analysed. Equal amounts of proteins were loaded and transferred on to a PVDF membrane at 4 °C. Subsequently, the membranes blocked with 4% BSA in TBST (137 mM NaCl, 20 mM Tris pH 7.6 and 0.05% Tween-20) for 3 h at room temperature and later incubated with primary antibodies against TLR2, p65 (NF-κβ) and β-actin antibodies (Santa Cruz Biotechnology Inc., USA). PVDF membranes were perceived by chemiluminescence (ECL). The band intensity in TLR2 assay was quantified densitometrically by Image J software37 and normalized to β-actin.
Statistical analysis
For all the experiments, wherever required, Student’s t-test and one way ANOVA was executed for the analysis of the results. The data were represented as the mean of triplicates ± SEM. p < 0.05 was considered as significant. Data analysis was carried out using GraphPad Prism 5 software.
Results
Rv3131 induces pro-inflammatory cytokines
To determine whether recombinant Rv3131can induce pro-inflammatory responses, THP-1 cells and human PBMCs were treated with varying concentrations of this protein and the secreted cytokines (IL-1β, IFN-γ, TNF-α and IL-8) were measured in the supernatants. The positive control, LPS (100 ng) induced the production of these cytokines at similar conditions tested. Treatment of PBMCs with rRv3131 resulted in increased production of IFN-γ, TNF-α, IL-1 β and IL-8 in a dose dependent manner (Fig. 1). Similarly in the THP-1 cells rRv3131 was observed to induce TNF-α, IL-1 β and IL-8 production in a time and dose dependent manner (Fig. 2A). The negative control (rRv3131 digested with proteinase K) did not affect the cytokine production. In order to analyse whether the increased production of pro-inflammatory cytokines in THP-1 cells after rRv3131 treatment was due to modulation at the transcriptional level, real-time PCR was performed. Significant increase in the mRNA levels of IL-8 and TNF-α was observed (Fig. 2B).
Rv3131 stimulates the secretion of pro-inflammatory cytokines in PBMCs.
Isolated PBMCs from human blood were treated with 10–2500 ng/ml of rRv3131 or LPS (100 ng/ml) or proteinase K digested Rv3131 protein for 24 h. The levels of secreted cytokines like IL-1β, IFN-γ, TNF-α and IL-8 were quantified by ELISA. Data represent the mean ± SEM of three experimental replicates.
(A) Rv3131 stimulates the secretion of pro-inflammatory cytokines in THP-1 cells. THP-1 cells differentiated by PMA were stimulated with 10–2500 ng/ml of rRv3131 or LPS (100 ng/ml) or proteinase K digested rRv3131 for 24 and 48 h. Supernatants were collected and the levels of secreted cytokines were estimated by ELISA. Data represent the mean ± SEM of three technical replicates. *p < 0.05; **p < 0.005; ***p < 0.0005. (B) Cytokine gene expression: relative expression of TNF-α and IL-8 in THP-1 cell lines treated with rRv3131 (1000 ng) and LPS (100 ng) was evaluated by quantitative real time-PCR (qRT-PCR). PPIB was used as endogenous control for normalization. The data represent mean ± SEM of triplicates.
