Abstract
MicroRNAs (miRNAs) have important roles in post-transcriptional regulation of gene expression. During viral infection, viruses utilize hosts to enhance their replication by altering cellular miRNAs. The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway plays crucial roles in the antiviral responses. In this study, two miRNAs (miR-9041 and miR-9850) from Macrobrachium rosenbergii were found to promote white spot syndrome virus (WSSV) replication. The up-regulation of miR-9041 or miR-9850 suppresses STAT expression in the gills of M. rosenbergii, which subsequently down-regulates the expression of its downstream dynamin (Dnm) genes: Dnm1, Dnm2, and Dnm3. Knockdown of miR-9041 and miR-9850 restricts WSSV replication by up-regulating STAT and Dnm gene expression. The silencing of STAT, Dnm1, Dnm2, or Dnm3 led to an increase of the number of WSSV copies in shrimp. The injection of recombinant Dnm1, Dnm2, or Dnm3 proteins could inhibit WSSV replication in vivo. Overall, our research indicates the roles of host miRNAs in the enhancement of WSSV replication by regulating the host JAK/STAT pathway.
Similar content being viewed by others
Introduction
The giant fresh water prawn, Macrobrachium rosenbergii, has become an important cultured species in China and other southern East Asian countries1,2. With the development of intensive culture and environmental deterioration, serious infectious diseases caused by viruses have been reported in M. rosenbergii3. Among these viruses, the white spot syndrome virus (WSSV) has emerged as one of the most prevalent and widespread viruses4,5. WSSV is a bacilliform, non-occluded enveloped virus, which belongs to the genus Whispovirus of the Nimaviridae family6,7. WSSV is a large double-stranded DNA virus with a virion that consists of a nucleocapsid, tegument, and envelope and includes at least 39 structural proteins8,9. WSSV remains a major threat to shrimp aquaculture10.
The Toll, immune deficiency (IMD), and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways are reported to regulate the humoral immunity of shrimp11. Limited information is available regarding the role of the JAK/STAT pathway during WSSV infection11. The JAK/STAT pathway was first identified as a cytokine-induced signaling pathway in mammals; to date, the pathway is known to be involved in immune response and inflammation, particularly in the interferon-mediated antiviral response in mammals12,13. Infectious viruses induce the production of interferons, interleukins, and growth factors, among others14. These compounds are recognized by cytokine receptors, which activate the JAK/STAT pathway and leads to the transcription of interferon-inducible genes with antiviral functions15. In humans, the four JAKs (JAK1, JAK2, JAK3, and TYK2) and seven STATs (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6) mediate responses to a number of cytokines and activate different downstream genes16. In Culex mosquitoes, the activation of the JAK/STAT pathway can induce the expression of Vago, which is regarded as an antiviral factor, to restrict the West Nile virus infection17. In Drosophila, this pathway activates at least two gene families: thioester-containing protein (TEP) and TOT, which are involved in innate immunity18. In Penaeus monodon, STAT directly transactivates the expression of the WSSV immediate-early gene ie1 and contributes to its high promoter activity19.
In humans, the myxovirus resistance gene MxA belongs to the superfamily of dynamin-like large GTPases. MxA is reported to be JAK/STAT-dependent and can inhibit the replication of orthomyxoviruses20. Dynamin (Dnm) is a large multi-domain GTPase essential for the membrane fission leading to clathrin-mediated endocytosis21. Dnm has three isoforms in mammalian cells: Dnm1, Dnm2, and Dnm3, which are encoded by distinct genes that are differentially expressed in various tissues22. Dnm is a key mediator of cell-autonomous innate immunity against a broad range of viruses23. In addition to its involvement in virus endocytosis, Dnm has also been proposed to participate in membrane fusion between viruses and endosomes after endocytosis24.
MicroRNAs (miRNAs) have roles in the post-transcriptional regulation of gene expression25 and various biological processes, such as proliferation, cell differentiation, apoptosis, tumorigenesis, and immune defense25,26. The production of mature miRNAs requires the participation of several molecules27,28. Typically, targeted mRNA leads to translation repression and/or mRNA degradation29. More miRNAs have been shown to participate in innate and adaptive immune response during virus infection by regulating the viral or host gene expression30. In humans, the Epstein–Barr virus (EBV)-encoded viral miR-BART22 modulates the viral gene product expression of the EBV latent membrane protein 2A (LMP2A), which may facilitate nasopharyngeal carcinoma carcinogenesis by evading the host immune response31. A human herpes virus miR-K12-11 attenuates IFN signaling and contributes to maintenance of viral latency by targeting I-κ-B kinase epsilon (IKKɛ)32. In Marsupenaeus japonicus shrimp, the viral miRNAs WSSV-miR-66 and WSSV-miR-68 could target WSSV genes and further promote WSSV infection33. However, the host miRNA-mediated regulations of the STAT gene and its downstream genes have not been well studied in the giant freshwater prawn to date.
In this study, we demonstrated that miR-9041 and miR-9850 played positive roles in WSSV replication. The up-regulation of miR-9041 or miR-9850 suppresses STAT expression in the gills of M. rosenbergii, which subsequently down-regulates the expression of its downstream Dnm genes. The RNA interference (RNAi) of Dnm genes or the overexpression of Dnms by injection of recombinant proteins could enhance or inhibit virus replication, respectively. Our research describes the roles of host miRNAs in enhancing WSSV replication by regulating the host JAK/STAT pathway.
Results
Effects of miR-9041 and miR-9850 on virus infection in shrimp
These 2 microRNAs (miR-9041 and miR-9850) was inducibly expressed in the WSSV challenged group in relative to the normal group (without WSSV challenge) based on the small RNA high-throughput sequencing data. So, we selected these 2 microRNAs for functional study. To further elucidate the roles of the host miR-9041 and miR-9850 in virus infection, both miRNAs were overexpressed in shrimp. When miR-9041 or miR-9850 was overexpressed in shrimp, the number of WSSV copies was examined. As shown in Fig. 1A, the overexpression of miR-9041 significantly increased the number of WSSV copies from 24 h to 48 h post-infection compared with the controls (miR-9041-scrambled and WSSV only). The overexpression of miR-9850 yielded similar results (Fig. 1C). By contrast, when miR-9041 or miR-9850 expression was knocked down by sequence-specific AMO-miR-9041 and AMO-miR-9850 in vivo, respectively, the WSSV copies were significantly decreased as compared with the controls (AMO-miR-9041-scrambled, AMO-miR-9850-scrambled, and WSSV only; Fig. 1BD). These findings indicated that the host miRNAs play a positive role in virus replication.
