Abstract
In the tropics, shifting cultivation has long been attributed to large scale forest degradation, and remains a major source of uncertainty in forest carbon accounting. In the Philippines, shifting cultivation, locally known as kaingin, is a major land-use in upland areas. We measured the distribution and recovery of aboveground biomass carbon along a fallow gradient in post-kaingin secondary forests in an upland area in the Philippines. We found significantly higher carbon in the aboveground total biomass and living woody biomass in old-growth forest, while coarse dead wood biomass carbon was higher in the new fallow sites. For young through to the oldest fallow secondary forests, there was a progressive recovery of biomass carbon evident. Multivariate analysis indicates patch size as an influential factor in explaining the variation in biomass carbon recovery in secondary forests after shifting cultivation. Our study indicates secondary forests after shifting cultivation are substantial carbon sinks and that this capacity to store carbon increases with abandonment age. Large trees contribute most to aboveground biomass. A better understanding of the relative contribution of different biomass sources in aboveground total forest biomass, however, is necessary to fully capture the value of such landscapes from forest management, restoration and conservation perspectives.
Similar content being viewed by others
Introduction
Secondary forests comprise more than half of the total forest area in tropical regions and are the dominant forest type1. In the tropics, secondary forests also represent a major global carbon sink that rapidly accumulates carbon in aboveground biomass2,3,4. Because they cover a large area in the tropics, accurate estimates of carbon in secondary forests are critical for quantifying the global carbon balance as well as for the successful implementation of climate change mitigation projects5. However, there remains a high level of uncertainty in tropical forest carbon accounting, firstly due to the unknown amount of deforestation and forest degradation6, and secondly due to a limited number of field studies that have estimated standing biomass in secondary forests7. In fact, globally tropical deforestation and forest degradation accounts for approximately 15–35% of anthropogenic carbon emissions8, and reversing this trend has a clearly recognized potential for recovering the stocks of forest biomass carbon and for other forest conservation outcomes9.
Shifting cultivation or ‘slash-and-burn agriculture’ is a traditional land-use practice in tropical forested landscapes, and is a dominant land-use in rural upland areas in the developing countries10. In the tropics, shifting cultivation has also been seen as the primary source of deforestation and forest degradation for many years11,12. Historically, shifting cultivation has been viewed negatively as contributing to many forms of environmental degradation, including loss of biodiversity and biomass carbon in forests13. Accordingly, throughout much of the tropics, governments have developed policies to control or reduce the practice of shifting cultivation by smallholder rural farmers12.
In Southeast Asia, secondary forests constitute around 63% of the total forest area14, with an estimated 14–34 million people dependent on shifting cultivation for their livelihoods10. The extent of land under shifting cultivation however has declined in recent years due to government policies restricting shifting cultivaton and economic factors that promoted other land-use systems12,13. Consequently, in many parts of this region regenerating secondary forests following shifting cultivation are becoming prominent10,11,15. Due to the dynamic nature of the landscape, shifting cultivation and its changes over time have been very difficult to delineate using satellite based earth observation systems9,15. In Southeast Asia there is also a lack of spatially explicit knowledge of the forest biomass carbon stocks and carbon dynamics associated with shifting cultivation landscapes. This has limited the inclusion of these landscapes in current negotiations on Reducing Emissions from Deforestation and Forest Degradation (REDD+) in the region to achieve the dual objectives of community development and forest conservation16.
In this paper, we report aboveground biomass carbon distribution along a fallow gradient in an upland secondary forest of the Philippines regenerating after shifting cultivation. The Philippines, is both a mega-diverse country and a global biodiversity hotspot, placing it amongst the top priority countries for global conservation17,18. As in other parts of the developing tropics, shifting cultivation, known as kaingin in the Philippines, is a common and controversial land-use in the country19. In fact, secondary forests developed after shifting cultivation forms the second largest group of forest in the country after post logging secondary forests20. In this paper we also investigate the determinants of biomass carbon recovery in fallow secondary forests, which remain largely overlooked throughout the tropics4,21. We believe the present study helps address the existing gaps in knowledge on biomass carbon changes and recovery after forest degradation in Southeast Asia which is still largely biased towards the neotropics (see Ngo et al.22, Saner et al.23, Kenzo et al.24 for example). The study is one of the first attempts to systematically assess the carbon in secondary forests associated with slash-and-burn fallows; and will thus substantially improve our understanding of the role that such landscapes play as a sink of atmospheric carbon for both the Philippnes and other tropical developing countries.
Results
Distribution of biomass carbon in fallow secondary forests
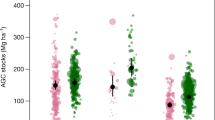
We measured the biomass of 2918 living tree stems (representing 131 species), 184 tree ferns and 124 Abaca plants (Musa textilis, a species of banana native to the Philippines) in our study sites covering a total sample area of 2.5 ha. Tree ferns and Abaca are characteristic species in fallow secondary forests in the area and we include them due to their common occurrence in our study sites. Within our transects we also encountered 1281 pieces of dead woody debris that met the criteria for inclusion for biomass measurement. Using existing allometric models our study thus across all sites finds 328.2 Mg C in the living woody biomass (LWBC), 1.18 Mg C in other living biomass (OLBC; i.e. tree fern and Abaca), 88.83 Mg C in coarse dead wood biomass (CDWBC) and 0.02 Mg C in undergrowth (UBC) and litter biomass (LBC). Aboveground total biomass carbon (AGTBC) was significantly (F4 = 6.07, p < 0.01) higher in old-growth forest than the secondary forests of all fallow categories. We found that, carbon in both living woody biomass and coarse dead wood biomass varied significantly (F4 = 9.54, p < 0.01) across the sites of different kaingin history as expressed by their fallow age (i.e. post kaingin period) and in our control old-growth forest (Table 1). There were however no significant differences among the sites when we considered the carbon stored in other living biomass and in undergrowth and litter biomass (Fig. 1). Our post-hoc analysis using Tukey’s HSD revealed significantly higher (321.29 ± 130.96 Mg C; p < 0.01) aboveground total biomass carbon in old-growth forest followed by our new (i.e. SA 0–5) and oldest (i.e. SA 21–30) kaingin fallow sites. LWBC was also significantly higher (316.96 ± 130.63 Mg C; p < 0.01) in old-growth forest sites accounting for 98.65% of the AGTBC, whilst CDWBC was highest (126.65 ± 22.58 Mg C; p < 0.01) in our new kaingin fallow sites with an estimated 79% contribution to the AGTBC (Fig. 2).
