Abstract
Fusarium graminearum is an important pathogen of wheat and barley. In addition to severe yield losses, infested grains are often contaminated with harmful mycotoxins. In this study, we characterized the functions of FgSSN3 kinase gene in different developmental and infection processes and gene regulation in F. graminearum. The FgSSN3 deletion mutant had a nutrient-dependent growth defects and abnormal conidium morphology. It was significantly reduced in DON production, TRI gene expression and virulence. Deletion of FgSSN3 also resulted in up-regulation of HTF1 and PCS1 expression in juvenile cultures and repression of TRI genes in DON-producing cultures. In addition, Fgssn3 was female sterile and defective in hypopodium formation and infectious growth. RNA-seq analysis showed that FgSsn3 is involved in the transcriptional regulation of a wide variety genes acting as either a repressor or activator. FgSsn3 physically interacted with C-type cyclin Cid1 and the cid1 mutant had similar phenotypes with Fgssn3, indicating that FgSsn3 and Cid1 form the CDK-cyclin pair as a component of the mediator complex in F. graminearum. Taken together, our results indicate that FgSSN3 is important for secondary metabolism, sexual reproduction and plant infection, as a subunit of mediator complex contributing to transcriptional regulation of diverse genes.
Similar content being viewed by others
Introduction
Wheat head blight (FHB) disease is one of the most destructive diseases of wheat. Fusarium graminearum is a major causal agent of FHB in the world1 and it also infects other small grain crops, including barley and maize1,2. In addition to yield losses, this pathogen is a producer of deoxynivalenol (DON), zearalenone and other mycotoxins. DON is a potent inhibitor of eukaryotic protein synthesis and zearalenone is an estrogenic mycotoxin. Both of them are harmful to human and animals.
F. graminearum initiates plant infection when ascospores land on flowering wheat heads. The fungus could form hyphopodia for direct penetration of plant tissues. DON is a phytotoxin and it is, in fact, the first virulence factor identified in F. graminearum3,4. The TRI5 trichodiene synthase gene is expressed as early as in hyphopodia5. Other important pathogenicity factors that have been characterized in F. graminearum include genes involved in various signal transduction pathways, metabolism and developmental processes6,7,8,9,10,11,12,13,14,15,16. Interestingly, a number of them, such as the protein kinase genes related to cAMP signaling and three mitogen-activated protein (MAP) kinase pathways also are involved in the regulation of DON biosynthesis and sexual reproduction10,14,15. Other protein kinase genes that are important for DON production, plant infection and sexual reproduction include SNF117, FGK318 and several genes characterized in the kinome study of F. graminearum19. One of them is FGSG_04484 that encodes a protein orthologous to the cyclin-dependent protein kinase (CDK) Ssn3 (=Srb10 or Ume5) in yeast and CDK8 in human.
Orthologs of Ssn3 are conserved from yeast to humans and involved in regulation of RNA polymerase II (Pol II)-dependent gene transcription20,21. Ssn3 is the only protein kinase in the yeast mediator complex, which functions as a bridge between gene-specific transcription regulators and Pol II machinery at the promoter region22. Like Cdk8 in humans, kinase activity of Ssn3 is related to binding with C-type cyclin Ssn8 (=Srb11). In yeast, Ssn3 is a regulator of global transcription and affects many important cellular processes, such as filamentous growth and cell cycle progression23,24,25. In humans, Cdk8 has been shown to repress the transcription of immune response genes26 and activate genes within the serum response network27.
In S. cerevisiae, SSN3 was originally identified as a suppressor of the C-terminal domain (CTD) truncation of Pol II28,29. Together with its cyclin Ssn8, yeast Ssn3 forms a stable complex with Srb8 and Srb9, which is one sub-module of the mediator complexes30. As a nonessential subunit of the mediator complex, Ssn3 regulates gene transcription probably by phosphorylation of the CTD of Pol II20. Deletion of SSN3 decreases the stability of meiotic mRNAs and induces the expression of genes repressed by glucose and mating type-specific genes. SSN3 also is involved in the regulation of genes related to stress responses and nutrient utilization23,31.