The effect of miR-9041 (A) or miR-9850 (C) overexpression on virus infection in the gills of shrimp. To overexpress the host miRNA, miR-9041-mimic or miR-9850-mimic and WSSV were co-injected into shrimp. At different time post-infection (0, 24, 36, and 48 h), the WSSV copies in shrimp were examined. U6 was used as a control. The influence of miR-9041 (B) or miR-9850 (D) silencing on the virus infection in vivo. AMO-miR-9041 or AMO-miR-9850 and WSSV were co-injected into shrimp. At various time post-infection (0, 24, 36, and 48 h), the WSSV copies in shrimp were evaluated. The numbers showed the time points after WSSV infection in shrimp. Asterisks indicated significant differences (*p < 0.05; **p < 0.01) between treatments.
Interaction between the host miRNAs and the host STAT gene
Increasing evidence has indicated that host miRNAs has important roles in host–virus interactions. To reveal the pathways mediated by host miRNAs, the target genes of miR-9041 and miR-9850 were analyzed. The predictions by the TargetScan, miRanda, and Pictar algorithms showed that miR-9041 and miR-9850 could target the host STAT gene (Fig. 2AD), which is a gene involved in the antiviral immunity of shrimp19. Therefore, these host miRNAs may have significant effects on WSSV infection in shrimp.
The predicted target gene of miR-9041 (A) or miR-9850 (D). The 3′UTR of the STAT gene could be targeted by miR-9041 and miR-9850. The direct interaction between miR-9041 (B) or miR-9850 (E) and host gene. The synthesized miR-9041-mimic or miR-9850-mimic and the plasmid consisting of EGFP and shrimp STAT 3′UTR were co-transfected into insect High Five cells. The mutant of STAT 3′UTR (EGFP-∆STAT-9041 or EGFP-∆STAT-9850) was included in the co-transfection as a control. At 48 h after cotransfection, the fluorescence of cells was examined. The seed sequences targeted by miR-9041 and miR-9850 were underlined. Effects of miR-9041 (C) or miR-9850 (F) on the STAT gene expression. The relative fluorescence intensity of cells was determined. Statistically significant differences between treatments were indicated by asterisks (**p < 0.01).
To evaluate the interaction between the host miR-9041 or miR-9850 and the host STAT gene, we constructed the EGFP-STAT plasmid, which contained EGFP and the 3′-UTR of STAT in M. rosenbergii shrimp. The synthesized miR-9041 or miR-9850 mimic and the plasmid EGFP-STAT were co-transfected into insect High Five cells (Fig. 2BE). The results showed that the fluorescence intensity in the co-transfected cells was significantly reduced compared with the intensity in the EGFP-STAT-transfected cells (Fig. 2CF), thereby indicating that the synthesized miR-9041 or miR-9850 mimic repressed the expression of the STAT gene by targeting its 3′-UTR. However, the control miRNA mimic had a negligible effect on the expression of EGFP-STAT.
To further explore the interaction between miR-9041 or miR-9850 and STAT in vivo, the expression of miR-9041 and miR-9850 was overexpressed or silenced in shrimp, followed by the detection of STAT mRNA levels. The results indicated that miR-9041 or miR-9850 overexpression significantly decreased the STAT expression compared with the positive control (WSSV only), whereas the control miRNAs had no effect on the expression of STAT, thereby showing that miR-9041 and miR-9850 inhibited STAT expression in shrimp (Fig. 3AC). When the expression of miR-9041 or miR-9850 was knocked down by AMO-miR-9041 or AMO-miR-9850, the expression of STAT was significantly up-regulated (Fig. 3BD). These findings showed that miR-9041 and miR-9850 could interact with the host STAT gene in vivo.
The influence of miR-9041 (A) or miR-9850 (C) overexpression on the expression of shrimp STAT gene in vivo. miR-9041-mimic or miR-9850-mimic and WSSV were co-injected into shrimps. As a control, WSSV only was included in the injections. At different time post-infection (0, 24, 36, and 48 h), the expression of STAT was examined with quantitative real-time PCR. The effect of miR-9041 (B) or miR-9850 (D) silencing on the STAT expression in vivo. AMO-miR-9041 or AMO-miR-9850 was injected into WSSV-infected shrimp to knock down the miR-9041 or miR-9850 expression. WSSV only was used as a control. At different time post-infection (0, 24, 36, and 48 h), the expression of STAT was detected with quantitative real-time PCR. Statistically significant differences between treatments were indicated by asterisks (**p < 0.01).
Role of host STAT in virus infection
The abovementioned data showed that the host STAT gene is the target of miR-9041 and miR-9850. Therefore, the role of STAT in virus infection was explored in shrimp. Results of qRT-PCR indicated that the STAT mRNA was detected in all the examined tissues and mainly expressed in the heart and intestine tissues of shrimp (Fig. 4A). In response to WSSV infection, the expression level of STAT was up-regulated from 24 h to 48 h, which was significantly higher than that of the untreated controls (Fig. 4B). The results suggested that the STAT gene may play an important role in virus infection.
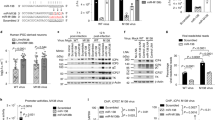
(A) The distribution of STAT in various tissues of shrimp. The shrimp STAT mRNA levels of heart, hemocyte, hepatopancreas, gill, stomach, intestine and nerve were examined using quantitative real-time PCR. Shrimp GAPDH was used as a control to calibrate the cDNA template for all the samples. (B) The expression profile of STAT in shrimp in response to virus infection. Shrimp were infected with WSSV. At different time post-infection (0, 6, 12, 24, 36, and 48 h), the STAT expression in gills was detected with quantitative real-time PCR. (C) The silencing of the STAT expression in shrimp. The sequence-specific STAT-siRNA was injected into shrimp to knock down the expression of STAT. As a control, STAT-siRNA-scrambled was included in the injections. WSSV alone was used as a positive control. (D) The influence of STAT silencing on virus infection. The WSSV copies in gills of STAT-siRNA-treated shrimp were quantified using quantitative real-time PCR. In all panels, statistically significant differences between treatments were indicated by asterisks (**p < 0.01).