Parashorea malaanonan had the highest contribution (33.23%) to the overall LWBC, and had relatively greater contribution to all of our fallow sites and old-growth forest. Other than P. malaanonan, Lithocarpus llanosii, Ficus balete and Shorea contorta contributed respectively 6.39%, 5.32% and 3.89% to the overall LWBC (see Supplementary Table 1). In old-growth forest Calophyllum blancoi (5.69%), Petersianthus quadrialatus (5.26%) and Bischofia javanica (4.79%) were other major sources of biomass carbon in living woody stems. When considering species successional guild, climax species were the highest contributers (48.93%; p < 0.01) to LWBC in old-growth forest sites followed by the oldest kangin fallow sites (i.e. SA 21–30) (Fig. 3). Similarly, the contribution of native species to LWBC was also significantly higher in the old-growth forest (80.81%; p < 0.01) (Fig. 3). As expected, large diameter stems had the greatest contribution to the LWBC in our fallow sites, and a significantly high contribution in the old-growth forest (43.28%; p < 0.01) (Supplementary Fig. 1). It was a similar case when considering stem heights, where woody stems attaining heights between 30–50 m constituted about 30.11% of the LWBC, which was a significantly higher (p < 0.01) contribution than other height classes (Supplementary Fig. 2). In the case of OLBC as measured for tree fern and Abaca, we found no significant difference in biomass carbon allocation across the sites.
In the case of CDWBC, carbon stored in standing dead wood was significantly higher (p < 0.01) in our new fallow sites contributing about 85.49% to the CDWBC (Fig. 4). There were no significant differences in the carbon stored in downed dead wood in our sites of different fallow categories. Post hoc analysis however revealed significantly different (p < 0.05) carbon in downed dead wood in new fallow sites and old-growth forest. In new fallow sites the amount of carbon stored in the freshly cut wood was also highest (91.03%; p < 0.01), and there were no significant differences in carbon stored in moderately decomposed, highly decomposed and burnt dead wood in different fallow sites and in old-growth forest (Fig. 4). Similarly, we found no significant difference in biomass carbon in litter and undergrowth between our fallow secondary forest sites and in old-growth forest.
Recovery of biomass carbon in fallow secondary forests
When compared with old-growth forests used as our control, overall we found that AGTBC was highest (49.89 ± 17.39) in the new (i.e. SA 0–5) fallow sites, followed by our oldest fallow sites (41.25 ± 17.76), middle-aged (i.e. SA 11–20) sites (38.10 ± 23.33), and in the young (i.e. SA 6–10) sites (31.46 ± 17.42). The high amount of AGTBC in our new fallow sites was mainly driven by the large amount of CDWBC remaining in the sites after being cleared and/or used for kaingin. Although there was no significant difference, the recovery of LWBC was highest in the oldest fallow sites (37.86%), followed by the middle-aged sites (35.28%), young fallow sites (23.4%) and the new fallow sites (10.54%) (Fig. 5). There was no significant difference in the recovery of OLBC (in tree fern and Abaca) across our sites, except in the case of new kaingin fallow sites and young fallow sites where it was significantly different (p < 0.05). The amount of CDWBC in relation to that in the control old-growth forest sites was significantly different (F3 = 47.42, p < 0.01) across the fallow categories, and was significantly higher in the new kaingin fallow sites (i.e. SA 0–5). In fallow secondary forests, woody debris is ultimately lost from the ecosystem with the increasing fallow age but at the same time the regrowth of vegetation offsets the large loss in dead wood in the area. There was however no significant difference in the recovery of undergrowth and litter biomass carbon (ULBC) across our fallow sites (of different age categories) compared with the control old-growth forest.
Factors influencing the recovery of aboveground biomass carbon in fallow secondary forests
Patch size was found to consistently explain the variation in the response variables (i.e. percentage recovery of living woody biomass carbon, LWBC; other living biomass carbon, OLBC; coarse dead wood biomass carbon, CDWBC; and undergrowth and litter biomass carbon, ULBC). It also explained the similar amount of variation (models within ∆AIC = 4 are considered equivalent) found in other complex models with more interactions among explanatory variables (Table 2; Table 3). Soil organic carbon, fallow age and the slope of a site were also important in explaining the variation in the recovery of different parameters investigated. Distance from the nearest control forest sites was not retained in any of the best fit candidate models.
Discussion
Biomass carbon distribution in tropical fallow secondary forests
We show that secondary forests after shifting cultivation are significant sinks for above-ground carbon. We also show the relatively greater contribution of older fallow areas over young fallow areas as a carbon sink after being used for shifting cultivation in the upland Philippines. In this area, carbon stored in old-growth forests, oldest fallow areas and moderately-aged fallow areas were 321.29 (±130.96) Mg ha−1, 132.54 (±57.07) Mg ha−1 and 122.41 (±74.95) Mg ha−1 respectively, which is comparable to the carbon pools reported from other forests in the Philippines26,27,28. These studies found carbon stored in aboveground biomass ranged between 117.9–305.5 Mg ha−1. This estimate is also greater than the upland forests that were selectively logged27.