Although SSN3 orthologs are well conserved in plant pathogenic ascomycetes, none of them has been functional characterized. This study aims to determine the function of FgSSN3 in plant infection and other developmental processes in F. graminearum. Deletion of FgSSN3 resulted in medium-dependent growth defects, loss of female fertility, reduced hyphopodium formation and defects in infectious growth. In DON-producing cultures, the Fgssn3 mutant was repressed in TRI gene expression but increased in the transcription of genes related to aurofusarin biosynthesis. RNA-seq analysis also showed that FgSsn3 negatively or positively regulated the transcription of different subsets of genes. FgSsn3 physically interacted with C-type cyclin Cid1 and likely functions as the CDK-cyclin pair in the mediator complex to regulate the expression of various genes important for growth, differentiation and pathogenesis in F. graminearum.
Results
The Fgssn3 mutant has nutrient-dependent growth defects
The SSN3 ortholog in F. graminearum FGSG_04484.3 named as FgSSN3 in this study encodes a 453 amino acid protein. Sequence alignment revealed that SSN3 orthologs are well conserved in filamentous fungi. The Fgssn3 mutant was generated with the split-marker approach in a previous study of the F. graminearum kinome19. In this study, three putative Fgssn3 mutants, M5, M7 and M9 were further confirmed by Southern blot analysis (Fig. S1). All the Fgssn3 mutants had the same phenotype although only data for M9 were described below. Compared with the wild type, the Fgssn3 mutant was reduced in growth rate and produced fewer and shorter aerial hyphae (Fig. 1A). Interestingly, the growth defect of the Fgssn3 mutants was nutrient-dependent. In comparison with the growth rate of PH-1, the Fgssn3 mutants had the most significant reduction (56%) on 5 × YEG and less reduction (13%) on oatmeal agar (OTA) and PDA (27%) (Table 1). Whereas 5 × YEG is a synthetic medium, OTA and PDA are medium with natural substrates.
Defects of the Fgssn3 mutant in growth and sexual reproduction.
(A) Colonies of wild-type (PH-1), Fgssn3 deletion mutant (M9), complemented strain (C1) cultured on PDA, OTA and 5 × YEG medium for 3 days. (B) Self-crossing plates of PH-1, M9 and C1 at 14 days post-fertilization. Arrows point to perithecia. (C) Mating cultures of the Fgssn3 mutant used as the male (left) or female (right) crossed with the mat1-1-1 mutant were examined for perithecia and ascospore formation 2 weeks post- fertilization. Bar = 20 μm.
FgSSN3 is essential for female fertility
When assayed for sexual reproduction on carrot agar medium, the wild type formed small black perithecia 7 days post-perithecial induction and produced cirrhi after 10 days. However, no perithecia were observed in Fgssn3 mutant (Fig. 1B), indicating the importance of FgSSN3 during sexual reproduction. To determine whether its mating defects were related to male or female fertility, the Fgssn3 mutant was out-crossed with the mat1-1-1 deletion mutant9. When Fgssn3 was used as the male, fertile perithecia with normal ascospores were produced (Fig. 1C). In contrast, no perithecia were formed when Fgssn3 was used as the female (Fig. 1C), indicating that FgSSN3 is essential for female fertility but dispensable for male fertility.
The Fgssn3 mutant is de-repressed in conidiophore development
Although the Fgssn3 mutant was reduced in growth rate, it produced the same amount of conidia as the wild type (Table 1). Microscopic examination revealed that the Fgssn3 mutant tended to produce phialides and conidia earlier than the wild type. After 12 h incubation in CMC medium, clusters of phialides were observed in Fgssn3 but not in the wild type (Fig. 2A). However, mutant conidia were shorter and had fewer septa than the wild type (Fig. 2B). Approximately one-third of the mutant conidia also lacked typical tip or foot cells. Nevertheless, Fgssn3 conidia could germinate normally although germ tube growth was reduced (Fig. 2B), which is consistent with its reduced growth rate.
