Abstract
Exhaled carbon monoxide (eCO) and fractional exhaled nitric oxide (FeNO) could reflect underlying inflammatory and oxidative stresses, which play important roles in pathogenetic pathways of metabolic syndrome (MetS). However, epidemiologic evidence was limited. We conducted a study in Wuhan-Zhuhai (WHZH) cohort of 3649 community participants to investigate the association between eCO, FeNO and MetS in both cross-sectional and prospective ways. The results showed that higher eCO and FeNO were associated cross-sectionally with a higher prevalence of MetS. The multivariable-adjusted odds ratios for MetS at baseline were 1.22 (95% confidence interval [CI]: 1.11 to 1.35) associated with per log eCO and 1.14 (95% CI: 1.00 to 1.30) associated with per log FeNO. During a follow-up of 3 years, 358/2181 new developed MetS cases were identified. Compared with lowest quartile of eCO and FeNO, the multivariable-adjusted risk ratios (95% CI) for MetS were 1.48 (1.06 to 2.06) related to the highest quartile of eCO. These findings remained consistent across sex but not smoking status, eCO was only associated with MetS in non-smokers when stratified by smoking status. In conclusion, our study demonstrated that eCO and FeNO were independently and positively associated with the prevalence of MetS cross-sectionally, while only eCO was positively related with the incidence of MetS prospectively.
Similar content being viewed by others
Introduction
Exhaled carbon monoxide (eCO) and fractional exhaled nitric oxide (FeNO)1 are both small gas molecules produced endogenously in human body. The endogenous eCO is the by-product of the degradation of heme through the heme oxygenase (HO) enzyme system in cells and tissues2,3,4. HO catalyzes the first and rate-limiting step in the oxidative degration of heme to ferrous iron, biliverdin-IXα and CO5. Although, the biochemical characterization of endogenous CO production is precise, eCO have been regarded as metabolic waste for a long time till the discovering of physiologically similarity with nitric oxide (NO)5,6. FeNO is synthesized by one of the three forms of nitric oxcide synthases (NOS), which catalyze the transformation of L-arginine to L-citrulline and NO in the presence of oxygen and several cofactors7,8,9. And it has been recommended as a monitor of airway inflammation in asthma patients by US Food and Drug Administration (FDA)1. In addition, NO is a mediators of vasodilation, regulators of endothelial cell proliferation and maintainers of vascular health10,11.
Accumulating evidences suggest eCO and FeNO are structurally and biologically similar in the ability to modulate vascular functions and cellular homeostasis5,12,13,14 and are evaluated as candidate breath biomarker of pathophysiological states4,13,14,15. Both eCO and FeNO can be cytoprotective and exert antioxidant, antiinflammatory and antiapoptotic properties at normally physiological levels1,8,16, while, excess eCO and FeNO can reflect underlying inflammatory and oxidative stress.
Metabolic syndrome (MetS), characterized by a constellation of multiple cardiometabolic abnormalities including central obesity, hypertension, dyslipidemia and impaired glucose tolerance, has been recognized as a condition strongly predicting type 2 diabetes and cardiovascular disease (CVD)17. MetS shares common pathogenetic pathways with CVD and type 2 diabetes, where oxidative stress and inflammation play central roles8,9,18,19. Therefore, exhaled biomarkers like endogenously CO and NO, reflecting increment in oxidative stress, systemic inflammation and endothelial dysfunction can be studied as potential non-invasive tools to predict MetS.
However, there are limited epidemiologic studies to investigate the relationship between eCO, FeNO and MetS up to date. Additionally, the concentrations of eCO and FeNO were indicated to be significant affected by sex and smoking status13. But no study has been conducted to examine the effects of these factors on the association of eCO and FeNO with MetS. Therefore, we sought to investigate the association of eCO, FeNO with MetS in both cross-sectionally and longitudinally ways, as well as evaluating whether the association is modified by sex and smoking status in a community-based cohort in China.
