Abstract
Coral reefs have been declining considerably in recent years because of changes to the environment and climate. The cryopreservation of coral gametes is an essential alternative method that yields immense success in preserving corals. This study focuses on developing vitrification techniques for Junceella fragilis and Ellisella robusta oocytes and presents a comparison on the cryotolerance of their vitrified oocytes. The results revealed that these coral oocytes could be preserved for a longer period in equilibration solution 2 and vitrification solution (VS) 2 at 5 °C than at 26 °C. Oocyte viability decreased significantly when VS2 was used for >4 min at 26 °C compared with the control. Cryoprotectant tolerance was higher in E. robusta oocytes than in J. fragilis oocytes. However, E. robusta was determined to be more cryo-tolerant, with differences attributed to their habitats, thus making E. robusta is likely a superior candidate species for further study. The results of this study on the effects of coral cryopreservation provide a foundation for developing protocols further for the cryopreservation of the oocytes of gorgonian corals.
Similar content being viewed by others
Introduction
Corals are most essential to the marine ecosystem because they provide both food and shelter to several aquatic organisms. In recent years, corals have been disappearing rapidly, consequently affecting the entire ecosystem. Therefore, it is urgent that both research and preservation efforts be devoted to saving coral reefs for conserving marine life1,2. Several factors have contributed to the major depletion of corals in recent years, such as environmental and climate changes as well as diseases and most prominently, human influence3,4. Overfishing has led to the loss of larger predators and an abundance of smaller organisms, resulting in competition and dominance over other species5. Furthermore, heavy pollution in oceans has drastically altered the marine habitat. In the event of natural disasters and damages, corals can regenerate; however, in recent years, this capability has decreased because of human disturbances6.
Cryopreservation involves preserving germ cells through freezing techniques for future breeding purposes. Because low temperatures are highly damaging to gametes, the primary concern in cryopreservation is in refining the freezing and thawing techniques for determining optimal conditions7,8. With current technologies, a variety of biological materials, including DNA, gametes, somatic cells, embryos and tissues can be cryopreserved9,10. Although the field of invertebrate cryobiology lags behind that of the respective vertebrate field, freezing techniques have nevertheless been employed to cryopreserve eggs and embryos of a diverse array of invertebrate taxa, such as cnidarians (e.g. corals, Junceella juncea)11, mollusks (e.g. oysters, Crassostrea virginica and greenshell mussel, Perna canaliculus)12,13, polychaetes (e.g. Nereis virens)14 and echinoderms (e.g. sea urchins, Cvechinus chloroticus)15.
Vitrification is a technique used in cryopreservation, where cells are suspended in a mixture of cryoprotectant (CPA) or equilibration solution (ES) and vitrification solution (VS) and then submerged in liquid nitrogen. Under rapid freezing conditions, this mixture forms a glass-like structure with minimal ice crystal formation16. Vitrification is becoming a preferred technique for cryopreservation because it is efficient, economic and minimises freezing injuries to the cells11. Vitrification differs from crystallisation because it does not involve molecule rearrangements in a crystal lattice structure. Regarding the former, it is clear from prior works that the oocytes of scleractinian corals are more sensitive to sub-zero temperatures than those of gorgonian corals, an observation that suggests that vitrification may be a preferred technique for the preservation of gorgonian oocytes11; briefly, this approach bypasses the hypothmic zone through direct contact of biological samples with liquid nitrogen. As such, the objective of the present study was to develop vitrification techniques for the ultimate cryopreservation of oocytes from gorgonian corals, Ellisella robusta and Junceella fragilis, with a focus on the cryotolerance of their vitrified oocytes.