Defects of the Fgssn3 mutant in conidiogenesis and conidium morphology.
(A) CMC cultures of the wild type (PH-1) and Fgssn3 mutant (M9) after incubation for 12 h. Bar = 10 μm. (B) Conidia of PH-1, M9 and the complemented transformant C1 were examined for difference in morphology (upper row) and germ tube growth after incubation in YEPD for 6 h (lower row). Bar = 10 μm. (C) Expression levels of genes related to conidiation were assayed by qRT-PCR assays. RNA samples were isolated from 12 h YEPD cultures of PH-1 and M9. For each gene, its expression level in PH-1 was arbitrarily set to 1. Mean and standard deviation were calculated with data from three biological replicates.
We transformed the full-length FgSSN3 allele into the Fgssn3 mutant. The resulting Fgssn3/FgSSN3 transformant had the wild-type growth rate (Fig. 1A) and normal conidium morphology (Fig. 2B). The defects of the mutant in sexual reproduction and plant infection also were complemented by the ectopic integration of FgSSN3, indicating that deletion of FgSSN3 was directly responsible for defects of Fgssn3 mutants.
HTF1 and PCS1 are up-regulated in the Fgssn3 mutant
In F. graminearum, a number of genes, including FgMCM1, FgSTUA, PCS1, HTF1, COM1, FgCOS1, RAC1 and CON2 are known to be important for conidiation32,33,34,35,36,37,38,39. To assay their expression in the Fgssn3 mutant, RNA samples were isolated from 12 h CMC cultures. Compared with PH-1, transcript abundance of other genes was not affected, but the expression levels of HTF1 and PCS1 were up-regulated over 3- and 5-fold, respectively, in the Fgssn3 mutant (Fig. 2C). The PCS1 transcription factor gene plays a role in regulating proper production of conidia. Overexpression of PCS1 increased the formation of intercalary phialides34. HTF1 encodes a conserved homeobox transcription factor important for conidiogenesis and phialide formation35. The up-regulation of these two genes in Fgssn3 may be responsible for the de-repression of phialide formation and conidiation in 12 h CMC cultures.
FgSSN3 is important for plant colonization and infectious growth
In infection assays with flowering wheat heads, the Fgssn3 mutant developed typical disease symptoms only on the inoculated kernels but never spread to neighboring spikelets at 14 days post-inoculation (dpi) (Fig. 3A). The average disease index of the Fgssn3 mutant M9 and PH-1 was 0.7 and 11.3, respectively (Table 1), which was approximately a 90% reduction in virulence. Therefore, FgSSN3 is essential for disease spreading and colonization of wheat head tissues. Similar results were obtained in infection assays with corn stalks (Fig. 3B) and silks (Fig. 3C). Stalk rot and discoloration were restricted to the inoculated sites. These results suggest that FgSSN3 may play an important role in infectious growth in plant tissues.
Defects of the Fgssn3 mutant in plant infection.
(A) Flowering wheat heads were drop-inoculated with conidia from the wild type (PH-1), Fgssn3 mutant (M9) and complemented strain (C1). Black dots mark the inoculated spikelets. Photographs were taken 14 days post-inoculation (dpi). (B) Corn stalks were inoculated with toothpicks dipped in conidia of the same set of strains and examined for stalk rot symptoms 14 dpi. (C) Corn silks were inoculated with blocks of cultures of the same set of strains. Photographs were taken 5 dpi. (D) Lemma from the spikelets inoculated with PH-1 and M9 were examined by SEM 24 hpi. Hyphopodia formed on the inner surface are marked with white arrows. Bar = 10 μm. (E) Infectious hyphae (IH) formed by PH-1 and M9 inside lemma tissues 48 hpi. Bar = 50 μm. (F) Thick sections of rachis tissues directly below the inoculated spikelet were examined for infectious growth 5 dpi. In samples inoculated with PH-1, abundant hyphal growth was observed. No infectious hyphae (IH) were observed in the rachis inoculated with M9. Bar = 50 μm.