To evaluate the influence of STAT in WSSV infection, the STAT expression was silenced by gene-specific siRNA (STAT-siRNA) in shrimp in vivo, followed by the detection of virus copies. The expression of the STAT gene was significantly knocked down at 24 and 48 h post-infection compared with the WSSV-only control group; by contrast, STAT-siRNA-scrambled had no effect on the STAT expression (Fig. 4C), thereby showing that the siRNA was highly specific. When the expression of the STAT gene was knocked down, the number of WSSV copies in shrimp gills was significantly increased compared with the control (WSSV only; Fig. 4D). The data indicated that the host STAT gene plays a negative role in WSSV infection in vivo.
Dnm1, Dnm2, and Dnm3 expression is regulated by STAT
The human Mx homolog MxA belongs to the class of interferon (IFN)-stimulated genes (ISGs); this gene is JAK/STAT-dependent and can inhibit the replication of orthomyxoviruses and other RNA viruses21. Haller and Kochs reported that Mx proteins belong to the superfamily of Dnm-like large GTPases21. Consequently, we predicted that Dnms are important effectors of the JAK-STAT signaling pathway in M. rosenbergii. To reveal the relationship between STAT and the Dnm genes (Dnm1, Dnm2, and Dnm3), the expression of STAT was knocked down by STAT-siRNA in shrimp. After the expression of the STAT gene was silenced, the Dnm1, Dnm2, and Dnm3 expression levels were detected by qRT-PCR. The results are shown in Fig. 5, wherein the three Dnms were induced by 1.4-fold to 3.5-fold after the WSSV challenge. However, the expression levels of these Dnms were significantly decreased in the STAT-siRNA experimental group compared with the controls (Fig. 5A–C). Therefore, Dnm1, Dnm2, and Dnm3 are likely the downstream genes of STAT.
The WSSV-infected shrimp were injected with STAT-siRNA to silence the expression of STAT gene, followed by the quantification of Dnm1 (A), Dnm2 (B) or Dnm3 (C) mRNA using quantitative real-time PCR. The results are shown as mean and standard deviation from three independent experiments (**p < 0.01).
Dnm1, Dnm2, and Dnm3 mediated by miR-9041 or miR-9850
The abovementioned data implied that miR-9041, miR-9850, STAT, Dnm1, Dnm2, and Dnm3 are involved in the virus infection of shrimp in vivo. Therefore, the pathway mediated by the host miR-9041 and miR-9850 was further explored. The results indicated that the expression levels of Dnm1, Dnm2, and Dnm3 are significantly down-regulated when miR-9041 or miR-9850 is overexpressed in shrimp (Fig. 6A,C,E,G,I,K). When the expression of miR-9041 or miR-9850 is knocked down by AMO-miR-9041 or AMO-miR-9850, the expression levels of Dnm1, Dnm2, and Dnm3 are up-regulated (Fig. 6B,D,F,H,J,L), thereby suggesting that the host miRNAs, Dnm1, Dnm2, and Dnm3 shared the same pathway.
Effects of miR-9041 overexpression on the expressions of Dnm1 (A), Dnm2 (C) or Dnm3 (E) in vivo. Effects of miR-9850 overexpression on the expressions of Dnm1 (G), Dnm2 (I) or Dnm3 (K). miR-9041-mimic or miR-9850-mimic and WSSV were co-injected into shrimp. As a control, WSSV only was included in the injections. At different time after injection (0, 24, 36, and 48 h), the mRNA levels of Dnm1, Dnm2 and Dnm3 were quantified by quantitative real-time PCR. Influence of miR-9041 silencing on the Dnm1 (B), Dnm2 (D) or Dnm3 (F) expressions. Influence of miR-9850 silencing on the Dnm1 (H), Dnm2 (J) or Dnm3 (L) expressions. AMO-miR-9041 or AMO-miR-9850 was injected into WSSV-infected shrimp to knock down the miR-9041 or miRv-9850 expression. WSSV only was used as a control. At different time post-infection (0, 24, 36, and 48 h), the expressions of Dnm1, Dnm2 and Dnm3 were detected with quantitative real-time PCR. Statistically significant differences between treatments were indicated by asterisks (**p < 0.01).
Effects of Dnm1, Dnm2, and Dnm3 in virus infection
As one of the key effectors of the JAK/STAT pathway, Dnm genes are downstream of STAT. To evaluate the roles of Dnm1, Dnm2, and Dnm3 in viral infection, these genes were characterized in shrimp. As shown in Figs. S1A, S1C, and S1E, the mRNAs of Dnm1, Dnm2, and Dnm3 were detected in all the examined tissues, including the hemocyte, heart, hepatopancreas, gills, stomach, intestine, and nerve tissues. In response to WSSV infection, the expression levels of Dnm1, Dnm2, and Dnm3 were significantly up-regulated from 24 h to 48 h (Figs. S1B, S1D, and S1F). Furthermore, when the expression of Dnm1, Dnm2, and Dnm3 was knocked down by sequence-specific siRNAs (Fig. 7A–C), the number of WSSV copies significantly increased as compared with the controls (WSSV only). By contrast, the Dnm1-siRNA-scrambled, Dnm2-siRNA-scrambled, and Dnm3-siRNA-scrambled treatments had no effect on the virus infection (Fig. 7D–F). The data demonstrated that Dnm1, Dnm2, and Dnm3 had significant effects on virus infection in shrimp.
The gene expression silencing of Dnm1 (A), Dnm2 (B) or Dnm3 (C) in shrimp. The sequence-specific Dnm1-siRNA, Dnm2-siRNA or Dnm2-siRNA was injected into shrimp. Then the gene expression was examined. As a negative control, siRNA-scrambled was included in the injections. WSSV alone was used as a positive control. The detection of WSSV copies after Dnm1 (D), Dnm2 (E) or Dnm3 (F) knocked down in shrimp. The gills of siRNA-treated shrimp were subjected to the quantitative real-time PCR analysis to examine the WSSV copies. WSSV alone was used as a positive control. In all panels, asterisks indicated significant differences between treatments (**p < 0.01).