The allometric models and sampling approach used may introduce errors in the estimates of carbon, and thus both may have substantial influences on the results of groundbased forest carbon estimates7,29,30. Locally developed and calibrated allometeric models have the potential to minimize this uncertainty in tropical forest carbon accounting7. In our study, we used the most recent allometric model developed by Chave et al.5 for estimating living woody biomass in tropical forests. Studies on biomass dynamics and forest carbon stocks are biased towards the neotropics, with very limited systematic inventory reported so far from Southeast Asian secondary forests22,23,24. The model developed by Chave et al.5 is reported to underestimate the aboveground living biomass by 20% when observed biomass exceeded 30 Mg for individual stems, although this trend disappears when a stem’s biomass is between 10–30 Mg5. We found that stem density was not the main factor in determining carbon stored in living woody biomass, but diameter and height of individual stems had a better ability to control biomass carbon distribution in forests which is consistent with the observations made by Lasco et al.27, Rozendaal and Chazdon31, Marin-Spiotta et al.32 and Lawrence33 respectively in the Philippines, Costa Rica, Puerto Rico and Indonesia. We also found that, in old-growth forests and in older fallow secondary forests climax species contributed the most in terms of aboveground living woody biomass carbon, which is also in accordance with the finding of Rozendaal and Chazdon31. This may reflect the persistence of mature remnant trees in fallow forest when converted from old-growth stands33. For example, in all of our fallow sites and old-growth forest Parashorea malaanonan consistently made the highest contribution to living woody biomass. In contrast, McNamara et al.34 have found limited difference in the occurrence of old-growth forest specialist species in secondary forests with different disturbance history in Lao PDR, and argued that it may due to the high resilience capacity of certain species and their quick resprouting ability.
In young kaingin fallow areas the ‘other living standing biomass’ (i.e. tree ferns and Abaca in the present study) and coarse dead biomass constitute a major part of the aboveground biomass carbon. This depicts a very different composition in landscape scale total aboveground biomass carbon than that of old-growth forest and older kaingin fallow areas. This is largely due to the long disturbance (and use) history and the large remaining amount of dead wood on sites after being used for shifting cultivation3. However, Orihuela-Belmonte et al.35 have found that in Mexico coarse dead wood biomass carbon is higher in older fallow areas and also significantly different across sites of different fallow age. Similar to our findings, Pelletier et al.15 also reported that aboveground forest biomass carbon is not very different between old-growth forest and older fallow areas, but is different between young fallow areas and old-growth forest.
Factors influencing the biomass carbon recovery in fallow secondary forests
Recovery of aboveground living tree biomass carbon was highest in the oldest fallow sites and lowest in the new kaingin fallow areas, although living tree biomass is the first pool to be affected when forests are converted for shifting cultivation use. Coarse dead biomass carbon was high in the new kaingin fallow sites compared to the control old-growth forest site, reflecting the high amount of coarse dead wood in new and relatively young kaingin fallow sites remaining after clearing these areas. Other living biomass carbon was highest in the young kaingin fallow areas representing the dynamic nature of such landscapes, where undergrowth and litter biomass carbon is found to increase gradually from new to oldest kaingin fallow sites. Several studies have found that aboveground biomass carbon recovers rapidly during early successional years after abandonment, followed by a relatively slow recovery rate after reaching a peak or intermediate stage24,33,36,37. Such recovery may take place at a rate of between 3.75–9.4 Mg C ha−1 year−1 and may take as long as 55–95 years24,38,39. In tropical old-growth forests, annual rates of biomass carbon change are typically lower than forests that have been subject to different levels of anthropogenic disturbance40, and in such forests the biomass carbon accumulation rates also decrease with an increasing stand age after reaching an intermediate age31,32.
We found that biomass carbon recovery was constrained mainly by landscape patchiness. In our LMEM patch size showed a consistent control in determining the recovery of biomass carbon at different aboveground levels. In tropical forests, environmental determinants of such recovery as well as its magnitude are still poorly quantified4,21. Changes in biomass carbon are also driven by the growth and mortality of trees, although such changes are difficult to monitor and require long-term monitoring41. It is however clear from our study that aboveground living tree biomass is the most vulnerable carbon pool in tropical secondary forests. A similar observation is also made by Kotto-Same et al.39. Other environmental factors that also influence the variation in recovery rates are soil organic carbon, fallow age and slope of a site. Distance from the nearest control old-growth forest was found to be unimportant in our LMEM. In the case of other living biomass carbon and coarse dead wood biomass carbon recovery, there was no notable pattern in the LMEM, which may be attributed to the fact that these components have the smallest contribution to our old-growth forest total aboveground biomass carbon.
Chronosequence studies are a widely used approach to investigate secondary forest and successional developments after disturbances31,42,43. Both fallow age and fallow cycles offers an indication of previous forest use33, although the present study was limited to only fallow age as the number of cycles was unknown. We found that recovery of standing living woody biomass carbon was distinct across sites of different categories and was superior in older kaingin fallow sites. In young fallow areas, although the number of stumps was higher, biomass carbon recovery was higher in sites with larger diameter trees as also mentioned by Rozendaal and Chazdon31. Many environmental factors influence secondary forest recovery after disturbances11,44, and studies have demonstrated different recovery rates depending on the site’s geographic position together with biotic and abiotic attributes11,38,45.