To further determine the function of FgSSN3 in plant infection, we examined the infection processes by scanning electron microscopy (SEM) and light microscopy. At 24 hours post infection (hpi), the wild-type strain formed penetration structures on wheat glumes (Fig. 3D). Under the same conditions, Fgssn3 was significantly reduced in hyphopodium formation. In fact, hyphopodia were rarely observed in samples inoculated with the mutant (Fig. 3D). Nevertheless, infectious hyphae were observed in lemma tissues inoculated with both the wild type and Fgssn3 mutant at 48 hpi although the extent of invasive growth was significantly reduced in the latter (Fig. 3E). By 5 dpi, fungal growth was not observed in the rachis below or above the spikelets inoculated with the Fgssn3 mutant (Fig. 3F). Under the same conditions, abundant intracellular hyphae were observed in the vascular and other tissues of the rachis in samples inoculated with PH-1 (Fig. 3F). These results further indicate that FgSSN3 is important for infectious growth and spreading from the inoculated spikelet to the rachis and nearby spikelets, which is consistent with its disease index being less than 1.
FgSSN3 positively regulates DON biosynthesis during plant infection and in DON-inducing culture conditions
Because of its importance as a virulence factor, we assayed DON production in the Fgssn3 mutant. In wheat kernels with scab symptoms collected 14 dpi, over 1000 ppm DON was detected in samples inoculated with PH-1 (Table 1). In contrast, DON concentration was less than 50 ppm in samples inoculated with the Fgssn3 mutants (Table 1). To confirm this observation, we assayed DON production in rice grain cultures as described40. DON production was barely detectable in rice grains inoculated with Fgssn3 (Table 1).
We also assayed the expression levels of the TRI4, TRI5, TRI6, TRI10 and TRI11 genes in DON-inducing cultures containing 5 μM arginine by qRT-PCR assay. In comparison with that of the wild type, the expression level of TRI4, TRI5, TRI6, TRI10 and TRI11 was reduced in the Fgssn3 mutant for approximately 20, 2.5, 2, 3 and 30 folds, respectively (Fig. 4A). These results showed that FgSSN3 plays an important role in the regulation of DON biosynthesis under DON-inducing conditions.
Assays for expression levels of selected genes related to trichothecene and aurofusarin biosynthesis by qRT-PCR.
The expression level of each gene in PH-1 was arbitrarily set to 1. Mean and standard deviation were calculated with data from three biological replicates. (A) Expression of TRI4, TRI5, TRI6, TRI10 and TRI11 in the wild-type strain PH-1 and Fgssn3 mutant M9. RNA samples were isolated from DON-producing cultures (containing 5 mM arginine). (B) Expression of PKS12, GIP1 and GIP2 in PH-1 and M9. RNA samples were isolated from DON-producing cultures containing 5 mM arginine.
Aurofusarin biosynthesis is negatively regulated by FgSSN3
Because the Fgssn3 mutant appeared to have enhanced reddish pigmentation than PH-1 on PDA and OTA cultures (Fig. 1A), we assayed the expression levels of three genes related to aurofusarin synthesis, the polyketide synthase genes PKS12 and two putative laccase genes GIP1 and GIP241, with RNA samples used for assaying TRI gene expression. In comparison with the wild type, the expression levels of these three genes were increased in the Fgssn3 mutant (Fig. 4B). The expression of GIP1 and GIP2 was upregulated over 30-folds (Fig. 4B), suggesting that FgSSN3 negatively regulates aurofusarin biosynthesis.