Recombinant plasmids (pET30a-Dnm1, pET30a-Dnm2, and pET30a-Dnm3) were respectively transformed into E. coli BL21 (DE3). After IPTG induction for 4 h, the whole cell lysates were analyzed by SDS–PAGE. In Fig. 8A, a distinct band with a molecular weight (MW) of approximately 100 kDa was detected, which was roughly consistent with the predicted MW of Dnm1 (theoretical MW, 96.4 kDa) and the ~5 kDa His tag at the N-terminal. The recombinant Dnm2 contained an additional N-terminal His Tag (5 kDa); thus, its size was relatively larger (~90 kDa) than the theoretical MW (84.8 kDa; Fig. 8B). Furthermore, a distinct band with an 80 kDa molecular mass was revealed, which agreed with the predicted molecular mass of the recombinant Dnm3 protein (77.6 kDa) with a His tag (Fig. 8C).
SDS–PAGE analysis of rDnm1 (A), rDnm2 (B) and rDnm3 (C). Lane M: protein molecular weight standards (kDa); lane 1: negative control (without induction); lane 2: IPTG induced rDnms; lane 3: purified rDnms. Recombined Dnm1 (D), rDnm2 (E) and rDnm3 (F) proteins affect the WSSV copies in shrimp. Purified rDnm1, rDnm2 or rDnm3 was individually incubated with WSSV at 28 °C for 30 min with rotation. BSA as control was incubated with WSSV. Four groups of mixtures were injected into the shrimps. The gills of shrimps were collected at different post injection times (0, 24, and 48 h) to detect the WSSV copies by quantitative real-time PCR. Asterisks indicated significant differences (*p < 0.05; **p < 0.01) between treatments.
Given that Dnm1, Dnm2, and Dnm3 could be involved in virus infection at the mRNA level, we further hypothesized whether or not recombined Dnm1, Dnm2, and Dnm3 proteins affect the WSSV copies in shrimp. To test this hypothesis, the inhibition of WSSV replication was performed. The number of WSSV copies was replicated at a slower rate in the gills of the rDnm1, rDnm2, or rDnm3 injection groups compared with the BSA control and WSSV only groups at all post-injection times (24 and 48 h; Fig. 8D–F). These findings indicated that rDnm1, rDnm2, and rDnm3 had important functions in the inhibition of WSSV replication.
Discussion
MicroRNAs play an important role in the regulation of gene expression25. First discovered in the nematode Caenorhabditis elegans, miRNAs have been identified in all multicellular eukaryotes and some viruses34. Recent studies demonstrate that miRNAs can also strongly affect the replication of pathogenic viruses. Viruses are known to encode their own miRNAs and/or trigger changes in cellular miRNA expression to target mRNAs of virus and/or host genes, which further results in either mRNA degradation or translational repression33. The viral miRNAs WSSV-miR-66 and WSSV-miR-68 can promote WSSV infection by targeting the WSSV genes (the WSSV wsv094 and wsv177 genes are the targets of WSSV-miR-66; the wsv248 and wsv309 genes are the targets of WSSV-miR-68)33. WSSV-miR-N24 is employed by WSSV to regulate the expression of shrimp caspase 8 and facilitate viral replication by inhibiting the host antiviral apoptotic activity35. Cellular miRNAs can influence hepatitis B virus (HBV) replication directly by binding to HBV transcripts and indirectly by targeting cellular factors relevant to the HBV life cycle36. In human hepatocytes, the host miR-373 is up-regulated by the hepatitis C virus during its infection and negatively regulated by the type I IFN signaling pathway via suppression of JAK1 and IFN regulating factor 9 (IRF9)37. The host miR-146 targets the interleukin 1 receptor-associated kinase (IRAK1) and TNF receptor-associated factor 6 (TRAF6) as a negative regulator of constitutive nuclear factor-κB (NF-κB) activity in breast cancer38. However, the regulation of transcription factor expression by the simultaneous mediation of two different host miRNAs has not been explored to date. Our study highlights a novel aspect of two host miRNAs, which target the mRNA of host STAT gene and suppress the corresponding JAK/STAT-Dnm1/Dnm2/Dnm3 signaling pathways in the virus–host interactions in vivo.
To date, more than 10,000 miRNAs have been annotated in 96 species39, including over 2500 human miRNAs (miRBase ver. 21; http://www.mirbase.org/, released in June 2014). Each miRNA can regulate hundreds of different mRNAs, whereas a single mRNA can be conversely targeted by several miRNAs40. Cellular miRNAs play crucial roles in several biological processes, such as the innate and adaptive immune response; the deregulation of miRNA expression and function is also involved in various diseases41. Viruses can alter cellular miRNA expression; cellular miRNAs can positively or negatively influence virus replication during viral infection by regulating the expression of viral genes and/or cellular factors relevant to the course of virus-induced disease42. Viruses are equipped with complex machinery to exploit and manipulate the host pathways to establish an environment favorable for their persistence43.
In this study, shrimp miR-9041 and miR-9850 can regulate the expression of the host gene STAT, which is one of key components of the JAK/STAT pathway. A WSSV-encoded miRNA (WSSV-miR-22) could also promote WSSV infection in Marsupenaeus japonicas shrimp by targeting the host STAT gene44. The JAK-STAT signaling pathway plays a critical role in the initiation of antiviral response. An IFN-like antiviral cytokine known as Vago can activate the JAK/STAT pathway to control viral loads in West Nile virus-infected Culex quinquefasciatus cells17. The up-regulation of miR-9041 or miR-9850 suppresses the expression of STAT and its downstream molecules to promote WSSV replication. However, the downstream genes and their precise mechanisms are not yet fully understood.
In our previous study, thioester-containing protein TEP1 and TEP2 were the effectors of JAK-STAT signaling pathway44. Whereas in this study, Dnm genes were found to be downstream of STAT. Dnms are involved in antiviral immune defense. The Mx dynamin-like GTPases are key antiviral effector proteins of the types I and III IFN systems23. Human Mx2 or MxB are ISGs that contribute to the inhibition of the human immunodeficiency virus (HIV-1) replication by interferons45. A Dnm-dependent protein-trafficking pathway can mediate Ebola virus glycoprotein toxicity46. Dnm is required for recombinant adeno-associated virus type 2 (rAAV-2) infections, whereas the overexpression of mutant DnmI significantly inhibited AAV-2 internalization and gene delivery47. Therefore, Dnm is a key mediator involved in a broad range of viral infections.