Protein sequence analysis of Rv3131 and docking study with TLR2
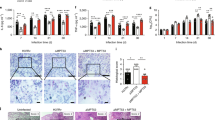
Computational analysis demonstrated that Rv3131 possessed conserved nitroreductase domain spanning 236–301 residues, while TOPCONS suggested the lack of signal peptide or transmembrane region in this protein. As crystallographic/solution structure of this protein is not available, BLASTp was used to find the structural templates and a template (PDB ID: 2YMV, nitroreductase from Mycobacterium smegmatis) with 37% identity was retrieved from PDB data bank. Modeller was used to generate structures using single template and the best structure was selected on the basis of different parameters including low DOPE score. To analyse the quality of structure, Ramachandran plot was generated and it revealed two residues (L327 and L328) in disallowed region. To improve quality of the structure, modelling was performed further with two templates i.e. PDB ID: 2YMV and 2B67. The quality of the structure was evaluated using Ramachandran plot acquired via Procheck and results of the validated model were as follows: most favoured regions contained 89.9%; additional allowed regions had 9.4%; generously allowed regions had 0.7% and disallowed regions contained 0.0% residues. Secondary structure of modelled Rv3131 protein revealed fourteen alpha helices and eight beta sheets (Fig. 3A). Identification of possible binding interface residues was performed using MetaPPISP programme. Protein-protein docking was performed with receptor as TLR2 and ligand as Rv3131 model using different online servers including GRAMM-X38 and PatchDock. Among the used programmes, Patchdock provided several solutions based on shape complementarity criteria, where Rv3131 was placed besides TLR2. Out of obtained complexes, best 10 were further subjected to FireDock to refine the solutions based on global energy. However, complex 7 was evolved as the best solution for residues interaction between the two proteins. As shown in Fig. 3B, Rv3131 emanates close to TLR2 and shows strong interaction due to the presence of several non-covalent bonds between them.
Modelled 3D-structure of Rv3131 and its interaction with TLR2.
(A) Three dimensional structure of Rv3131 determined using homology modelling: the elements of protein secondary structure were coloured and labelled (helices and sheets displayed in cyan and pink, respectively). (B) Docking study of Rv3131 with TLR2: the residues of TLR2 and Rv3131 are coloured in green and cyan, respectively. The residues presenting interaction among both the proteins are labelled and shown as stick model in element colours (green/cyan colour represents carbon, blue represents nitrogen and red represents oxygen). The black dashed lines represent hydrogen bonds. (C) Rv3131 interacts with TLR2: HEK-Blue 293 hTLR-2 cells were treated with various concentrations (10 ng–250 ng) of rRv3131 and interaction of Rv3131 with TLR2 was measured spectrophotometrically by the colour change read at 620 nm due to the activity of SEAP. *p < 0.05; ***p < 0.0005.
Rv3131 interacts with TLR2
To lend further credence to the in silico observations, the interaction between Rv3131 and TLR2 was analysed using a cell based assay. In HEK-Blue 293 hTLR2 cells expressing TLR2 on their cell surface, alkaline phosphatase activity was found to be augmented with the increase in rRv3131 treatment. Similar increase in activity was observed in cells treated with the positive control FSL-1. The negative control, Rab5 did not cause any increase in alkaline phosphatase activity, thus validating the assay (Fig. 3C). Such an increase in activity was not observed in HEK-Blue 293 hTLR-4 cells expressing TLR4 on their cell surface when treated with Rv3131 protein, suggesting that the interaction of this protein was specific to TLR2 (data not shown).
Rv3131 induces TLR2 expression
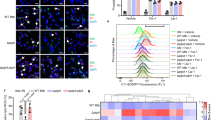
The possible role of Rv3131 in modulating TLR2 expression, besides its interaction on the cell surface was analysed by real time PCR. In THP-1 cells treated with rRv3131 protein, TLR2 mRNA levels were significantly upregulated (Fig. 4A) similar to that of the positive control TSA. Besides the gene expression, TLR2 expression levels on THP-1 cell surface following rRv3131 treatment were also analysed using flow cytometry analysis. A significant increase in the expression of TLR2 (Fig. 4B) but not TLR4 (data not shown) was observed in THP-1 cells when treated with rRv3131. Western blot analyses also indicated an increased expression of TLR2 in rRv3131 treated THP-1 cells (Fig. 4C). To determine whether the interaction of Rv3131 with TLR2 initiates NF-κB signalling pathway, western blot was performed. Analysis of whole cell protein isolated from THP-1 cells revealed activated form of NF- κB (p65) in rRv3131 and LPS treated preparations, but not in untreated cells (Fig. 4D).
Rv3131 induces TLR2 expression and NF-κB activation.