Results
The baseline characteristics of our participants were presented in Table 1 according to quartiles of eCO and FeNO. Rug plots displaying the distribution of eCO and FeNO of our sample were shown in Supplementary Fig. S1 and Supplementary Fig. S2. All the participants aged from 18 to 80 years and the average age was 52.7. Female participants took up 65.1% of the whole population. During the 3 years of follow-up, we identified 358 new MetS cases out of 2,181participants. We observed increases of waist circumferences, low-density lipoprotein cholesterol (LDL), serum triglycerides and blood glucose across increasing eCO and FeNO quartiles. In this study, the levels of eCO and FeNO were higher in men and smokers. There were also increases in age and BMI across increasing FeNO quartiles.
The cross-sectional associations of eCO and FeNO with MetS were shown in Table 2. After adjusting for multiple potential confounders, a positive relationship was indicated between eCO, FeNO and the prevalence of MetS. Comparing with the lowest quartile, the multi-variate adjusted ORs (95%CI) for MetS were 0.99 (0.80–1.23), 1.03 (0.83–1.28) and 1.71 (1.34–2.18) from the second quartile to the fourth quartile of eCO and the OR (95%CI) was 1.22 (1.11–1.35) correspond to 1-unit change in log eCO. Compared with the lowest quartile, the ORs (95%CI) for MetS were 1.37 (1.07–1.74), 1.16 (0.91–1.49) and 1.45 (1.13–1.86) across the increasing quartiles of FeNO and the OR (95%CI) was 1.14 (1.00–1.30) for 1-unit increase in log FeNO.
The associations were further investigated longitudinally (Table 3). There was still a positive relationship between eCO and the development of metabolic syndrome. The risk ratio (RR) and 95%CI were 1.23 (0.87–1.74), 1.35 (1.01–1.82) and 1.48 (1.06–2.06) as the increasing of eCO quartiles in multi-variable model. And the RR (95%CI) correspond to 1-unit change in log eCO was 1.12 (1.02–1.26). However, there was no significance in the relationship between FeNO and MetS across the increasing quartiles of FeNO. In the spline regression analysis, the associations were mainly the same (Fig. 1). Additionally, the association between FeNO and MetS remained consistent in stratified analysis according to sex and smoking status, but the association between eCO and MetS was modified by smoking status; eCO was related to MetS only in non-smokers (see Supplementary Table S3).
Discussion
To our knowledge, this was the first cohort study to investigate the associations of eCO and FeNO with MetS. Our principle findings showed that: higher eCO and FeNO were associated cross-sectionally with a higher prevalence of MetS under adjustment for possible confounders; and, higher eCO was also associated with further development of MetS. In stratified analysis, eCO was related to MetS in non-smokers, but the association was not modified by sex and smoking status for FeNO and MetS.
The eCO and FeNO has evolved rapidly as markers of breath analysis in the past decade. Breath analysis has the potential to offer relatively inexpensive, rapid, noninvasive methods for detecting and monitoring a variety of diseases8,20. And the ideal requirement of an exhaled marker needs to be easy to perform measurement with a simple methodology accomplishing reproducibility and repeatability of measurement9. Therefore, eCO and FeNO have been taken as markers of pulmonary and airway inflammation for a long time2,11,12,21,22. In recent years, the relationship between eCO, FeNO and metabolic diseases has drawn great attention9,19,23,24,25.