Results
Vitrification properties of cryopreservant solutions
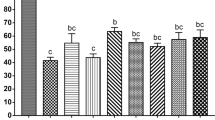
Figure 1 shows the results of the effects of various cryopreservants (i.e. propylene glycol [PG], ethylene glycol [EG], methanol, glycerol and dimethyl sulfoxide [DMSO]) along with different carriers (i.e. open pulled straw [OPS], cryotop, cyroloop and fibre plug) used for the vitrification process. The order of minimum concentrations required for an effective outcome was as follows: PG < DMSO = glycerol < EG < methanol. PG was the solution that required the lowest concentration and methanol was the solution that required the greatest concentration for vitrification. Fibre plug and cryotop were efficient carriers when used with methanol and glycerol, respectively, because vitrification was possible with a low concentration of these CPAs.
Vitrification properties of VS mediums
The vitrifying properties of the PG-based VSs were examined and the results are shown in Fig. 2. PG-based VS1 exhibited positive vitrification when used with all carriers. PG-based VS2 yielded successful vitrification with cryotop, cryoloop and fibre plug. Although PG-based VS3 could vitrify when used with all carriers, it was devitrified upon thawing. By contrast, PG-based VS4 and VS5 used with OPS and Cryoloop did not exhibit vitrification under cooling and thawing conditions.
ES1, ES2, VS2 on the viability of oocytes
The oocytes of J. fragilis and E. robusta submerged in ES1 did not exhibit any significant difference (P > 0.05) in viability at 26 °C and 5 °C, nor when these conditions were compared against those of the controls and also in the comparisons for the final ATP counts at 20 min (Fig. 3a,d). When the J. fragilis oocytes were submerged in ES2, no difference was observed between their starting and final ATP counts at 26 °C and 5 °C. However, after 10 min, the viability of the J. fragilis oocytes at 5 °C was significantly higher than at 26 °C (P < 0.05), indicating that the oocytes endured better when vitrification was performed at 5 °C. This phenomenon was not observed in the E. robusta oocytes. The results revealed that the oocytes of J. fragilis and E. robusta could be preserved for longer in VS2 without a considerable decrease in viability at 5 °C than at 26 °C. The viability of the J. fragilis oocytes in VS2 did not vary significantly at 5 °C; however, the final viability decreased at 8 min. By contrast, the viability of the E. robusta oocytes decreased significantly after 4 min of incubation in VS2 at 5 °C (P < 0.05). The viability of the oocytes of both species started decreasing after incubation in VS2 for 1 min at 26 °C, indicating that at ≥1 min the oocytes were affected by VS2 treatment.
Effects of PG-based ES1, ES2 and VS2 on the J. fragilis and E. robusta oocytes at 5 °C and 26 °C for different time periods.
Different letters and numbers represent statistical differences between exposure time points for oocytes treated with the same solutions (*P < 0.05 difference between temperatures).
Comparison of cryopreservant tolerance of the J. fragilis and E. robusta oocytes
Figure 4 shows a comparison between the oocytes of J. fragilis and E. robusta after being submerged in ES1 solution for 20 min at 26 °C and 5 °C. Because ES1 was used at lower concentrations compared with ES2 and VS2, the effect of ES1 on the oocytes was less obvious; consequently, determining a notable trend for the comparisons between viabilities of the J. fragilis and E. robusta oocytes under different conditions was difficult (Fig. 4a,d). The J. fragilis oocytes exhibited a high tolerance for ES2 at 26 °C and at 5 °C (Fig. 4b,e). The oocytes of J. fragilis and E. robusta initially exhibited similar responses to ES2; however, at 20 min, the viability of the J. fragilis oocytes was significantly higher than that of the E. robusta oocytes at both 26 °C and 5 °C (P < 0.05). The difference between the cryopreservant tolerance of the J. fragilis and E. robusta oocytes was more evident when VS2 was used. The ATP count of J. fragilis oocytes was significantly higher than that of the E. robusta oocytes at 2 min, 4 min and 8 min (P < 0.05; Fig. 4c). Although initially no substantial difference was observed between the viabilities of the 2 types of oocytes, at 8 min the J. fragilis oocytes exhibited higher viability, indicating that they could tolerate the toxic effects of VS2 compared with the E. robusta oocytes. In summary, J. fragilis oocytes exhibited higher tolerance to VS than did the E. robusta oocytes (Fig. 4f).