In conclusion, we demonstrated that the host miR-9041 and miR-9850 play positive roles in WSSV replication by targeting the host JAK/STAT signal pathway. Dnm genes are regulated by STAT and are involved in antiviral immune response. Therefore, WSSV infection could induce host miRNAs, which inhibit the JAK/STAT pathway and finally enhance virus replication.
Materials and Methods
Shrimp culture and WSSV challenge
The M. rosenbergii shrimp (approximately 15 g each) were purchased from an aquaculture market in Nanjing, Jiangsu Province, China, and acclimatized under laboratory conditions in the freshwater tanks at room temperature (25 °C) for a week before processing. To ensure that the shrimp were WSSV-free before experiments, PCR was conducted with WSSV-specific primers (5′-TATTGTCTCTCCTGACGTAC-3′and 5′-CACATTCTTCACGAGTCTAC-3′). Various tissues (hemocyte, heart, hepatopancreas, gills, stomach, intestine, and nerve; from 5 virus-free shrimp as parallel samples) were collected to determine the tissue distribution of STAT, Dnm1, Dnm2, and Dnm3 transcripts. Hemolymph was collected from untreated shrimp by mixing half the volume of anticoagulant buffer (0.14 M NaCl, 0.1 M glucose, 30 mM trisodium citrate, 26 mM citric acid, and 10 mM EDTA; pH 4.6)48. Samples were immediately centrifuged at 800 × g at 4 °C for 15 min to harvest the hemocytes. A total of 20 healthy shrimp were infected with 100 μL of WSSV virus solution at 105 copies/mL by intramuscular injection into the lateral area of the fourth abdominal segment. At different times post-infection (0, 24, 36, and 48 h), gills of 5 shrimp were randomly collected for each treatment and stored for future use.
Overexpression or silencing of miR-9041 and miR-9850 in shrimp
Based on their sequences, miR-9041 (5′-GAAUUCACCAAGCGUUGGAUUGUU-3′) and miR-9850 (5′-UGUAAAUUAGAUCGGGUAGGA-3′) were synthesized with the in vitro transcription T7 kit for siRNA synthesis (Takara, Japan). The sequences of miR-9041 and miR-9850 were scrambled to generate the miR-9041-scrambled (5′-UCAUCAGCAUCGAUAUGUAGUUGG-3′) and miR-9850-scrambled (5′-AUAGGAUAUGGGGUAUCAGUA-3′) controls. The synthesized miRNA was dissolved in miRNA solution (50 mM Tris-HCl, 100 mM NaCl, pH 7.5) and quantified by obtaining the absorbance at 260 nm with a spectrophotometer. To overexpress the host miRNAs, the miR-9041 or miR-9850 (15 μg) and WSSV (105 copies/mL) were co-injected into virus-free shrimp at a volume of 100 μL/shrimp. At 16 h after co-injection, the miRNAs (15 μg; 100 μL/shrimp) were injected into the same shrimp. miR-9041-scrambled, miR-9850-scrambled, WSSV alone (105 copies/mL), and phosphate buffer saline PBS (0.14 M NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4) were injected as controls.
To knock down miR-9041 or miR-9850 expression, an anti-miRNA oligonucleotide (AMO) was injected into WSSV-infected shrimp. AMO-miR-9041 (5′-AACAATCCAACGCTTGGTGAATTC-3′) was synthesized (Sangon Biotech, Shanghai, China) with a phosphorothioate backbone and a 2′-O-methyl modification at the 6th, 18th, and 22nd nucleotides. AMO-miR-9850 (5′-TCCTACCCGATCTAATTTACA-3′) was synthesized with a phosphorothioate backbone and a 2′-O-methyl modification at the 6th, 16th, and 18th nucleotides. AMO-miR-9041 or AMO-miR-9850 (10 nM) and WSSV (105 copies/mL) were co-injected into virus-free shrimp at 100 μL/shrimp. At 16 h after the co-injection, AMOs (10 nM; 100 μL/shrimp) were injected into the same shrimp. AMO-miR-9041-scrambled (5′-CATGGTAAATCGACACTTCGCTAA-3′), AMO-miR-9850-scrambled (5′-TACGATCTACTCCTACAATCT-3′), WSSV alone (105 copies/mL), or PBS were injected into shrimp as controls.
For each treatment, 20 shrimp were used. At different times post-infection (0, 24, 36, and 48 h), 5 shrimp were randomly collected for each treatment and subjected to the subsequent analyses. All the experiments were biologically replicated three times.
Detection of WSSV copies by quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed to examine the WSSV copies in gills of WSSV-infected shrimp. The viral DNA was extracted from shrimp gills with the EasyPure Marine Animal Genomic DNA Kit (TransGen Biotech, Beijing, China). The WSSV copies were detected by qRT-PCR with WSSV-specific primers (5′-TTGGTTTCAGCCCGAGATT-3′and 5′-CCTTGGTCAGCCCCTTGA-3′) and the WSSV-specific TaqMan probe (5′-FAM-TGCTGCCGTCTCCAA-TAMRA-3′). qRT-PCR was performed in a total volume of 25 μL, containing 12.5 μL of Premix ExTaq (TaKaRa, Japan), 0.5 μL of 10 μM forward primer, 0.5 μL of 10 μM reverse primer, 1 μL of 10 μM TaqMan fluorogenic probe, 1 μL of the DNA template, and 9.5 μL of distilled water. The predenaturation stage of the PCR program was 95 °C for 1 min, followed by the amplification stage consisting of 40 cycles of 95 °C for 30 s, 52 °C of 30 s, and 72 °C for 30 s. The assays were performed three times.
Prediction of target genes
To predict the genes targeted by host miRNAs, the shrimp genome sequence (data unpublished) was employed with three independent computational algorithms TargetScan 5.1 (http://www.targetscan.org), miRanda (http://www.microrna.org/), and Pictar (http://pictar.mdc-berlin.de/). TargetScan was used to search for miRNA seed matches (nucleotides 2–8 from the 5′-end of miRNA) in the 3′-untranslated region (UTR) sequences. miRanda was used to match the entire miRNA sequences. The miRanda parameters were set as free energy < −20 kcal/mol and score > 50. Pictar was employed to search the combined effects of microRNA target microRNA-based or other characteristics, with score > 20. Finally, the results predicted by the three algorithms were combined, and the overlaps were calculated33,49.