In this study, eCO was cross-sectionally and prospectively associated with Mets and the association remained consistent across different sex and modified by smoking status subgroups. Similar association was also indicated by Cheng and his collegues in a community-based study, which reported that interindividual variation in CO was associated with metabolic derangements and the development of manifest CVD13. Several mechanisms may contribute to the associations. Firstly, the HO-dependent response to oxidative stress and inflammation may be a main common pathway by which CO is associated with metabolic traits. Endogenous CO is a byproduct of heme oxygenase (HO) activity and oxidative stress and inflammatory cytokines can increase the activity of HO3,26,27. Excess levels of CO could disrupt the mitochondrial electron transport chain, resulting in the generation of reactive oxygen species. On the other hand, studies suggested that both hyperglycemia and the progressive accumulation of advanced glycation end-products in diabetes mellitus can be drivers of increased oxidative activity and, in turn, long-term activation of HO and oxidized lipids also activate HO may be related to the relation of dyslipidemia with excess CO levels28. Totally, excess endogenous CO may be both the cause and results of oxidative stress and inflammation. Secondly, CO has also been shown to stimulate insulin and glucagon release from islet cells as well as modulate insulin sensitivity and glucose tolerance in metabolic disorder animals29,30. In our study, eCO levels were also indicated to be higher in smoker groups which were consistent with former studies13,31,32. Cheng and his collegues also found the differences in their study, but they didn’t investigate whether the association between eCO and MetS differed by gender and smoking status13. Although, the association between eCO and MetS was not modified by sex, it was modified by smoking status. eCO was only significantly related with MetS in non-smokers after adjusting for multiple potential confounders. This can be partly explained by the relatively smaller population of smokers compared to non-smokers in this population, for we also observed increased but not significantly risk in smokes. Another mechanism might be that the dependence of endogenous CO production on HO activity, for HO activity can be modulated by smoking and smoking can lead to the changes of microenvironment which also have impact on the expression and inducibility of HO33.
To our knowledge, no previous study was conducted to explore the association between FeNO levels with MetS, although endogenous NO is regarded as a regulatory factor that plays a key role in many different physiologic and pathophysiological processes. In our study, FeNO was found to be positively associated with MetS cross-sectionally but not in prospective analysis. As FeNO was also found to be higher in male and smoker groups, which was indicated in previous studies10,21,34,35. The association between FeNO and MetS remained consistent when stratified by sex and smoking status in our study. FeNO associated with metabolic disease mainly through the endothelium dysfunction36, which has been advocated as a pathogenic mechanism of metabolic diseases9,37. FeNO can also associate with metabolic disease through asymmetric dimethylarginine (ADMA), a common link between oxidative stress, inflammation and metabolic syndrome, by competing for binding to Endothelial Nitric Oxide Synthase (eNOS) with L-arginine and synthesizing high levels of NO and alteration in NO has also been related to mitochondrial dysfunction, a potential pathogenic mechanism for metabolic dysfunction38. Several potential mechanisms may account for the insignificant relationship after follow-up. Firstly, compared with eCO, FeNO may exhibit less involvement in metabolic pathways30, since eCO can stimulate insulin and glucagon release from islet cells as well as modulate insulin sensitivity and glucose tolerance in metabolic disorder animals29. Secondly, the average level of FeNO in the whole samples is relatively lower when compared with the recommended high risk range: 50 ppb of adults in some studies1,35 and we also observed a sharp increase of RR for MetS during follow-up when FeNOs were more than 100 ppb in the regression splines. Furthermore, Salonen I. found FeNO were inversely associated with plasma concentration of triglycerides and blood concentration of glycated haemoglobin A1c which was conflicted with this study39. The conflicted results might be explained by the difference in study sample: the study sample of Salonen, I’s study was very small and were all ischaemic heart disease patients while our study sample were healthy community residents. And studies indicated that heart diseases might lead to abnormal production of NO and pills to cure heart diseases may leads to the release large quantity of NO40.
Although our study examined the relationship between exhaled CO, FeNO and MetS in a cohort study, there are still some limitations. At first, exhaled CO and NO measurements in this study were obtained at a single point, but the use of a standardized protocol and routinely calibrated instrumentation likely served to reduce excess intraindividual and interindividual variability. Secondly, although we adjusted lifestyle factors, like smoking, we could not exclude the effect of ambient air and environment while the concentrations of eCO and FeNO might be influenced by air pollution, home heating apparatus, distance from major roadway, or transportation method and we also could not accurately calculate current number of cigarettes per day or time between the last cigarette and the CO measurement. However, all the participants lived in two communities from Wuhan city where the ambient air and living conditions were similar, so the effect of environmental factors might be homogenous in the whole population. Thirdly, although diet frequencies for seven kind of food were adjusted in multi-variate models, we cannot evaluate the effect of high-carbonhydrate diet and high-fat diet accurately without accurate information on food intake for every participant. Fourthly, MetS was only assessed at baseline and at 3 years of follow-up and that 40% of individuals in the study sample were not included in the prospective analyses. In addition, all participants in this study are of Chinese Han ethnicity, which minimizes the confounding effects by ethnic background but doesn’t allow us to explore whether the associations of exhaled CO and NO and MetS is different between ethnicities.