Comparison of the effects of ES1 (a,d), ES2 (b,e) and VS2 (c,f) at 26 °C and 5 °C at different time points for the J. fragilis and E. robusta oocytes. The black line represents E. robusta oocytes and the grey line represents J. fragilis oocytes. * represents a significant difference between the 2 species.
Vitrification
Among the 4 trials (Trials 1–4), none were found to yield a significant difference for oocyte viability between the J. fragilis and E. robusta oocytes (P < 0.05). Figure 5 shows a comparison between the 4 trials and controls. The viability of the J. fragilis oocytes was significantly lower than that of the control for all 4 treatments (P < 0.05), indicating that a stepwise vitrification process using ES1, ES2 and VS2 for the J. fragilis oocytes was not optimal. By contrast, the E. robusta oocytes responded better to the vitrification process; the results of Trial 1 and 2 revealed that the viability of the oocytes of E. robusta was lower than that of the controls; however, the difference was nonsignificant (P > 0.05). Trial 2 included the oocytes submerged in ES1 for the longest duration (15 min) before being transferred to ES2, indicating that treatment in ES1 for a long duration was required for protecting the oocytes before submerging them in a higher-concentration solution. The results of Trials 3 and 4 varied significantly compared with those of the controls (P < 0.05); however, the E. robusta oocytes endured better than the J. fragilis oocytes under the same conditions. These results revealed that the J. fragilis oocytes were more cryosensitive compared with the E. robusta oocytes.
Discussion
Of the 4 types of carriers examined in this study, cryotop and fibre plug performed the best. The factors necessary for optimal vitrification are a fast cooling rate, a high-viscosity VS and a small VS volume17. By reducing the VS volume and using carriers with a minimum capacity, heat conduction improves greatly for fast freezing18. Compared with OPS, cryotop and fibre plug use a substantially less amount of VS. Cryotop had the highest cooling rate compared with the other carriers used in this study. Fibre plug is typically used as a closed-carrier system; however, in this study, it was used as an open-carrier system. The cryotop carrier has been proven to be efficient for cattle, sheep, buffalo and sensitive porcine and cattle oocytes18. The cryotop design allows several samples to be loaded simultaneously with minimal VS; thus, it was the preferred carrier for our study.
A crucial step in vitrification is in evaluating the cryoprotectant, a solution that shields cells from being exposed to extremely low temperatures. VS must be nontoxic to cells and it must permeate through the cell membrane16. However, VS must be used at proper concentrations for complete vitrification; it is typically used at high concentrations because ice formation is reduced by increasing the amount of solutes7. In this study, VS2 was composed of 3.5M PG, 1.5M EG and 2M methanol. The examination of the CPAs with the 4 carriers revealed that PG required the lowest concentration for completing vitrification, whereas methanol required the highest concentration compared with the other carriers. Previous studies have shown that methanol permeates throughout the cell membrane and protects coral oocytes, although it is used at high concentrations for complete vitrification19,20. As mentioned, a CPA with high viscosity is preferable for vitrification; thus, glycerol should have been a good candidate. However, in our previous experiments, we had compared glycerol- and PG-based VS under the same conditions as used in this study and the results had shown that the PG-based VS yielded a suitable performance11. A similar study on zebrafish oocytes reported that DMSO was toxic, leading to various concerns, including protein denaturation and cytoskeletal reassembly21. However, another study on starfish oocytes reported that DMSO was an efficient CPA when used at low concentrations22.
VSs can also be toxic and detrimental to oocytes if they are directly submerged in VSs. It is essential to create ESs at low concentrations to ease interactions between the biological materials and the final VS in a stepwise manner. The viability of the oocytes submerged in ES1 did not exhibit significant differences at 5 °C and 26 °C; however, an observable difference was noted with ES2 and VS2. This result was noted for camel oocytes as well, which retained their metabolic levels at 4 °C than at 20 °C after 24 h23. At warm temperatures, oocytes metabolise and their organelles develop. If an oocyte is subjected to experimentation at 26 °C under toxic conditions, it is still metabolically active, despite losing its major functions23. Storing oocytes at low temperatures slows their metabolic process, thus slowing the degenerative process23. CPA tolerance is conditional and it depends strongly on the components used in a VS, the temperature of the treatment facility, the animal species and the stage of oocyte development7. This explains the major difference between the viability of the E. robusta and J. fragilis oocytes after exposure to ES2 and VS2. Any combination of these factors could have affected our results.