(A) Real time expression of TLR2: RNA isolated from differentiated THP-1 cells treated with rRv3131 (1000 ng) or TSA (250 ng) was reverse transcribed and real time PCR was carried out using gene specific primers. The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control. Data are presented as mean ± SEM of three independent experiments. (B) Surface expression of TLR2 by flow cytometry: PMA-differentiated THP-1 cells were treated with 100 ng of rRv3131 for 24 h followed by incubation with primary and secondary FITC labelled antibodies and analysed by flow cytometry. Data are expressed as mean ± SEM of percentage of cell population/MFI values from three independent experiments. (C) TLR2 protein expression: total protein isolated from differentiated THP-1 cells treated with rRv3131 (1000 ng) or LPS (100 ng) was electrophoresed on polyacrylamide gel, transferred onto PVDF membrane and probed with antibodies against TLR2 and β-actin (internal control). Signal corresponding to the intensity of the band was measured using chemiluminiscence. The graph represents relative TLR2 expression levels quantified densitometrically (D) NF-κB phosphorylation: the total protein isolated was analysed using western blot with antibodies specific to phosphorylated p65.
Discussion
Despite existence of tuberculosis infection worldwide, since ancient times, its pathology remained complicated even after extensive research. Majority of the tuberculosis patients experience latent phase infection before the active disease sets in. During this phase, immune responses to latency associated antigens are detected. The existing treatment approaches turn out to be ineffective due to a unique ability of this pathogen to remain dormant by vesting in granulomas, impermeable to the anti-tuberculosis drugs39. Adding to this is the lack of accurate in vitro or in vivo models that can mimic M. tuberculosis latency and/or resuscitation. Thus, development of effective vaccines using M. tuberculosis antigens is essential. Though classical antigens of M. tuberculosis were proposed to be potential vaccine candidates, because of the complicated life cycle of this pathogen, which includes latency, developing vaccines using antigens that are specifically expressed in latent stage is gaining prominence40. Recent studies identified that genes of DosR, upregulated during latency, also serve as antigens eliciting immune response4,41. Rv3131 was demonstrated to be significantly upregulated (up to 40 folds) under in vitro dormancy conditions21. Thus, such antigens were projected to be potential vaccine candidates due to their stage specific expression and immunogenicity.
We observed increased pro-inflammatory cytokine induction in THP-1 cells and human PBMCs with the treatment of recombinant rRv3131. Though a dose dependent increase in cytokine levels was observed in THP-1 cells and PBMCs treated with Rv3131, the highest dose (2500 ng) did not augment the release of IL-1β over 100 ng. This variation in IL-1β response could be due to physiological and phenotypic differences between the two cell types. The ability of Rv3131 to elicit immune response in M. tuberculosis specific T-cells obtained from tuberculin skin test positive patients was demonstrated4. In an Ugandan population, Rv3131 was demonstrated to be one of the most immunogenic antigens by virtue of its ability to induce interferon-γ19. TNF-α levels were found to be high in the lungs of mice with persistent tuberculosis42 and is known to contribute to many immune regulatory functions such as macrophage activation and granuloma formation5,43. The ability of Rv3131 to induce pro-inflammatory cytokines suggests that it might activate macrophages during latency to govern the micro environment of the granuloma and favor the survival of latent M. tuberculosis. Recognition of M. tuberculosis in the host is executed by TLRs, especially TLR2 and to some extent by TLR4. The role of TLR2 in mycobacterial recognition and the downstream signalling during M. tuberculosis infection was well proven in a variety of TLR knock out animal models44 and the susceptibility observed due to genetic polymorphisms in their genes45 was demonstrated. Interaction of mycobacterial products with TLR2 leads to the secretion of pro-inflammatory23,46 and anti-inflammatory cytokines47. It should be noted that M. tuberculosis is an intracellular pathogen and the proteins secreted by it could also enter or be located in extracellular spaces during infections. We earlier characterized the mycobacterial Hsp65 and found it to have dual roles in autoimmunity and inflammation48. It is possible that Rv3131 could also reach the extracellular space and presented to immune cells to trigger cytokine secretion. Though our study proves that cytokine secretion induced by Rv3131 involves TLR2 and NF- κB, the possibility that cytokine secretion is also due to presentation of Rv3131 to immune cells cannot be ruled out. It is worthwhile to mention that cytokine response in isolated PBMCs could also be contributed by other cells such as dendritic cells, T and B cells, etc. Analysing cytokine expression using purified fractions of each of these cells would provide information on the individual cell type contribution and/or cell specificity, if any, in Rv3131 induced cytokine response.