Conclusion
Our study demonstrated that eCO and FeNO were independently and positively associated with the prevalence of MetS, while only eCO was positively and significantly related with the incidence of MetS in nonsmokers prospectively. It may contribute to our new understanding of the importance eCO and FeNO, in the pathogenesis of MetS. Further studies are warranted to confirm our findings and elucidate the potential mechanisms.
Methods
Study Sample
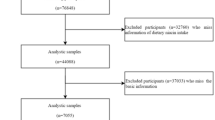
In the present study, we used data form a community-based prospective cohort: the Wuhan-Zhuhai (WHZH) cohort study (established in 2011, n = 4812). The design and enrollment criteria of this cohort have been well described previously41. In this cohort, standard questionnaires, anthropometry and physical examination were administered at baseline and three years later to collect and update information on lifestyles and occurrence of diseases. For the cross-sectional investigation, we excluded 298 participants with former cardiovascular diseases and lung diseases, as well as 865 people with missing information or outliers (>mean ± 3SD) on indexes of blood test (n = 100), eCO (n = 209) and FeNO (n = 556). After exclusion, a total of 3649 participants were included in our cross-sectional analysis. And, for the longitudinal investigation, we further excluded people lost to follow-up (n = 390), participants with metabolic syndrome (n = 484) at baseline and individuals with missing information on indexes of blood test (n = 594) at follow-up. Finally, 2,181 participants were included in the longitudinal analysis. However, individuals included and excluded in these analysis were similar with respect to age, sex, body mass index (BMI) and smoking status (P > 0.05).
Ethics Statement
The study protocol was approved by the institutional review boards of Tongji Medical College Institutional review Board, School of Public Health, Tongji Medical College, Huazhong University of Science & Technology (Wuhan, Hubei, China). The methods were carried out in accordance with the approved guidelines. All participants provided written informed consent.
Exhaled CO and FeNO Assessment
Exhaled CO and FeNO were measured following the standard protocol in resting state in every participant. Exhaled CO was measured for each participant with a MicroCO Meter (Carefusion, Kent, UK). After a 5-minute rest, the participants were instructed to inspire fully and hold the breath for 20 seconds, then seal their lips around the mouthpiece and exhale slowly and fully. The calibrations were performed with standard gas weekly. And FeNO was tested by a Nano Coulomb Nitric Oxide Analyzer (SV-02E, Sunvou Medical Electronics CO., Ltd., Wuxi, China) following the recommendations of American Thoracic Society/European Respiratory Society (2005)1. The tests were conducted at least 2 hours after food eating and 30 minutes after strenuous exercise. Calibrations were performed with standard bottle gas (15, 75 and 150 ppb) on a weekly basis.
Ascertainment of Metabolic Syndrome
MetS was defined according to the diagnostic criteria proposed by the Adult Treatment Program III of the National Cholesterol Education Program (NCEP ATP III, 2005). Participants were defined as MetS patients if they met three or more of the following variables and cutoff points: (1) Fasting triglyceride ≥1.69 mmol/L (150 mg/dL); (2) HDL cholesterol: Men <1.04 mmol/L (40 mg/dL), Women <1.29 mmol/L (50 mg/dL); (3) Fasting glucose: ≥5.5 mmol/L (100 mg/dL); (4) Waist circumference: men ≥102 cm, women ≥88 cm; (5) Systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg. And MetS was only ascertained at baseline and at 3 years of follow-up.