Biomass accumulation specifies the carbon stored in aboveground biomass, and was reported to be declining by 9.3% with each fallow cycle in Indonesia11,33,46. This decline was mainly driven by the density and biomass of woody stems >10 cm dbh as well as soil phosphorus availability33. Burning also has a positive influence on biomass carbon accumulation in fallow secondary forests24,47. Recovery may also depend on remaining forest cover in a landscape, although intensity of past land-use rather than edaphic variables is the strongest predictor of biomass recovery48. Rapid biomass recovery during secondary forest succession was also reported by Letcher and Chazdon37 and Martin et al.44.
Forest management and landscape restoration implications
In tropical forests, aboveground biomass carbon dynamics are important in net primary productivity, and regardless their large contribution to the global carbon balance, uncertainty yet remains regarding their quantitative contribution to the atmospheric carbon cycle7,49. In Southeast Asia there are large areas of secondary forest as a result of past anthropogenic disturbances. The existing carbon measurement uncertainties create critical data gaps that limit our understanding of the important role of these forests as sources and sinks of terrestrial carbon16. In recent years, it is also increasingly recognized that although undervalued, tropical secondary forests can provide the same important ecosystem goods and services as primary or old-growth forests32,50,51. In tropical regions deforestation has been a large contributor of greenhouse gas emissions, and reversing these trends with suitable land-use(s) has a clearly recognized potential for recovering biomass carbon stock in forests9,52. Compared to other climate mitigation options, regenerating secondary forests offers a low-cost approach to reducing greenhouse gas emissions in the tropics, although a greater understanding of and capability to quantify carbon dynamics in such novel and emerging ecosystems is necessary at landscape scales50. For instance, in many tropical countries monocultures have been preferred for reforestation, but our study along with others suggest that considerable net primary productivity and carbon storage could be achieved if more species diversity is secured in tropical landscapes53. Current remote sensing based techniques using satellite imagery offer promise for estimation of ecosystem carbon exchange in complex forested landscapes, although large variability exists depending on forest conditions and landscape type54,55. A combination of field-based inventory and remote sensing techniques can be used to reduce such variability and to cover large areas of tropical forests15.
Conclusion
Our results highlight that the secondary forests regenerating following shifting cultivation are important carbon sinks in tropical ecosystems. Allowing development of such secondary regrowth has clear potential for carbon storage in the aboveground forest biomass. Biomass carbon distribution differs across sites with different land-use histories, and a large amount of carbon is stored in living woody biomass in older fallow areas indicating the dynamic nature of the landscape and succesional development towards undisturbed forests. In young fallow areas, large amounts of carbon are stored in coarse dead wood material which ultimately provided inputs to the soil for biomass accumulation in living trees. We found that patch size is an important factor in biomass carbon recovery together with soil organic carbon, fallow age and the slope of a site, and after thirty years a site may achieve more than 40% of the biomass carbon found in old-growth forest without any history of major disturbances. The extensive deforestation and forest degradation in tropical regions caused by shifting cultivation and other land-uses is being blamed for biodiversity loss and global warming. We found that regenerating secondary forests have the potential to mitigate the impacts of such deforestation and forest degradation and to contribute to global carbon sequestration. However, it remains necessary to determine exactly where in the aboveground forest biomass the carbon is being sequestered (e.g. in the present study, we found that coarse woody debris in new kaingin fallow sites has the highest contribution to aboveground total biomass carbon, and in oldest kaingin fallow sites living woody biomass carbon had the highest contribution).
Methods
Study area
The study was conducted in Barangay (the smallest administrative unit in the Philippines and native Filipino term for village) Gaas on Leyte Island, the Philippines (Fig. 6). Leyte is the eighth largest island in the Philippines, and our study site was located on the western side of the island. Geographically, Leyte is located between 124°17′ and 125°18′ East longitude and between 9°55′ and 11°48′ North latitude, and covers an area of about 800,000 ha. Forest cover on the island is about 10%, although the once dipterocarp-rich rainforests now comprise mainly patches of old-growth and primary forests, and coconut (Cocos nucifera) and Abaca plantations56. The relatively flat lowlands of the island are being used for agricultural crop production, especially rice (Oryza sativa) and corn (Zea mays)56.
Spatial position of the site locations were plotted in global geo-political boundary available from Esri (http://www.arcgis.com/) using ArcMap (version 10.3) software.
Leyte Island was formed from tectonic movement and plate convergence which started during the tertiary and quaternary age56,57. Based on the Coronas Classification of Climate, Leyte has a ‘type IV’ climate with two distinct season58. The area enjoys a relatively even distribution of rainfall throughout the year with annual rainfall totalling approximately 4,000 mm59. Mean annual temperature is 28 °C which remains constant throughout the year58. Relative humidity ranges between 75 to 80 percent during the dry and the wettest months60. The soil in our study area in Gaas was an Andisol type which possesses a markedly higher soil organic carbon content than rest of the islands58.
Site selection
We chose Barangy Gaas (also refer to as Gaas) purposively. This area of Leyte is situated in a comparatively high altitudinal range and compared to other parts of the island it has a relatively greater extent of undisturbed forests. It also has a low population density. These critieria are prerequisites for the kaingin fallow to regrow as secondary forests61. For our study we consider only the dahilig kaingin system which is identical to the most common practice of shifting cultivation in the tropics (see Olofson62 for details of the Philippines kaingin systems). Smallholder farmers living in the area usually grow Abaca or coconut in their fallow kaingin area in order to receive financial benefits during the time of abandonment. Our study was however confined to the areas where farmers cultivated only Abaca since coconut plantations generally involve more intensified land-management during the fallow periods and this is not conducive for secondary forest development.