One of the most frequent problems when performing a vitrification assay is in reducing osmotic shock, cell shrinkage, or expansion caused by the rapid loss or gain of water because of the differences in solute concentrations24. When an oocyte is submerged in the VS, the CPA essentially replaces the water present inside the oocyte24. If the solution is extremely hypertonic, the oocytes equilibrate through osmosis and undergo osmotic shock; however, this osmotic shock can be attenuated substantially with the slow introduction of the CPA24. Therefore, it was necessary to perform stepwise vitrification by using ES1 and ES2, 2 solutions at less toxic concentration levels, before adding VS2. This could also explain the high yield in viabilities obtained in Trials 1 and 2, which involved ES1 exposure for 15 min, compared with the rates obtained in Trials 3 and 4, which involved ES1 exposure for 10 min. Submerging cells in ES1 for a long period was beneficial because the oocytes had more time to equilibrate before being submerged in ES2.
In this study, the E. robusta oocytes were more cryotolerant than were the J. fragilis oocytes; however, the E. robusta oocytes were more CPA-sensitive. Our previous study reported that J. juncea oocytes have a higher lipid content compared with J. fragilis oocytes25. These differences were attributed to dissimilarities in their habitat, where J. fragilis is primarily found at depths of 3–5 m, whereas E. robusta is found at depths <20 m. Presumably, E. robusta has adapted to living under greater depths because its membrane has a high lipid composition, which allows for better membrane fluidity and protection from colder temperatures25,26. The lipid content of late-stage coral oocytes was also less than that of their early-stage counterparts; however, late-stage E. robusta oocytes had a higher lipid composition compared with late-stage J. fragilis oocytes. This can explain why the E. robusta oocytes were more cryotolerant than were the J. fragilis oocytes. A similar study was performed on starfish oocytes by using CPAs, DMSO, EG and glycerol. First, CPA toxicity was examined22. In that study, the VS was composed of trehalose, DMSO and artificial seawater in aluminum pans and cryostraws and the optimal treatment led to a 34% survival rate22. Sugar, which is impermeable through the cell membrane, offsets the osmotic effect through a toxic VS and many organisms naturally produce sugar when under stress. Furthermore, the surviving oocytes are fertilised and exhibit signs of normal development5. Instead of a stepwise addition of the VS, in the study on the cryopreservation of starfish oocytes, stepwise removal was performed during the thawing process. Although the methods differed, in our study, VS2 did not contain any sugar, which could be a potential area of improvement for future experiments.
Our study substantiated the difficulty in cryopreservation; no fixed method exists for optimal cryopreservation because multiple factors could affect cell survival. However, it was necessary to perform a series of trial experiments to identify the most efficient conditions. The results of our study revealed that the J. fragilis and E. robusta oocytes maintained viability better when treated at 5 °C than at 26 °C. After comparing the 2 species of gorgonian corals, we found J. fragilis to be more CPA-tolerant, whereas E. robusta was more cryotolerant, The J. fragilis and E. robusta colonies were typically found at 3–5 and <20 m depth, respectively. The collection depth difference between the former and latter species may have contributed to the variation in cryotolerance; specifically, E. robusta was determined to be more cryo-tolerant, and, as such, is a better candidate along with J. juncea for further studies. The results of this study on the effects of coral cryopreservation provide a foundation for developing protocols further for the cryopreservation of the oocytes of gorgonian corals.