Ascertainment of Covariates
Physical activities contain many aspects of activities, such as, climbing, walking, dancing, cycling, running, swimming and so on. Physical activity is defined as “yes” if the participant exercises ≥2 times per week and each time ≥20 minutes. Diet frequency include the consumption frequency of seven main kind of food: grain, coarse, fruits and vegetables, meat and poultry, fishery product, egg and milk, bacon. The frequency was recorded in 4 categories: times per day, times per week, times per month and times per year in questionnaires and then we transformed consumption frequency of these seven kind of food into times per month in the final analysis respectively.
Statistical Analyses
Distribution of information on demographic, lifestyles and some biochemical markers were demonstrated according to quartile of eCO and FeNO, separately. The quartiles of eCO and FeNO were determined using whole number approximate quartiles based on log-transformed values. And the P-Value for trend was tested by linear regression for continuous variables and Chi-square tests for category variables. eCO and FeNO were log-transformed in all analysis because of abnormal distribution.
Logistic regression model was used to calculate the odds ratio (OR), risk ratio (RR) and 95% confidence interval (CI) for MetS according to quartile of eCO and FeNO with the lowest quartile as the referent. In model 1, we adjusted for sex and age (continuous). In model 2, we adjusted for age (continuous), sex, body mass index (BMI) (continuous), race (Han, others), marital status (single or divorced, married), education (junior high school or below, senior high school or above), current drinking status (no, yes), diet frequency (times per month, for the following seven kind of food: grain, coarse, fruits and vegetables, meat and poultry, fishery product, egg and milk, bacon ) and physical activity (no, yes). In model 3, we further adjusted for passive smoking (no, yes) and current smoking status (no, yes) as well as variables in model 2. All p-values were two sided with a significant level at 0.05 and data were analyzed with SAS 9.1 (SAS Institute Inc. Cary, NC, USA). Rug plots displaying the distribution of eCO and FeNO were generated with R software (R 3.2.1). The display of multivariable-adjusted RR of MetS versus eCO and FeNO was generated with SAS 9.1 using spline regression, the knots were placed at the 5%, 25%, 50%, 75% and 95%42.
Additional Information
How to cite this article: Guo, Y. et al. Associations of Exhaled Carbon Monoxide and Fractional Exhaled Nitric Oxide with Metabolic Syndrome: A cohort study. Sci. Rep. 6, 24532; doi: 10.1038/srep24532 (2016).
References
Raed, A. et al. on behalf of the American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. Am J Respir Crit Care Med 184, 602–615, 10.1164/rccm.912011ST (2011).
Buszewski, B., Kesy, M., Ligor, T. & Amann, A. Human exhaled air analytics: biomarkers of diseases. Biomedical chromatography: BMC 21, 553–566, 10.1002/bmc.835 (2007).
Dulak, J., Deshane, J., Jozkowicz, A. & Agarwal, A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117, 231–241, 10.1161/CIRCULATIONAHA.107.698316 (2008).
Wu, L. & Wang, R. Carbon monoxide: endogenous production, physiological functions and pharmacological applications. Pharmacological reviews 57, 585–630, 10.1124/pr.57.4.3 (2005).
Stefan W. Ryter, D. M. & Augustine, M. K. C. Carbon Monoxide:To Boldly Go Where NO Has Gone Before. Science’s STKE 2004, re6 (2004).
Zhuo M, S. S., Kandel, E. R. & Hawkins, R. D. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science 260, 1946–1950 (1993).
Forstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. European heart journal 33, 829–837, 837a–837d, 10.1093/eurheartj/ehr304 (2012).
Cikach, F. S. Jr. & Dweik, R. A. Cardiovascular biomarkers in exhaled breath. Progress in cardiovascular diseases 55, 34–43, 10.1016/j.pcad.2012.05.005 (2012).