Biomass Inventory
A series of extensive field surveys were undertaken at the sites between May and October 2013. For the biomass inventory we followed a modified Gentry plot approach63. This method has been reported as the most efficient for monitoring secondary forest development in tropical regions64. We categorized our sites into four different fallow categories; i.e. less than 5 year old fallow (SA0–5), also referred to as new, 6–10 year old fallow (SA6–10) also referred to as young, 11–20 year old fallow (SA11–20) also referred to as middle-aged, and 21–30 year old fallow (SA21–30) also refered to as oldest. We limited our study to fallow forest sites that were at least 1 ha in size45. Additionally, we sampled old-growth forest (SF) as control forest sites. These forests had no history of kaingin and logging and were located close to our fallow sites. Our control forest sites were structurally and floristically similar to primary forests although they may have undergone a limited level of anthropogenic use (e.g. source of firewood, wild fruits etc.) like most of the forest in the tropical forest-agriculture frontiers.
We identified a total of 25 sites (four fallow categories +old-growth forest x five replicates). Both the fallow age and fallow cycles have been reported to influence the biomass dynamics in secondary forests3. In our study we were only able to consider the fallow age and not the fallow cycles due to a lack of reliable information about past site history. At each site, four transects of 50 m × 5 m were established parallel to each other and with a minimum of 5 m distance between transects, representing a total area of 0.1 ha per site. For standing live trees and palms ≥5 cm diameter at breast height (dbh) we recorded diameter and height of each individuals at 1.3 m from the ground or above stem abnormalities (e.g. buttresses, stilt roots etc.). All individuals were identified to the species level and named with the help of a local expert from Visyas State University (VSU). In the case of unknown species we used the most common Filipino name for that species. We also measured all tree ferns and Abaca ≥5 cm dbh in our transects as other living biomass because they represent a major component of secondary forest succession in post-kaingin secondary forests in the Philippines19. Lianas were not included in our study. For measuring the dbh of individual tree stems we used diameter tapes. We used a hypsometer for tree height measurements, although the closely-structured canopy in tropical forests sometimes made it difficult to measure tree heights with a high reliability.
Since slashing and burning is a common practice in shifting cultivation landscapes, coarse dead wood biomass in the form of felled, degraded and burnt trees comprise a significant part of the total aboveground biomass in such areas10,15. Consequently we censused all dead, cut and burnt trees ≥5 cm at dbh that fell within in our transects. For each individual stem, we recorded whether it was standing or downed (i.e. fallen), and the degradation staus, categorized as – freshly cut, moderately decomposed, highly decomposed and burnt23. For litter and undergrowth (i.e. seedlings, saplings, shrubs and herbaceous plants) we followed a destructive sampling approach. A 1 m × 1 m rectangular plot was laid in the centre of each of our 100 transects distributed in 25 sites, and all litter and undergrowth samples were collected and weighed in the plot using a measuring balance.
Additionally, for each site we recorded the site geographic position, elevation (E), distance from the nearest control forest (D), patch size (PS), slope (SL), leaf area index (LAI) and soil organic carbon (SOC) as a percentage. We used a digital plant canopy imager (Model: CID Bio-Science, CI-110/120) for measuring leaf area index and a hand-held global positioning system (Model: Garmin eTrex) for elevation.
Biomass calculation in forest ecosystems
There is no allometric equation which is specifically developed for the secondary forests in the Philippines28. Consequently we used the generic allometric model develeoped by Chave et al.5.

where AGB or aboveground dry biomass is in kg, D is the dbh in cm, H is the height of the tree and/or palm in m and ρ is the species specific wood density (g cm−3). This model performed better than the widely accepted previous model by Chave et al.7, and performed well across all forest types and bioclimatic conditions5. The inclusion of tree height in this model provides more reliable estimates of biomass, compared to the pervious models that used only diameter in the model5,65. Moreover, this model is based on 58 global sites distributed across the tropics where the previous model was based on 27 global sites5,7. For species specific wood density we used the World Agroforestry Centre’s wood density database where wood density was the ratio of dry mass to the green volume66 (Supplementary Table 3). In the case of unknown species or where wood density was not available, we took the mean wood density of the genus as a substitute23.
For tree ferns and Abaca we used the following allometric models developed by Stanley et al.67 and Armecin and Coseco68 respectively.


where AGB for ferns and abaca is respectively in g and kg, and D is the diameter of individuals in cm.
The volume of coarse woody debris per area was calculated from transect data, and we used the following equation to obtain the volume of individual stems that fell within or intersected our transects.

where V is the volume per stem, L is the total length of the stem (of coarse woody debris) that fell within or intersected our transects in m and D is the diameter of coarse woody debris. Wood density was determined locally by the water displacement method taking representative samples (n = 5) for each of the four wood degradation status (i.e. freshly cut, moderately decomeposed, highly decomposed and burnt), and were 0.48 g cm−3, 0.35 g cm−3, 0.25 g cm−3 and 0.19 g cm−3 respectively for our freshly cut, moderately decomposed, highly decomposed and burnt wood samples69. All biomass measurements were first made at the site level (Mg), and then converted to a per hectare (ha) value after correcting plot size or transect length for the slope15.
Estimating carbon in aboveground forest biomass
In our study total aboveground biomass carbon (AGTBC) is the sum of aboveground living woody (tree and palms) biomass carbon (LWBC), other living biomass carbon (OLBC) measured for tree ferns and Abaca, coarse dead wood biomass carbon (CDWBC), undergrowth biomass carbon (UBC), and litter biomass carbon (LBC). To convert the aboveground biomass of trees we assumed that 50% of the dry mass was carbon30. Studies at nearby sites with similar forest types have found average carbon content close to 50%23,24,28. For tree ferns and Abaca, carbon content was assumed to be 50% and 47.3% respectively by total dry biomass after Armecin and Coseco68 and Beets et al.70.