Interaction of mycobacterial classical antigens such as peptidoglycan, with TLR2 marks the commencement of downstream signaling7. We previously demonstrated that the DosR regulon antigen DATIN can interact and activate TLR2 resulting in secretion of pro inflammatory cytokines23. Similarly, PE35 (Rv3872), a mycobacterial protein physically interacts with TLR2 resulting in a dose dependent increase in the secretion of pro-inflammatory cytokine secretion49. The interaction of Rv3131 predicted in this study was based on computational modeling that generates models with a high degree of confidence. However, detailed analyses of the binding pockets and the associated specificity need to be addressed using site directed mutagenesis of both the ligand and the receptor.
In the current study, besides interacting with TLR2, Rv3131 induced TLR2 mRNA and protein expression. Modulation of TLR2 expression by the classical M. tuberculosis antigens was reported50. For example, the lipid fractions of three different strains of M. tuberculosis had differential effects on the expression profile of TLR2 and TLR4 cytokines and MHC class II components8. Another study indicated that the ESAT-6 antigen of M. tuberculosis modulates T helper cell responses via TLR-251. Further, in our studies, phosphorylation of NF- κB was evident in macrophages treated with recombinant Rv3131. Activation of NF-κB in the macrophages by the mycobacteria has been reported52. Given this, it is possible that Rv3131 might modulate the expression of TLR2 and may interact with it to influence the downstream signaling via NF-κB leading to the secretion of cytokines that favor the survival of M. tuberculosis in the granulomas. We used an in vitro system to study the immunogenic properties of Rv3131 protein at varying concentrations. The physiological concentration of Rv3131 in the granuloma during latency is very difficult to predict or estimate. However, it is an accepted practice to analyze the immunogenic properties of antigens using in vitro models. Hence, the results presented in our study strongly support that Rv3131 might induce pro-inflammatory cytokine expression via TLR2 signaling pathway and give an impetus to the understanding of the molecular mechanisms of regulation of DosR antigens.
In conclusion, we report that the DosR antigen, Rv3131 may possibly influence the innate immune responses to facilitate the survival of M. tuberculosis in the granulomatous microenvironment. This is brought about by induction of cytokine secretion mediated by its interaction with TLR2. Given paucity of knowledge on the mechanisms by which DosR antigens influence the milieu of the granuloma and support M. tuberculosis survival in a latent state, results of this study contribute suggestively to the understanding of the survival of M. tuberculosis executed via its latency specific antigens.
Additional Information
How to cite this article: Peddireddy, V. et al. A putative nitroreductase from the DosR regulon of Mycobacterium tuberculosis induces pro-inflammatory cytokine expression via TLR2 signaling pathway. Sci. Rep. 6, 24535; doi: 10.1038/srep24535 (2016).
References
Forrellad, M. A. et al. Virulence factors of the Mycobacterium tuberculosis complex. Virulence 4, 3–66 (2013).
Guirado, E. et al. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. MBio 6, e02537–02514 (2015).
Watanabe, S. et al. Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog 7, e1002287 (2011).
Leyten, E. M. et al. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect 8, 2052–2060 (2006).
Sasindran, S. J. & Torrelles, J. B. Mycobacterium Tuberculosis Infection and Inflammation: what is Beneficial for the Host and for the Bacterium? Front Microbiol 2, 2 (2011).
Basu, J., Shin, D. M. & Jo, E. K. Mycobacterial signaling through toll-like receptors. Front Cell Infect Microbiol 2, 145 (2012).