Plasmid construction
To characterize the direct interaction between miR-9041 or miR-9850 and the shrimp STAT gene, the 3′-UTR of STAT and the enhanced green fluorescent protein (EGFP) gene were cloned into a pIZ/EGFP V5-His vector (Invitrogen, USA). The EGFP gene was amplified from the pEGFP vector (BD Biosciences, USA) with EGFP-specific primers (5′-AAGAGCTCGGATCCCCGGGTA-3′and 5′-AATCTAGAGTCGCGGCCGCTTTA-3′). Subsequently, the STAT 3′-UTR was cloned into the pIZ vector downstream of the EGFP gene with the XbaI and SacII restriction sites and the sequence-specific primers (5′-GCTCTAGATAATAGGTTCTAGCACATG-3′and 5′-TCCCCGCGGATGTATATTATAAAAGTTTC-3′). As controls, the STAT 3′-UTR sequence (GTGAATT) complementary to the miR-9041 seed sequence was mutated to TGTCCGG, thereby yielding the EGFP-∆STAT-9041 construct, whereas the STAT 3′-UTR sequence (TAATTTAC) complementary to the miR-9041 seed sequence was mutated to GCCGGGCA, thereby yielding the EGFP-∆STAT-9850 construct. The mutant constructs of 3′-UTRs were obtained by DpnI-mediated site-directed mutagenesis (New England BioLabs, USA). All the recombinant plasmids were confirmed by sequencing.
Cell culture, transfection, and fluorescence assays
Insect High Five cells (Invitrogen, USA) were cultured at 28 °C in Express Five serum-free medium (Invitrogen) containing l-glutamine (Invitrogen). When the cells reached 70–80% confluence, they were transfected with 6 μg of EGFP, EGFP-STAT, EGFP-∆STAT-9041, or EGFP-∆STAT-9850. Simultaneously, the cells were transfected with 300 nM of synthesized miR-9041, synthesized miR-9850, or synthesized control miRNA. All the miRNAs were synthesized by Shanghai Gene Pharma Co., Ltd. (Shanghai, China). The transfection reactions were performed in triplicate with Cellfectin transfection reagent (Invitrogen) according to the manufacturer’s protocol. After 12 h of incubation, the transfected cells were seeded into 96-well plates at a concentration of 2.0 × 104 cells per well. At 48 h after transfection, the fluorescence of the cells was examined with a Flex Station II microplate reader (Molecular Devices, USA) at 490/510 nm for excitation and emission (Ex/Em), respectively. The fluorescence values were corrected by subtracting the autofluorescence of cells that did not express EGFP. All the experiments were biologically replicated three times.
Synthesis of siRNAs and RNAi assay in shrimp in vivo
Based on the sequences of the shrimp genes STAT, Dnm1, Dnm2, and Dnm3, siRNAs were separately synthesized according to the design rule for siRNA. The siRNAs used were STAT-siRNA (5′-GGTAACCAGAGAACACCTT-3′), Dnm1-siRNA (5′-CCTTGCAGGCAAGCCTTTA-3′), Dnm2-siRNA (5′-GGACAATCTCCAGAGCGAA-3′), and Dnm3-siRNA (5′-CCATCAAGGGCGATCCCAA-3′). The sequences of siRNAs were randomly scrambled to generate the control siRNAs (STAT-siRNA-scrambled: 5′-AAGTCAGTTAAGCACCCGA-3′; Dnm1-siRNA-scrambled: 5′-TGCCCTAGTAGCCCTAAGT-3′; Dnm2-siRNA-scrambled: 5′-CAGATCAGCAACGGATCGA-3′; Dnm3-siRNA-scrambled: 5′-TCACACGGAGAAATCGCCC-3′). The formation of double-stranded RNAs was monitored by determining the size in agarose gel electrophoresis.
The RNAi assay was conducted in shrimp by the injection of a siRNA at 30 μg/shrimp into the lateral area of the fourth abdominal segment with a 1 mL sterile syringe. The siRNA (15 μg) and WSSV (105 copies/mL) were co-injected into virus-free shrimp at a volume of 100 μL/shrimp. At 16 h after co-injection, the siRNA (15 μg; 100 μL/shrimp) was injected into the same shrimp. Simultaneously, the siRNAs-scrambled (15 μg; 100 μL/shrimp) was co-injected into virus-free shrimp. At 16 h after co-injection, siRNAs-scrambled (15 μg; 100 μL/shrimp) was injected into the same shrimp. WSSV (105 copies/mL; 100 μL/shrimp) alone was injected as a positive control. As a negative control, PBS was injected instead of the siRNAs. For each treatment, 20 shrimp were used. Shrimp gills were collected at different times after the last injection (0, 24, 36, and 48 h). From each treatment, 5 shrimp specimens were randomly selected and collected for later use. The assays were biologically replicated three times.
Detection of mRNA by qRT-PCR
SYBR Green fluorescent qRT-PCR was used to detect the expression of the shrimp genes STAT, Dnm1, Dnm2, and Dnm3 at the mRNA level. Shrimp tissues were collected from WSSV-infected shrimp with different treatments at different time points after WSSV infection (0, 6, 12, 24, 36, and 48 h). The total RNAs were extracted from the abovementioned tissues using RNApure high-purity total RNA rapid extraction kit (Spin-column, BioTeke, Beijing, China) following the manufacturer’s protocol. RNA quality was assessed by electrophoresis on 1.0% agarose gels. The total RNA concentration was determined by obtaining the absorbance at 260 nm with a spectrophotometer. First-strand cDNA synthesis was performed with the PrimeScript® 1st Strand cDNA Synthesis Kit (Takara, Dalian, China) and the Oligo dT Primer. The cDNA mix was diluted to 1:10 by PCR-grade water before storage at −80 °C until qRT-PCR. The shrimp glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a control. The gene-specific primers (STAT-RT-F: 5′-GTGTGAACGACATCAGACCAAG-3′, STAT-RT-R: 5′-TAACAAGTGAAGAGAGAAGACGAGT-3′; Dnm1-RT-F: 5′-TTTTACACAAGAAGGGCGACAGG-3′, Dnm1-RT-R: 5′-CACATTTGGCGAATAAACCCG-3′; Dnm2-RT-F: 5′-CACAACACCTACGCATCAGCTTC-3′, Dnm2-RT-R: 5′-CACAGTCCTTGGTCTCCCTCTCC-3′; Dnm3-RT-F: 5′-GGTCCATATCACATTGCATCAT-3′, Dnm3-RT-R: 5′-TTCTCAACTCAACCTCCTTTCTC-3′; GAPDH-RT-F: 5′-TGCCGCCCAGAACATCATT-3′, GAPDH-RT-R: 5′-TCGTCTTCGGTGTAGCCCA-3′) were used. qRT-PCR was performed according to the manufacturer’s instructions for the 2 × SYBR Premix Ex Taq Kit (Takara, Japan) with a real-time thermal cycler (Bio-Rad, Hercules, CA, USA). PCR was conducted with initial denaturation at 95 °C for 3 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 30 s. Data were quantified by the 2−ΔΔCT (ΔΔCT = ΔCT of sample − ΔCT of untreated control) method50 and subjected to statistical analysis.