Measuring of the dry biomass litter and undergrowth samples was undertaken in the VSU-ACIAR Analytical Chemistry Laboratory. The samples were air dried at room temperature and then grinded using an electric grinder. The samples were then oven dried at 70 °C for 24 hours and weighed. A representative subsample from each of the litter and undergrowth samples was analysed for estimating the carbon content (% C-Heanes) (Supplementary Table 4). In the case of coarse woody debris we took 5 subsamples from each of the degradation states as mentioned earlier, and the carbon content was measured locally in the laboratory.
Estimating the recovery of biomass carbon in forests
We compared the recovery of biomass carbon in different aboveground components with that of the control old-growth forests. We combined both tree fern and Abaca as other living biomass carbon (OLBC) during the analysis due to their relatively low contribution to AGTBC. Recovery (R) was expressed as a percentage (%) of biomass carbon using the following equation.

where X fallow is the measure of biomass carbon in a fallow site, and  is the mean of corresponding biomass carbon in a similar ecosystem in the control old-growth forest.
is the mean of corresponding biomass carbon in a similar ecosystem in the control old-growth forest.
Statistical analysis
We performed both the analysis of variance (ANOVA) and Tukey’s post-hoc test to test any significant difference between the variables. We developed linear mixed-effect models (also refered to as LMEM) to examine the effect of fallow age and selected site attributes (see Supplementary Table 2) on recovery of biomass carbon, using the package ‘nlme’. In our LMEM, fallow age (FA), slope (SL), distance from the nearest control forest (DIS), patch size (PS), leaf area index (LAI) and soil organic carbon (SOC) were used as explanatory variables (i.e. fixed factors), and biomass carbon in different forest strata was the response variable. We used sites nested in fallow categories as the random effect in our models. Due to their high collinearity with other explanatory variables, ‘elevation’ and ‘LAI’ were excluded from the final LMEM (Supplementary Table 5). All analyses were performed using ‘R’ Statistical package (version 3.0.1). We considered Akaike Information Criterion corrected for small sample sizes (AICc) for the selection of our top models, where the best models had the lowest AICc scores. We used the R-package ‘MuMin’ for our model selection, and to evaluate the contribution different fixed effects had on explaining the variabiont in the response variables25. We considered models within four AICc units to be equivalent models71.
Additional Information
How to cite this article: Mukul, S. A. et al. Tropical secondary forests regenerating after shifting cultivation in the Philippines uplands are important carbon sinks. Sci. Rep. 6, 22483; doi: 10.1038/srep22483 (2016).
References
FAO (Food and Agriculture Organization of the United Nations). Global Forest Resources Assessment 2010. FAO Forestry Paper 163. FAO, Rome, Italy (2010).
Pan, Y. D. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Eaton, J. M. & Lawrence, D. Loss of carbon sequestration potential after several decades of shifting cultivation in the Southern Yucatan. For. Ecol. Manage. 258, 949–958 (2009).
Malhi, Y. et al. The regional variation of aboveground live biomass in old-growth Amazonian forests. Glob. Change Biol. 12, 1107–1138 (2006).
Chave, J. et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 20, 3177–3190 (2014).
Malhi, Y. The carbon balance of tropical forest regions, 1990–2005. Curr. Opin. Environ. Sustain. 2, 237–244 (2010).
Chave, J. et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99 (2005).
DeFries, R. S. et al. Carbon emissions from tropical deforestation and regrowth based on satellite observations for the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 99, 14256–14261 (2002).
Houghton, R. Carbon emissions and the drivers of deforestation and forest degradation in the tropics. Curr. Opin. Environ. Sustain. 4, 597–603 (2012).
Mertz, O. et al. Swidden change in Southeast Asia: Understanding causes and cnsequences. Hum. Ecol. 37, 259–264 (2009).
Lawrence, D., Radel, C., Tully, K., Scmook, B. & Schneider, L. Untangling a decline in tropical forest resilience: constraints on the sustainability of shifting cultivation across the globe. Biotropica 42, 21–30 (2010).
Fox, J. et al. Policies, political economy, and swidden in Southeast Asia. Hum. Ecol. 37, 305–322 (2009).
van Vliet, N. et al. Trends, drivers and impacts of changes in swidden cultivation in tropical forest-agriculture frontiers: a global assessment. Global Environ. Chang. 22, 418–429 (2012).
Koh, L. P. Impending disaster or sliver of hope for Southeast Asian forests? The devil may lie in the details. Biodivers. Conserv. 16, 3935–3938 (2007).
Pelletier, J., Codjia, C. & Potvin, C. Trditional shifting agriculture: tracking forest carbon stock and biodiversity through time in western Panama. Glob. Change Biol. 18, 3581–3595 (2012).
Ziegler, A. D. et al. Carbon estimates of major land-cover transitions in SE Asia: great uncertainities and REDD+ policy implications. Glob. Change Biol. 18, 3087–3099 (2012).
Posa, M. R., Diesmos, A. C., Sodhi, N. S. & Brooks, T. M. Hope for threatened tropical biodiversity: lessons from the Philippines. Bioscience 58, 231–240 (2008).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Suarez, R. K. & Sajise, P. E. Deforestation, swidden agriculture and Philippine biodiversity. Philipp Sci Lett. 3, 91–99 (2010).
Lasco, R. D., Visco, R. G. & Pulhin, J. M. Secondary forests in the Philippines: formation and transformation in the 20th century. J. Trop. For. Sci. 13, 652–670 (2001).
Taylor, P. et al. Landscape-scale controls on aboveground forest carbon stocks on the Osa Peninsula, Costa Rica. PLoS One 10, e0126748 (2015).
Ngo, K. M. et al. Carbon stocks in primary and secondary tropical forests in Singapore. For. Ecol. Manage. 296, 81–89 (2013).