Esin, S. et al. Interaction of Mycobacterium tuberculosis cell wall components with the human natural killer cell receptors NKp44 and Toll-like receptor 2. Scand J Immunol 77, 460–469 (2013).
Rocha-Ramirez, L. M. et al. Mycobacterium tuberculosis lipids regulate cytokines, TLR-2/4 and MHC class II expression in human macrophages. Tuberculosis (Edinb) 88, 212–220 (2008).
Bocchino, M. et al. Role of mycobacteria-induced monocyte/macrophage apoptosis in the pathogenesis of human tuberculosis. Int J Tuberc Lung Dis 9, 375–383 (2005).
Tapping, R. I. & Tobias, P. S. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res 9, 264–268 (2003).
Jo, E. K., Yang, C. S., Choi, C. H. & Harding, C. V. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol 9, 1087–1098 (2007).
Collins, H. L. & Kaufmann, S. H. The many faces of host responses to tuberculosis. Immunology 103, 1–9 (2001).
Wilkinson, K. A. et al. Infection biology of a novel alpha-crystallin of Mycobacterium tuberculosis: Acr2. J Immunol 174, 4237–4243 (2005).
Geluk, A. et al. T-cell recognition of the HspX protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect Immun 75, 2914–2921 (2007).
Smith, S. M. et al. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J Immunol 165, 7088–7095 (2000).
Ottenhoff, T. H., Ab, B. K., Van Embden, J. D., Thole, J. E. & Kiessling, R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med 168, 1947–1952 (1988).
Demissie, A. et al. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol 13, 179–186 (2006).
Skjot, R. L. et al. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun 68, 214–220 (2000).
Black, G. F. et al. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin Vaccine Immunol 16, 1203–1212 (2009).
Timm, J. et al. Differential expression of iron-, carbon- and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci USA 100, 14321–14326 (2003).
Voskuil, M. I. et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198, 705–713 (2003).
Sander, C. & McShane, H. Translational mini-review series on vaccines: Development and evaluation of improved vaccines against tuberculosis. Clin Exp Immunol 147, 401–411 (2007).
Kumar, A. et al. Dormancy Associated Translation Inhibitor (DATIN/Rv0079) of Mycobacterium tuberculosis interacts with TLR2 and induces proinflammatory cytokine expression. Cytokine 64, 258–264 (2013).
Chauhan, S. & Tyagi, J. S. Powerful induction of divergent tgs1-Rv3131 genes in Mycobacterium tuberculosis is mediated by DevR interaction with a high-affinity site and an adjacent cryptic low-affinity site. J Bacteriol 191, 6075–6081 (2009).
Hu, Y. & Coates, A. R. Mycobacterium tuberculosis acg gene is required for growth and virulence in vivo. PLoS One 6, e20958 (2011).
Riano, F. et al. T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia. Tuberculosis (Edinb) 92, 148–159 (2012).
Kumar, A. et al. Mycobacterium tuberculosis DosR regulon gene Rv0079 encodes a putative, ‘dormancy associated translation inhibitor (DATIN)’. PLoS One 7, e38709 (2012).
Devi, S., Rajakumara, E. & Ahmed, N. Induction of Mincle by Helicobacter pylori and consequent anti-inflammatory signaling denote a bacterial survival strategy. Sci Rep 5, 150499 (2015).
Finn, R. D. et al. Pfam: the protein families database. Nucleic Acids Res 42, D222–230 (2014).
Tsirigos, K. D., Peters, C., Shu, N., Kall, L. & Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res 43, W401–407 (2015).
Webb, B. & Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr Protoc Bioinformatics 47, 561–5632 (2014).
Laskowski, R. A., Chistyakov, V. V. & Thornton, J. M. PDBsum more: new summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res 33, D266–268 (2005).
Qin, S. & Zhou, H. X. meta-PPISP: a meta web server for protein-protein interaction site prediction. Bioinformatics 23, 3386–3387 (2007).
Schneidman-Duhovny, D., Inbar, Y., Nussinov, R. & Wolfson, H. J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33, W363–367 (2005).