Recombinant expression and purification of Dnm1, Dnm2, and Dnm3 in Escherichia coli
The full-length cDNAs encoding the mature Dnm1, Dnm2, and Dnm3 were amplified by the primers (Dnm1-ex-F: 5′-TATCGGATCCGAATTCCTGCAAGATGCATTTACACAG-3′, Dnm1-ex-R: 5′-GGTGGTGGTGCTCGAGATGTGGGCGCTGAGGCACTTGA-3′; Dnm2-ex-F: 5′-TATCGGATCCGAATTCCAGGATGTCTTCAACACCGTC-3′, Dnm2-ex-R: 5′-GGTGGTGGTGCTCGAGGATTTCACCAATAATCACGTTAGC-3′; and Dnm3-ex-F: 5′-TATCGGATCCGAATTCGGAGTTAGCCAGTCACGGTG-3′, Dnm3-ex-R: 5′-GGTGGTGGTGCTCGAGTGACAGAGCATCTTCCCACTG-3′), with EcoRI and XhoI sites in the forward and reverse primers, respectively. The amplified products were cloned into pET30a(+) vector (Novagen) and transformed into competent cells of E. coli BL21 (DE3) (TransGen Biotech, Beijing, China). The positive clones were screened by PCR with specific primers and confirmed by further nucleotide sequencing. The cells were grown at 37 °C in LB medium under agitation until they reached an OD600 nm of 0.6–0.8. Recombinant expression was induced by the addition of isopropyl-b-d-1-thiogalactopyranoside (IPTG; 0.5 mmol/L), followed by incubation for another 4 h under the same conditions. The recombinant rDnm1, rDnm2, and rDnm3 proteins were separated by reducing 12.5% SDS polyacrylamide gel electrophoresis (SDS–PAGE) and visualized with Coomassie brilliant blue R250. rDnm1, rDnm2, and rDnm3 with a His tag were purified by His Bind resin chromatography (Novagen, USA) according to the manufacturer’s instructions.
Inhibition of WSSV copies
A total of 500 μL purified recombinant rDnm1 (600 μg/mL), rDnm2 (600 μg/mL), or rDnm3 (600 μg/mL) was individually incubated with 500 μL of WSSV (105 copies/mL) at 28 °C for 30 min with rotation. Bovine serum albumin (BSA; 600 μg/mL, 500 μL) was incubated with WSSV as the control. Four groups of mixtures (100 μL each) were respectively injected into the shrimp. The gills of 5 shrimp were collected at different post-injection times (0, 24, and 48 h) to detect the WSSV copies by qRT-PCR.
Statistical analysis
The data from three independent experiments were analyzed by one-way ANOVA to calculate the mean and standard deviation of the triplicate assays. Statistically significant differences between treatments were determined with the t-test, with significance defined as P < 0.05 or P < 0.01.
Additional Information
How to cite this article: Huang, Y. et al. Two host microRNAs influence WSSV replication via STAT gene regulation. Sci. Rep. 6, 23643; doi: 10.1038/srep23643 (2016).
References
Roustaian, P., Kamarudin, M. S., BinOmar, H., Saad, C. R. & Ahmad, M. H. Amino acid composition of developing larval freshwater prawn Macrobrachium rosenbergii . J World Aquacult Soc. 31, 130–136 (2000).
Tidwell, J. H., Coyle, S., Arnum, A. & Weibel, C. Production response of freshwater prawns Macrobrachium rosenbergii to increasing amounts of artificial substrate in ponds. J World Aquacult Soc. 31, 452–458 (2000).
Saurabh, S. & Sahoo, P. K. Major diseases and the defence mechanism in giant freshwater prawn, Macrobrachium rosenbergii (de Man). Proc Natl Acad Sci India B. 78, 103–121 (2008).
Sánchez-Paz, A. White spot syndrome virus: an overview on an emergent concern. Vet Res. 41, 43 (2010).
van Hulten, M. C. et al. The white spot syndrome virus DNA genome sequence. Virobgy 286, 7–22 (2001).
Dieu, B. T. et al. Molecular epidemiology of white spot syndrome virus within Vietnam. J Gen Virol 85, 3607–3618 (2004).
Escobedo-Bonilla, C. M. et al. A review on the morphology, molecular characterization, morphogenesis and pathogenesis of white spot syndrome virus. J Fish Dis. 31, 1–18 (2008).
Leu, J. H. et al. The unique stacked rings in the nucleocapsid of the white spot syndrome virus virion are formed by the major structural protein VP664, the largest viral structural protein ever found. J Virol 79, 140–149 (2005).
Tsai, J. M. et al. Genomic and proteomic analysis of thirty-nine structural proteins of shrimp white spot syndrome virus. J Virol 78, 11360–11370 (2004).
Lightner, D. V. Epizootiology distribution and the impact on international trade of two penaeid shrimp viruses in the Americas. Rev Sci Tech Oie. 15, 579–601 (1996).
Li, F. H. & Xiang, J. H. Signaling pathways regulating innate immune responses in shrimp. Fish Shellfish Immunol 34, 973–980 (2013).
Kiu, H. & Nicholson, S. E. Biology and significance of the JAK/STAT signalling pathways. Growth factors 30, 88–106 (2012).
Stark, G. R. & Darnell, J. E. Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514 (2012).