Methods
Coral collection and oocyte isolation
J. fragilis and E. robusta were collected during reproduction seasons (June–September, 2011–2014) by divers off the coast of Checheng Township, Pingtung County, Taiwan (21°5′60″N, 120°5′60″E) and transported to Coral Husbandry Center, where they were bound, secured to a substrate and placed in gravel bases of half-ton tanks with a water flow system at 25 °C. Using a knife, a thin tissue was cut from the surface of the corals, where the gametes were developed to determine whether they were viable for testing. The coral gametes were visible under a dissection microscope (SZ51; Olympus, Tokyo, Japan); sperm appeared smaller and transparent, whereas oocytes were larger and opaque. Polyps on the coral exterior indicate sites of coral germ cells. A thin layer was sliced from the coral surface and examined under the dissection microscope. With a pair of tweezers, the oocytes were prodded out of the strip and collected using a pipette. Oocytes >250 μm were used in this study. These corals are not regulated under Taiwanese law and coral collection was approved by Kenting National Park.
VSs and carriers
To determine the minimum concentration of each type of cryoprotectant for complete vitrification, 5 CPAs, namely methanol, DMSO, PG, EG and glycerol at different concentrations (1–10M) were prepared using 0.2-μm filtered seawater and frozen with liquid nitrogen. All chemicals were purchased from Sigma (St. Louis, MO, USA). A solution was considered vitrified if it appeared transparent and glass-like, indicating no ice crystal formation; however, it was considered devitrified if it formed ice crystals and appeared opaque. Different vitrification carriers, namely OPS (IMV Technologies, Paris, France), cryotop (Kitazato, Tokyo, Japan), cryoloop (Hampton research, Riverside, CA, USA) and fibre plug (CryoLogic, Melbourne, VIC, Australia), were evaluated with the solutions to examine the vitrification properties of the CPAs. Each solution was examined 3 times using each carrier. OPS, cryotop, cryoloop and fibre plug were loaded with a micropipette and then placed in liquid nitrogen. Based on these results, 5 VSs (VS1: 4M PG + 1M EG + 2M methanol; VS2: 3.5M PG + 1.5M EG + 2M methanol; VS3: 3M PG + 2M EG + 2M methanol; VS4: 2.5M PG + 2.5M EG + 2M methanol; VS5: 2M PG + 3M EG + 2M methanol) were prepared and examined with each of the 4 carriers for 3 times under the mentioned conditions.
ESs and VS on oocytes
Preparing ESs at low concentrations is essential because they facilitate the interaction between oocytes and the final VS in a stepwise manner. Oocytes were submerged in ES1 (1M PG + 0.5M EG + 0.5M methanol) and were examined at 5-min intervals for 20 min. After each interval, 5 oocytes were drawn from the solution and an ATP count was performed, indicating the viability of the oocytes. The experiments were performed both at room temperature (approximately 26 °C) and in an ice bath (approximately 5 °C) for 3 times. These conditions were repeated for evaluating ES2 (2M PG + 1M EG + 1M methanol) and VS2 (3.5M PG + 1.5M EG + 2M methanol), except that the intervals for the VS2 experiment were at 1 min, 2 min, 4 min and 8 min.
Vitrification using ES1, ES2 and VS2
The effect of stepwise vitrification on the oocytes was determined using ES1, ES2 and VS2 at 5 °C by using the cryotop carrier. Fifteen oocytes were submerged in ES1 and then transferred to ES2 for various time intervals in each trial. During Trial 1, the oocytes were in ES1 and ES2 for 15 min and 10 min, respectively; 15 min and 5 min in ES1 and ES2, respectively, during Trial 2; 10 min in ES1 and ES2 each during Trial 3; and 10 min and 5 min in ES1 and ES2, respectively, in Trial 4. Finally, the oocytes were submerged in VS2 for 2 min. The oocytes were then placed on the cryotop carrier by using a pipette suction and the carrier was submerged in liquid nitrogen for at least 10 min. After vitrification, the oocytes were immediately transferred back into VS2 at 5 °C for thawing and for preventing ice-crystal formation and an ATP assay was performed to confirm oocyte viability. Each trial experiment was performed in triplicate.