Saner, P., Loh, Y. Y., Ong, R. C. & Hector, A. Carbon Stocks and Fluxes in Tropical Lowland Dipterocarp Rainforests in Sabah, Malaysian Borneo. PLoS One 7, e29642 (2012).
Kenzo, T. et al. Changes in above- and belowground biomass in early successional tropical secondary forests after shifting cultivation in Sarawak, Malaysia. For. Ecol. Manage. 260, 875–882 (2010).
Bartoń, K. Package ‘MuMIn’. Available online at: http://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (Date of access: 21/2/2014) (2011).
Lasco, R. D. & Pulhin, F. B. Carbon budgets of forest ecosystems in the Philippines. J. of Environ. Sci. Manage. 12, 1–13 (2009).
Lasco, R. D. et al. Carbon stocks assessment of a selectively logged dipterocarp forest and wood processing mill in the Philippines. J. Trop. For. Sci. 18, 166–172 (2006).
Lasco, R. D., Guillermo, I. Q., Cruz, R. V. O., Bantayan, N. C. & Pulhin, F. B. Carbon stocks assessment of a secondary forest in Mount Makiling Forest Reserve, Philippines. J. Trop. For. Sci. 16, 35–45 (2004).
Ketterings, Q. M., Coe, R., van Noordwijk, M., Ambagau, Y. & Palm, C. A. Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. For. Ecol. Manage. 146, 199–209 (2001).
Brown, S. Estimating biomass and biomass change of tropical forests: A primer. FAO Forestry Paper 134. FAO, Rome, Italy (1997).
Rozendaal, D. M. A. & Chazdon, R. L. Demographic drivers of tree biomass change during secondary succession in northeastern Costa Rica. Ecol. Appl. 25, 506–516 (2015).
Marin-Spiotta, E., Ostertag, R. & Silver, W. L. Long-term patterns in tropical reforestation: plant community composition and aboveground biomass accumulation. Ecol. Appl. 17, 828–839 (2007).
Lawrence, D. Biomass accumulation after 10–200 years of shifting cultivation in Bornean rain forest. Ecology 86, 26–33 (2005).
McNamara, S., Erskine, P. D., Lamb, D., Chantalangsy, L. & Boyle, S. Primary tree species diversity in secondary fallow forests of Laos. For. Ecol. Manage. 281, 93– 99 (2012).
Orihuela-Belmonte, D. E. et al. Carbon stocks and accumulation rates in tropical secondary forest at the scale of community, landscape and forest type. Agric. Ecosyst. Environ. 171, 72–84 (2013).
Raharimalala, O., Buttler, A., Schlaepfer, R. & Gobat, J. M. Quantifying biomass of secondary forest after slash-and-burn cultivation in central Menabe, Madagascar. J. Trop. For. Sci. 24, 474–489 (2012).
Letcher, S. G. & Chazdon, R. L. Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in northeastern Costa Rica. Biotropica 41, 608–617 (2009).
Read, L. & Lawrence, D. Recovery of biomass following shifting cultivation in dry tropical forests of the Yucatan. Ecol. Appl. 13, 85–97 (2003).
Kotto-Same, J., Woomer, P. L., Appolinaire, M. & Louis, Z. Carbon dynamics in slash-and-bum agriculture and land use alternatives of the humid forest zone in Cameroon. Agric. Ecosyst. Environ. 65, 245–256 (1998).
Valencia, R., Condit, R., Muller-Landau, H. C., Hernandez, C. & Navarrete, H. Dissecting biomass dynamics in a large Amazonian forest plot. J. Trop. Ecol. 25, 473–482 (2009).
Lasky, J. R. et al. The relationship between tree biodiversity and biomass dynamics changes with tropical forest succession. Ecol. Lett. 17, 1158–1167 (2014).
Mukul, S. A. & Herbohn, J. The impacts of shifting cultivation on secondary forests dynamics in tropics: a synthesis of the key findings and spatio temporal distribution of research. Environ. Sci. Policy 55, 167–177 (2016).
Blanc, L. et al. Dynamics of aboveground carbon stocks in a selectively logged tropical forest. Ecol. Appl. 19, 1397–1404 (2009).
Martin, P. A., Newton, A. C. & Bullock, J. M. Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. R. Soc. B. 280, 20132236 (2013).
Piotto, D., Montagnini, F., Thomas, W., Ashton, M. & Oliver, C. Forest recovery after swidden cultivation across a 40-year chronosequence in the Atlantic forest of southern Bahia, Brazil. Plant Ecol. 205, 261–272 (2009).
Hulvey, K. B. et al. Benefits of tree mixes in carbon plantings. Nat. Clim. Chang. 3, 869–874 (2013).
d’Oliveira, M. V. N., Alvarado, E. C., Santos, J. C. & Carvalho Jr, J. A. Forest natural regeneration and biomass production after slash and burn in a seasonally dry forest in the Southern Brazilian Amazon. For. Ecol. Manage. 261, 1490–1498 (2011).
Castro-Luna, A. A., Castillo-Campos, G. & Sosa, V. J. Effects of selective logging and shifting cultivation on the structure and diversity of a tropical evergreen forest in South-Eastern Mexico. J. Trop. For. Sci. 23, 17–34 (2011).
Clark, D. A. et al. Net primary production in tropical forests: an evaluation and synthesis of existing field data. Ecol. Appl. 11, 371–384 (2001).
Bonner, M. T. L., Schmidt, S. & Shoo, L. P. A meta-analytical global comparison of aboveground biomass accumulation between tropical secondary forests and monoculture plantations. For. Ecol. Manage. 291, 73–86 (2013).
Chazdon, R. L. Tropical forest recovery: legacies of human impact and natural disturbances. Perspect. Plant Ecol. Evol. Syst. 6, 51–71 (2003).