Expression and localization of FgSsn3-GFP fusion
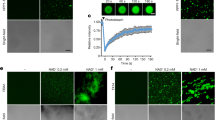
To determine the expression and localization of FgSsn3, we generated an FgSSN3-EGFP fusion construct under the control of its native promotor and transformed it into the Fgssn3 mutant. In the resulting transformant, although the mutant phenotypes were complemented, no or only faint GFP signals were observed in the nucleus. We then cloned the FgSSN3-EGFP fusion construct behind the strong constitutive promotor RP27 that is derived from the Magnaporthe grisea ribosomal protein 2742,43 and transformed it into the Fgssn3 mutant. In the resulting transformants, GFP signals were observed in the nucleus in conidia and hyphae (Fig. 5A).
Localization and expression of FgSSN3.
(A) Conidia and hyphae of the Fgssn3/FgSSN3-GFP H1-mCherry transformant were examined by DIC and epifluorescence microscopy. Bar = 20 μm. (B) Relative expression level of FgSSN3 in conidia (C), 4 or 12 h germlings and perithecia (P). Mean and standard deviation were calculated with data from three replicates.
We also assayed FgSSN3 expression in PH-1 by qRT-PCR with RNA samples isolated from conidia, 4 h, or 12 h germlings and mature perithecia. In comparison with conidia, the expression level of FgSSN3 was increased approximately 5-fold in 4 h or 12 h germlings and mature perithecia (Fig. 5B), indicating that FgSSN3 may be constitutively expressed in different growth and developmental stages except in conidia.
Kinase activity is essential for the function but not subcellular localization of FgSsn3
To determine whether the kinase activity is essential for FgSsn3 function and localization, we generated the FgSSN3D191A-GFP and FgSSN3K71R-GFP alleles and transformed them into the Fgssn3 mutant. The D191 and K71 residues of FgSsn3 are equivalent to D290 and K183 of S. cerevisiae Srb10, respectively, which are highly conserved amino acids in the kinase domain and essential for its kinase activity44,45. The Fgssn3/FgSSN3D191A-GFP transformants D8 and D24 and the Fgssn3/FgSSN3K71R-GFP transformants K4 and K8 had similar defects with the original Fgssn3 mutant in growth rate (Fig. 6A), conidium morphology (Fig. 6B), plant infection (Fig. 6C) and sexual reproduction (Fig. 6D). However, both FgSsn3D191A–GFP and FgSsn3K71R-GFP fusion proteins still localized to the nucleus (Fig. 6E). These results suggested that the kinase activity is essential for its function but dispensable for its subcellular localization.
Functions and Localization of FgSsn3D191A–GFP and FgSsn3K71R-GFP.
(A) Two-day-old 5 × YEG cultures of the wide type (PH-1), Fgssn3 mutant (M9), Fgssn3/FgSSN3D191A-GFP transformant (D24) and Fgssn3/FgSSN3K71R-GFP transformant (K4). (B) Conidia of PH-1, D24 and K4 in 4-day-old CMC cultures. Bar = 10 μm. (C) Corn silks inoculated with PH-1, D24 and K4 were examined 5 dpi. (D) Mating cultures of PH-1, D24 and K4 were examined 2 weeks post-induction for sexual reproduction. (E) Hyphae of D24 and K4 were examined by DIC and epifluorescence microscopy. Bar = 10 μm.
FgSsn3 is a component of the mediator complex in F. graminearum
In the budding yeast, Ssn3 interacts with Ssn8 (a C-type cyclin), Srb8 (Med12) and Srb9 (Med13) to form the kinase module of the mediator complex46. Orthologs of Ssn8, Srb8 and Srb9 and many other components of the yeast mediator complex are conserved in F. graminearum. The ortholog of yeast SSN8, CID1, has been characterized in an earlier study47. To confirm that FgSsn3 is also as a component of the mediator complex in F. graminearum, we constructed the Cid1 bait and FgSsn3 prey constructs and transformed them in pairs into yeast strain AH109. The resultant Trp+Leu+transformants were able to grow on the SD-Trp-Leu-His plate and had beta-galactosidase (LacZ) activities (Fig. 7). These results indicated that FgSsn3 directly interacts with Cid1, suggesting that FgSsn3 and Cid1 may function as a CDK-cyclin pair of the mediator complex in F. graminearum.