Viability assay
An ATP Assay was performed for determining the viability of the coral oocytes. The productivity of the mitochondria, as indicated by the number of ATPs, indicated the degree of harm for the oocytes in a treatment. Coral oocytes were placed in a test tube and seawater was drawn using a pipette. Oocyte viability was assessed through a luminescence assay (ApoSENSOR Cell Viability Assay Kit, BioVision, Cambridge BioScience, UK). In brief, a nucleotide-releasing buffer (100 μL) was added to the test tube and after 3–5 min, an ATP-monitoring enzyme (5 μL) was added. After approximately 30 s to 1 min, the test tube was placed into a luminometer (Lumat LB 9507, Berthold Technologies, Germany) and readings were taken27.
Statistics
The normal distribution of the data was confirmed by performing a one-sample Kolmogorov–Smirnov test. Two-way analysis of variance was then performed to determine significant differences between the groups. Variance homogeneity was confirmed by conducting the Levene test (P > 0.05). All P values reported in the results section correspond to Tukey’s post-hoc differences between individual means; such tests were conducted only when a significant treatment effect was detected in the overall ANOVA model. The data are presented in the tables and figures as the mean ± SEM and P values < 0.05 were considered statistically significant. All statistical data were generated using SPSS (version 17, IBM, Armonk, NY, USA).
Additional Information
How to cite this article: Tsai, S. et al. Comparison of the cryo-tolerance of vitrified gorgonian oocytes. Sci. Rep. 6, 23290; doi: 10.1038/srep23290 (2016).
References
Tsai, S., Kuit, V., Lin, Z. G. & Lin, C. Application of a functional marker for the effect of cryoprotectant agents on gorgonian coral (Junceella juncea J. fragilis) sperm sacs. CryoLetters 35(1), 1–7 (2014).
Tsai, S. et al. Ultrastructural observations of the early and late stages of gorgonian coral (Junceella juncea) oocytes. Tissue Cell 46(4), 225–232 (2014).
Tsai, S. et al. Development of cryopreservation techniques for gorgonian (Junceella juncea) oocytes through vitrification. PLoS ONE (2015). 10.1371/journal.pone.0123409.
Tsai, S., Spikings, E., Kuo, F. W. & Lin, C. Use of an adenosine triphosphate assay and simultaneous staining with fluorescein diacetate and propidium iodide, to evaluate the effects of cryoprotectants on hard coral (Echinopora ) oocytes. Theriogenology 73, 605–611 (2010).
Bellwood, D. R., Hughes, T. P., Folke, C. & Nyström, M. Confronting the Coral Reef Crisis. Nature 429, 827–33 (2004).
Tsai, S., Spikings, E., Huang, I. C. & Lin, C. Study on the mitochondrial activity and membrane potential after exposing later stage oocytes of two gorgonian corals (Junceella juncea Junceella fragilis) to cryoprotectants. CryoLetters 32(1), 1–12 (2011).
Pegg, D. E. Principles of Cryopreservation. Methods Mol Biol. 368, 39–57 (2007).
Lin, C., Zhang, T., Kuo, F. W. & Tsai, S. Studies on oocytes chilling sensitivity in the context of ATP response of two gorgonian coral species (J. juncea J. fragilis). CryoLetters 32, 141–147 (2011).
Tsai, S. & Lin, C. Advantages and applications of cryopreservation in fisheries science. Braz Arch Biol Technol. 55(3), 425–433 (2012).
Lin, C. & Tsai, S. The effect of cryopreservation on DNA damage, gene expression and protein abundance in vertebrate. Ital J Anim Sci. 11, 119–122 (2011).
Tsai, S., Thongpooe, P., Kuo, F. W. & Lin, C. Impacts of low temperature preservation on mitochondrial DNA copy number in oocytes of the hard coral Echinopora sp. Mitochondrial DNA. (2015). 10.3109/19401736.2015.1036254.