Rawlings, J. S., Rosler, K. M. & Harrison, D. A. The JAK/STAT signaling pathway. J Cell Sci. 117, 1281–1283 (2004).
Samuel, C. E. Antiviral actions of interferons. Clin Microbiol Rev. 14, 778–809 (2001).
Myllymäki, H. & Rämet, M. JAK/STAT pathway in Drosophila immunity. Scand J Immunol 79, 377–385 (2014).
Paradkar, P. N., Trinidad, L., Voysey, R., Duchemin, J. B. & Walker, P. J. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci USA 109, 18915–18920 (2012).
Lagueux, M., Perrodou, E., Levashina, E. A., Capovilla, M. & Hoffmann, J. A. Constitutive expression of a complement-like protein in Toll and JAK gain-of-function mutants of Drosophila. Proc Natl Acad Sci USA 97, 11427–11432 (2000).
Liu, W. J., Chang, Y. S., Wang, A. H., Kou, G. H. & Lo, C. F. White spot syndrome virus annexes a shrimp STAT to enhance expression of the immediate-early gene ie1 . J Virol 81, 1461–1471 (2007).
Haller, O. & Kochs, G. Interferon-induced Mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3, 710–717 (2002).
Ferguson, S. M. & De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13, 75–88 (2012).
Ferguson, S. M. et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17, 811–822 (2009).
Haller, O., Gao, S., von der Malsburg, A., Daumke, O. & Kochs, G. Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity. J Biol Chem. 285, 28419–28424 (2010).
Sun, Y. & Tien, P. From endocytosis to membrane fusion: emerging roles of dynamin in virus entry. Crit Rev Microbiol 39, 166–179 (2013).
Medina, P. P. & Slack, F. J. MicroRNAs and cancer: an overview. Cell Cycle 7, 2485–2492 (2008).
Xiao, C. & Rajewsky, K. MicroRNA control in the immune system: basic principles. Cell 136, 26–36 (2009).
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Winter, J., Jung, S., Keller, S., Gregory, R. I. & Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 11, 228–234 (2009).
Bellare, P. & Ganem, D. Regulation of KSHV lytic switch protein expression by a virus-encoded microRNA: an evolutionary adaptation that fine-tunes lytic reactivation. Cell Host Microbe 6, 570–575 (2009).
Lung, R. W. M. et al. Modulation of LMP2A expression by a newly identified Epstein-Barr virus-encoded microRNA miR-BART22. Neoplasia 11, 1174 (2009).
Liang, D. et al. A human herpesvirus miRNA attenuates interferon signaling and contributes to maintenance of viral latency by targeting IKKε. Cell Res. 21, 793–806 (2011).
He, Y. D. & Zhang, X. B. Viral microRNAs targeting virus genes promote virus infection in shrimp in vivo . J Virol 88, 1104–1112 (2014).
Qureshi, A., Thakur, N., Monga, I., Thakur, A. & Kumar, M. VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database (Oxford) (2014).
Huang, T. Z., Cui, Y. L. & Zhang, X. B. Involvement of viral microRNA in the regulation of antiviral apoptosis in shrimp. J Virol 88, 2544–2554 (2013).
Kitab, B., Alj, H. S., Ezzikouri, S. & Benjelloun, S. MicroRNAs as Important Players in Host-hepatitis B Virus Interactions. J Clin Transl Hepatol 3, 149–161 (2015).
Mukherjee, A., Di Bisceglie, A. M. & Ray, R. B. Hepatitis C virus-mediated enhancement of microRNA miR-373 impairs the JAK/STAT signaling pathway. J. Virol. 89, 3356–3365 (2015).
Bhaumik, D. et al. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene 27, 5643–5647 (2008).
Griffiths-Jones, S., Grocock, R. J., van Dongen, S., Bateman, A. & Enright, A. J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–144 (2006).
Kitab, B., Alj, H. S., Ezzikouri, S. & Benjelloun, S. MicroRNAs as Important Players in Host-hepatitis B Virus Interactions. J Clin Transl Hepatol 3, 149–161 (2015).
Ebert, M. S. & Sharp, P. A. Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515–524 (2012).
Skalsky, R. L. & Cullen, B. R. Viruses, microRNAs, and host interactions. Annu Rev Microbiol. 64, 123–141 (2010).
Sullivan, C. S. New roles for large and small viral RNAs in evading host defences. Nat Rev Genet. 9, 503–507 (2008).
Ren, Q., Huang, Y., He, Y. D., Wang, W. & Zhang, X. A white spot syndrome virus microRNA promotes the virus infection by targeting the host STAT. Sci Rep. 16, 5 18384 (2015).
Xu, B. et al. Structural insight into the assembly of human anti-HIV dynamin-like protein MxB/Mx2. Biochem Biophys Res Commun 456, 197–201 (2015).
Sullivan, N. J. et al. Ebola virus glycoprotein toxicity is mediated by a dynamin-dependent protein-trafficking pathway. J Virol 79, 547–553 (2005).
Duan, D. et al. Dynamin Is Required for Recombinant Adeno-Associated Virus Type 2 Infection. J Virol 73, 10371–10376 (1999).
Ren, Q., Li, M., Du, J., Zhang, C. Y. & Wang, W. Immune response of four dual-CRD C-type lectins to microbial challenges in giant fresh water prawn Macrobrachium rosenbergii . Fish Shellfish Immunol 33, 155–167 (2012).
Huang, T., Xu, D. & Zhang, X. Characterization of host microRNAs that respond to DNA virus infection in a crustacean. BMC Genomics 13, 159 (2012).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 (2001).
Acknowledgements
We appreciate Professor Xiaobo Zhang (Zhejiang University) for directing the siRNA experiments. The current study was supported by the National Natural Science Foundation of China (Grant Nos. 31572647), the Natural Science Foundation of Jiangsu Province (BK20131401), Natural Science Fund of Colleges and universities in Jiangsu Province (14KJA240002), Graduate students Research and Innovation Program of Jiangsu Province (KYZZ15_0214), and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Y.H., Q.R. and W.W. carried out the experiments. Y.H. and Q.R. designed the experiments and analysed the data. Y.H. wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, Y., Wang, W. & Ren, Q. Two host microRNAs influence WSSV replication via STAT gene regulation. Sci Rep 6, 23643 (2016). https://doi.org/10.1038/srep23643
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23643
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.