Mashiach, E., Schneidman-Duhovny, D., Andrusier, N., Nussinov, R. & Wolfson, H. J. FireDock: a web server for fast interaction refinement in molecular docking. Nucleic Acids Res 36, W229–232 (2008).
Konieczna, P. et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 61, 354–366 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675 (2012).
Tovchigrechko, A. & Vakser, I. A. GRAMM-X public web server for protein-protein docking. Nucleic Acids Res 34, W310–314 (2006).
Smith, T., Wolff, K. A. & Nguyen, L. Molecular biology of drug resistance in Mycobacterium tuberculosis. Curr Top Microbiol Immunol 374, 53–80 (2013).
Kaufmann, S. H. The contribution of immunology to the rational design of novel antibacterial vaccines. Nat Rev Microbiol 5, 491–504 (2007).
Serra-Vidal, M. M. et al. Immunogenicity of 60 novel latency-related antigens of Mycobacterium tuberculosis. Front Microbiol 5, 517 (2014).
Flynn, J. L., Scanga, C. A., Tanaka, K. E. & Chan, J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol 160, 1796–1803 (1998).
Cooper, A. M. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27, 393–422 (2009).
Noss, E. H. et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol 167, 910–918 (2001).
Thada, S., Valluri, V. L. & Gaddam, S. L. Influence of Toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand J Immunol 78, 221–229 (2013).
Basu, S. et al. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J Biol Chem 282, 1039–1050 (2007).
Nair, S. et al. The PPE18 of Mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J Immunol 183, 6269–6281 (2009).
Rani, P. S. et al. Mycobacterial Hsp65 potentially cross-reacts with autoantibodies of diabetes sera and also induces (in vitro) cytokine responses relevant to diabetes mellitus. Mol Biosyst 9, 2932–2941 (2013).
Tiwari, B., Soory, A. & Raghunand, T. R. An immunomodulatory role for the Mycobacterium tuberculosis region of difference 1 locus proteins PE35 (Rv3872) and PPE68 (Rv3873). FEBS J 281, 1556–1570 (2014).
Harding, C. V. & Boom, W. H. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol 8, 296–307 (2010).
Chatterjee, S. et al. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog 7, e1002378 (2011).
Kumar, P., Tyagi, R., Das, G. & Bhaskar, S. Mycobacterium indicus pranii and Mycobacterium bovis BCG lead to differential macrophage activation in Toll-like receptor-dependent manner. Immunology 143, 258–268 (2014).
Hayashi, F., Means, T. K. & Luster, A. D. Toll-like receptors stimulate human neutrophil function. Blood 102, 2660–2669 (2003).
Moore, X. L. et al. Endothelial progenitor cells’ “homing” specificity to brain tumors. Gene Ther 11, 811–818 (2004).
Acknowledgements
We would like to thank DST WOS-A [SR/WOS-A/LS-126/2013 (G)] to V.P; Department of Biotechnology, Government of India [BT/PR12817/COE/34/23/2015 to NA]; Senior Research Fellowship, Council for Scientific and Industrial Research, India [CSIR-SRF to S.N.D]; we would like to thank Naveen Kumar for his help in flow cytometry analysis. NA is an Adjunct Professor of the Academy of Scientific and Innovative Research, India.
Author information
Authors and Affiliations
Contributions
V.P. designed and performed all wet lab experiments and contributed to writing of the manuscript; S.N.D. helped in wet lab experiments and data analysis; I.A.Q. performed computational modeling of Rv3131 and its interactions and wrote the related portions of the manuscript; P.Y. helped in analyzing data and participated in discussions; N.A. conceptualized the study, provided reagents and materials, critically reviewed and refined the manuscript and provided overall supervision.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Peddireddy, V., Doddam, S., Qureshi, I. et al. A putative nitroreductase from the DosR regulon of Mycobacterium tuberculosis induces pro-inflammatory cytokine expression via TLR2 signaling pathway. Sci Rep 6, 24535 (2016). https://doi.org/10.1038/srep24535
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24535
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.