Mauro Maniscalco, F. P., Mauro, M. & Matteo, S. Exhaled nitric oxide and other major exhaled compounds for the diagnosis of metabolic diseases. Expert Opin. Med. Diagn. 3, 547 (2009).
Takao Kaneko, F. et al. ERZURUM. Biochemical Reaction Products of Nitric Oxide as Quantitative Markers of Primary Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine 158, 917–923 (1998).
Masahiko Tsuchiya et al. Interrelation between Oxygen Tension and Nitric Oxide in the Respiratory System. American Journal of Respiratory and Critical Care Medicine 162, 1257–1261 (2000).
Paolo Montuschi, M. S. A. K. & Peter J. Barnes Exhaled Carbon Monoxide and Nitric Oxide in COPD. Chest 120, 496–501 (2001).
Cheng, S. et al. Exhaled carbon monoxide and risk of metabolic syndrome and cardiovascular disease in the community. Circulation 122, 1470–1477, 10.1161/CIRCULATIONAHA.110.941013 (2010).
Ryter, S. W. & Choi, A. M. Carbon monoxide in exhaled breath testing and therapeutics. Journal of breath research 7, 017111, 10.1088/1752-7155/7/1/017111 (2013).
Leung, J. et al. Assessment of fractionated exhaled nitric oxide as a biomarker for the treatment of eosinophilic esophagitis. Allergy and asthma proceedings: the official journal of regional and state allergy societies 33, 519–524, 10.2500/aap.2012.33.3606 (2012).
Bauer, I. & Pannen, B. H. Bench-to-bedside review: Carbon monoxide–from mitochondrial poisoning to therapeutic use. Crit Care 13, 220, 10.1186/cc7887 (2009).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung and Blood Institute Scientific Statement. Circulation 112, 2735–2752, 10.1161/CIRCULATIONAHA.105.169404 (2005).
Grundy, S. M. et al. Definition of metabolic syndrome: Report of the National Heart, Lung and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109, 433–438, 10.1161/01.CIR.0000111245.75752.C6 (2004).
Minh Tdo, C., Blake, D. R. & Galassetti, P. R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes research and clinical practice 97, 195–205, 10.1016/j.diabres.2012.02.006 (2012).
Dweik, R. A. & Amann, A. Exhaled breath analysis: the new frontier in medical testing. Journal of breath research 2, 10.1088/1752-7163/2/3/030301 (2008).
Balint, B., L. E. D., Hanazawa, T., Kharitonov, S A. & Barnes, P J. Increased nitric oxide metabolites in exhaled breath condensate after exposure to tobacco smoke. Thorax 56, 456–461 (2001).
Malby Schoos, A. M., Chawes, B. L., Bonnelykke, K. & Bisgaard, H. Fraction of exhaled nitric oxide and bronchial responsiveness are associated and continuous traits in young children independent of asthma. Chest 142, 1562–1568, 10.1378/chest.12-0658 (2012).
Schivo, M., Aksenov, A. A., Yeates, L. C., Pasamontes, A. & Davis, C. E. Diabetes and the metabolic syndrome: possibilities of a new breath test in a dolphin model. Front Endocrinol (Lausanne) 4, 163, 10.3389/fendo.2013.00163 (2013).
Paolo Pared et al. Exhaled Carbon Monoxide Levels Elevated in Diabetes and Correlated With Glucose Concentration in Blood: A New Test for Monitoring the Disease? Chest 116, 1007–1011 (1999).
Cheng, S. et al. Association of exhaled carbon monoxide with subclinical cardiovascular disease and their conjoint impact on the incidence of cardiovascular outcomes. European heart journal, 10.1093/eurheartj/ehu052 (2014).
Ryter, S. W., Alam, J. & Choi, A. M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86, 583–650, 10.1152/physrev.00011.2005 (2006).
Wagner, C. T., W. D., Christodoulides, N., Hellums, J. D. & Schafer, A. I. Hemodynamic Forces Induce the Expression of Heme Oxygenase in Cultured Vascular Smooth Muscle Cells. The Journal of Clinical Investigation 100, 589–596 (1997).