Paniagua-Chavez, C. G. & Tiersch, T. R. Laboratory studies of cryopreservation of sperm and trochophore larvae of the eastern oyster. Cryobiology 43, 211–223 (2001).
Adams, S. L. et al. Toward cryopreservation of greenshell mussel (Perna canaliculus) oocytes. Cryobiology 58, 69–74 (2009).
Olive, P. J. W. & Wang, W. B. Cryopreservation of Nereis virens (Polychaeta, Annelida) larvae: the mechanism of cryopreservation of a differentiated metazoan. Cryobiology 34, 284–294 (1997).
Gakhova, E. N. et al. Embryonic development of sea urchin after low temperature preservation. Ontogenez 19, 175–180 (1988).
Cabrita, E. et al. Dimethyl Sulfoxide Influx in Turbot Embryos Exposed to a Vitrification Protocol. Theriogenology 60, 463–73 (2003).
Lin, T. et al. Cryotop Vitrification as Compared to Conventional Slow Freezing for Human Embryos at the Cleavage Stage: Survival and Outcomes. Taiwan J Obstet Gynecol. 49(3), 272–78 (2010).
Inaba, Y. et al. In-straw Cryoprotectant Dilution for Bovine Embryos Vitrified Using Cryotop. J Reprod Develop. 57(4), 437–43 (2011).
Lin, C. & Tsai, S. The effect of chilling and cryoprotectants on hard coral (Echinopora) oocytes during short-term low temperature preservation. Theriogenology 77, 1257–1261 (2012).
Tsai, S., Spikings, E., Haung, I. C. & Lin, C. Study on the mitochondrial activity and membrane potential after exposing later stage oocytes of two gorgonian corals (J. juncea J. fragilis) to cryoprotectants. CryoLetters 32, 1–12 (2010).
Tsai, S., Rawson, D. M. & Zhang, T. Studies on Cryoprotectant Toxicity to Early Stage Zebrafish (Danio Rerio) Ovarian Follicles. CryoLetters 29(6), 477–83 (2008).
Hamaratoglu, F., Eroglu, A., Toner, M. & Sadler, K. Cryopreservation of Starfish Oocytes. Cryobiology 50(1), 38–47 (2005).
Abd-Allah, S. M. Effects of Storage Conditions of Dromedary Camel Ovaries on the Morphology, Viability and Development of Antral Follicular Oocytes. Anim Reprod. 7(2), 65–69 (2010).
Shaw, J. M. & Jones, G. M. Terminology Associated with Vitrification and Other Cryopreservation Procedures for Oocytes and Embryos. Hum Reprod Update 9(6), 583–605 (2003).
Lin, C., Wang, L. H., Fan, T. Y. & Kuo, F. W. Lipid content and composition during the oocyte development of two gorgonian coral species in relation to low temperature preservation. PLoS ONE 7(7), e38689 (2012). 10.1371/journal.pone.0038689.
Lin, C. et al. Lipid content and composition of oocytes from five coral species: potential implications for future cryopreservation efforts. PLoS ONE 8(2), e57823 (2013). 10.1371/journal.pone.0057823.
Chong, G. et al. Cryopreservation of the gorgonian endosymbiont Symbiodinium. Sci. Rep. 6, 18816 (2016). 10.1038/srep18816.
Author information
Authors and Affiliations
Contributions
C.L. and S.T. conceived the experiment, V.Y. and S.T. conducted the experiment, V.Y. and S.T analysed the results, V.Y and C.L. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tsai, S., Yang, V. & Lin, C. Comparison of the cryo-tolerance of vitrified gorgonian oocytes. Sci Rep 6, 23290 (2016). https://doi.org/10.1038/srep23290
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23290
This article is cited by
-
Effects of cryopreservation on the ultrastructure of coral larvae
Coral Reefs (2022)
-
Cryopreservation of sperm from the brain coral Diploria labyrinthiformis as a strategy to face the loss of corals in the Caribbean
Coral Reefs (2021)
-
First instance of settlement by cryopreserved coral larvae in symbiotic association with dinoflagellates
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.