Budiharta, S. et al. Restoring degraded tropical forests for carbon and biodiversity. Environ. Res. Lett. 9, 114020 (2014).
Pichancourt, J. B., Firn, J., Chades, I. & Martin, T. G. Growing biodiverse carbon-rich forests. Glob. Change Biol. 20, 382–393 (2014).
Tang, X. et al. Monitoring the seasonal and interannual variation of the carbon sequestration in a temperate deciduous forest with MODIS time series data. For. Ecol. Manage. 306, 150–160 (2013)
Tang, X. et al. Estimating the net ecosystem exchange for the major forests in the northern United States by integrating MODIS and AmeriFlux data. Agric. For. Meteorol. 156, 75–84 (2012).
Asio, V. B., Jahn, R., Stahr, K. & Margraf, J. Soils of the tropical forests of Leyte, Philippines II: Impact of different land uses on status of organic matter and nutrient availability. In: A. Schulte & D. Ruhiyat, (eds.), Soils of Tropical Forest Ecosystems: characteristics, ecology and management. 37–44 pp (1998).
Aurelio, M. A. Shear partitioning in the Philippines: constraints from Philippine fault and global positioning. Isl. Arc. 9, 584–597 (2000).
Navarrete, I. A., Tsutsuki, K. & Asio, V. B. Characteristics and fertility constraints of degraded soils in Leyte, Philippines. Arch. Agron. Soil Sci., 59, 625–639 (2013).
Jahn, R. & Asio, V. B. Climate, geology, geomorphology and soils of the tropics with special reference to Leyte Islands (Philippines). In: 8th International Seminar and Worshop on Tropical Ecology. Visaya State College of Agroculture, Baybay, Leyte, 25–43 pp (2001).
Kolb, M. Silvicultural Analysis of “Rainforestation Farming” Area on Leyte Island, Philippines. Mag. Arbeit, University of Göttingen, Göttingen, Germany (2003).
Chokkalingam, U. et al. One century of forest rehabilitation in the Philippines: Approaches, outcomes and lessons. Center for International Forestry Research (CIFOR), Bogor, Indonesia (2006).
Olofson, H. . Swidden and kaingin among the southern Tagalog: a problem in Philippine upland ethno-agriculture. Philipp. Q. Cult. Soc. 8, 168–180 (1980).
Gentry, A. H. Patterns of neotropical plant species diversity. Evol. Biol. 15, 1–84 (1982).
Baraloto, C. et al. Rapid simultaneous estimation of aboveground biomass and tree diversity across Neotropical forests: a comparison of field inventory methods. Biotropica 45, 288–298 (2013).
Hunter, M. O., Keller, M., Victoria, D. & Morton, D. C. Tree height and tropical forest biomass estimation. Biogeosciences 10, 8385–8399 (2013).
World Agroforestry Centre. Wood density database. Available online at: http://www.worldagroforestry.org/regions/Southeast_asia/resources/db/wd (Date of access: 14/11/2014) (2014).
Stanley, W. et al. The climate action project research initiative. The Nature Conservancy, Arlington, V.A (2010).
Armecin, R. B. & Coseco, W. C. Abaca (Musa textilis Nee) allometry for above-ground biomass and fiber production. Biomass Bioenerg. 46, 181–189 (2012).
Delaney, M., Brown, S., Lugo, A. E., Torres-Lezama, A. & Quintero, N. B. The quantity and turnover of dead wood in permanent forest plots in six life zones of Venezuela. Biotropica 30, 2–11 (1998).
Beets, P. N. et al. Allometric equations for estimating carbon stocks in natural forest in New Zealand. Forests 3, 818–839 (2012).
Grueber, C. E., Nakagawa, S., Laws, R. J. & Jamieson, I. G. Multimodel inference in ecology and evolution: challenges and soulution. J. Evol. Biol. 24, 699–711 (2011).
Acknowledgements
We would like to thank Drs Nestor Gregorio (UQ/USC), Arturo Pasa (VSU) and VSU-ACIAR field staff for their heartiest cooperation in the field. Thanks are due to Professors Angela Ferraen (VSU) and Robert Congdon (JCU) for assistance with laboratory facilities, and to Professors Robin Chazdon (UConn), David Lamb (UQ/USC), and L.A. Bruinjeel (VU Amsterdam) for their valuable feedback at different stages of this work. This work was made possible by a scholarship support (International Postgraduate Research Scholarship) to S.A.M. from the Australian Government. This project was conducted as part of research funded by the Australian Centre for International Agricultural Research (ACIAR-ASEM/2010/50).
Author information
Authors and Affiliations
Contributions
S.A.M., J.H. and J.F. conceived and designed the study and wrote the paper. S.A.M. collected and analysed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mukul, S., Herbohn, J. & Firn, J. Tropical secondary forests regenerating after shifting cultivation in the Philippines uplands are important carbon sinks. Sci Rep 6, 22483 (2016). https://doi.org/10.1038/srep22483
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22483
This article is cited by
-
Biomass and carbon recovery of secondary forest in a Montane Subtropical Forest of North Eastern India
Tropical Ecology (2023)
-
Tree diversity and biomass carbon stock along an altitudinal gradient in old-growth secondary semi-evergreen forests in North East India
Tropical Ecology (2022)
-
New allometric equations for quantifying tree biomass and carbon sequestration in seasonally dry secondary forest in northern Thailand
New Forests (2022)
-
The role of secondary riparian forests for conserving fish assemblages in eastern Amazon streams
Hydrobiologia (2022)
-
Prognosis of aboveground woody biomass in a central Brazilian Cerrado monitored for 27 years after the implementation of management systems
European Journal of Forest Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.