Savini, I., Catani, M. V., Evangelista, D., Gasperi, V. & Avigliano, L. Obesity-associated oxidative stress: strategies finalized to improve redox state. International journal of molecular sciences 14, 10497–10538, 10.3390/ijms140510497 (2013).
Li, M. et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels and improves insulin sensitivity and glucose tolerance. Diabetes 57, 1526–1535, 10.2337/db07-1764 (2008).
Joe, Y. et al. The role of carbon monoxide in metabolic disease. Ann N Y Acad Sci 1229, 156–161, 10.1111/j.1749-6632.2011.06121.x (2011).
Jones, A. Y. & Lam, P. K. End-expiratory carbon monoxide levels in healthy subjects living in a densely populated urban environment. Sci Total Environ 354, 150–156, 10.1016/j.scitotenv.2005.02.018 (2006).
Cunnington, A. J. P. H. Breath analysis to detect recent exposure to carbon monoxide. Postgrad Med J 78, 233–238 (2002).
Yang, G. et al. Anti-oxidant effect of heme oxygenase-1 on cigarette smoke-induced vascular injury. Mol Med Rep 12, 2481–2486, 10.3892/mmr.2015.3722 (2015).
Holguin, F. Arginine and nitric oxide pathways in obesity-associated asthma. Journal of allergy 2013, 714595, 10.1155/2013/714595 (2013).
Ren, X. et al. Fractional exhaled nitric oxide in healthy Tibetans at high altitude. Med Sci Monit 20, 2565–2570, 10.12659/MSM.891369 (2014).
Puthucheary, Z. A. et al. Exhaled nitric oxide is decreased by exposure to the hyperbaric oxygen therapy environment. Mediators of inflammation 2006, 72620, 10.1155/MI/2006/72620 (2006).
Beaussier, M. et al. Role of nitric oxide and cyclooxygenase pathways in the coronary vascular effects of halothane, isoflurane and desflurane in red blood cell-perfused isolated rabbit hearts. British Journal of Anaesthesia 88, 399–407 (2002).
Ahmad, T., Mabalirajan, U., Ghosh, B. & Agrawal, A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am J Respir Cell Mol Biol 42, 3–8, 10.1165/rcmb.2009-0137RC (2010).
Salonen, I. et al. Exhaled nitric oxide and atherosclerosis. Eur J Clin Invest 42, 873–880, 10.1111/j.1365-2362.2012.02662.x (2012).
Liu, X. et al. Effect of asymmetric dimethylarginine (ADMA) on heart failure development. Nitric Oxide, 10.1016/j.niox.2016.02.006 (2016).
Song, Yuanchao et al. The Wuhan-Zhuhai (WHZH) cohort study of environmental air particulate matter and the pathogenesis of cardiopulmonary diseases: study design, methods and baseline characteristics of the cohort. BMC Public Health 14, 994–1003 (2014).
Desquilbet, L. & Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 29, 1037–1057, 10.1002/sim.3841 (2010).
Acknowledgements
We gratefully acknowledge all the study participants and all the support from the Zhuhai center for diseases control and prevention, Zhuankou district diseases control and prevention and the local community residents committees in Wuhan and Zhuhai city. This study was supported by the National Basic Research Program of China (2011CB503804) and Key Program of the National Natural Science Foundation of China (91543207). The funder had no involvement in the study design, study management, data collection, data analysis, data interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.G., J.M., W.L., J.H., R.Z., J.Y. and W.C. designed and conducted research; Y.G. and wrote the manuscript; J.M. and W.L. edited the manuscript; All authors read and approved the final manuscript. W.C. is the guarantor of this work and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Guo, Y., Ma, J., Lu, W. et al. Associations of Exhaled Carbon Monoxide and Fractional Exhaled Nitric Oxide with Metabolic Syndrome: A Cohort Study. Sci Rep 6, 24532 (2016). https://doi.org/10.1038/srep24532
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24532
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.