Yeast two-hybrid assays for the interaction between FgSsn3 and Cid1 or FgMed8.
Different concentrations (cells/ml) of the yeast transformants expressing the FgSSN3 prey and Cid1 or FgMed8 bait constructs were assayed for growth on SD-Leu-Trp-His plates and β-galactosidase (LacZ) activities. Positive and negative controls were provided in the BD Matchmaker library construct kit.
To test whether FgSsn3 also interacts with other mediator components, we generated the bait construct of FgMed846. In yeast transformants expressing the FgSsn3 prey and FgMed8 bait constructs, growth on SD-Trp-Leu-His plate and beta-galactosidase activities also were observed (Fig. 7), indicating that FgSsn3 may also interact with other components of the mediator complex in F. graminearum.
FgSsn3 positively and negatively regulates different subsets of genes in F. graminearum
To identify genes regulated by FgSsn3 in F. graminearum, we conducted RNA-seq analysis with RNA samples isolated from 36 h CM cultures of the wild-type strain PH-1 and the Fgssn3 mutant M9. In total, 2839 genes had over two-fold differences in expression levels between PH-1 and M9, including 1348 and 1491 genes that were up- and down-regulated, respectively, in the Fgssn3 mutant. Among them, 259 were specifically expressed in Fgssn3 and 196 were only expressed in PH-1. These results indicate that, similar to yeast Ssn3, FgSsn3 may be functionally related to the mediator complex to repress or activate the transcription of different subsets of genes in F. graminearum.
Among the 1348 genes that were up-regulated in Fgssn3, Blast2GO (https://www.blast2go.com) analysis showed that genes related to the cellular component ‘membrane, mitochondrion and mitochondrion envelope’ were enriched. Genes belonging to the molecular function go term ‘transmembrane transporter activity and oxidoreductase activity’ and the cellular process go term ‘monocarboxylic acid metabolic process, generation of precursor metabolites and energy, cofactor metabolic process and transmembrane transporter also were enriched (Fig. S2).
To our surprise, TRI13 and TRI14, two genes involved in DON biosynthesis were up-regulated over 19-folds in Fgssn3 mutant (Table S1). It is likely that the regulation of TRI genes was de-repressed in CM cultures when FgSSN3 is deleted. In fact, a number of genes related to secondary metabolism (Table S1), including PKS10 and NRPS148 also had up-regulated expression levels in the Fgssn3 mutant. These results suggest that FgSSN3 may be involved in suppressing the expression of genes related to secondary metabolism in vegetative hyphae harvested from 36 h CM cultures.
Alternative splicing of FgSSN3
In RNA-seq data of PH-1, two transcripts of FgSSN3 derived from alternative splicing of its only intron were detected (Fig. S3). Transcript A had the intron retention and was predicted to encode a protein that is 13-aa shorter than transcript B (Fig. S3A). RT-PCR analysis further verified the presence of transcript A and B in hyphae and perithecia (Fig. S3B). To determine differences in the abundance of two FgSSN3 transcripts, we examined the expression levels of transcript A and B in RNA-seq data of conidia, hyphae harvested from YEPD medium and perithecia collected as 8 days post-fertilization. Whereas transcript A was the predominant transcript of FgSSN3 in conidia and vegetative hyphae, transcript B was over 2 folds more abundant than transcript A in perithecia (Fig. S3C), suggesting that transcript A mainly functions in vegetative growth and B in sexual reproduction.
Discussion
In eukaryotic organisms, the mediator complex directly bind to RNA polymerase II to regulate the transcription of various genes22. Ssn3 is a nonessential subunit of the mediator complex that is conserved between yeast and humans46. Like SSN3 in yeast, deletion of FgSSN3 is not lethal but important for hyphal growth and germ tube elongation in F. graminearum. In the fission yeast, the generation time of the ssn3 mutant was longer than that of the wild-type strain24. It is possible that the Fgssn3 mutant also had a longer generation time, which may be related to reduction in growth rate. Interestingly, the growth rate reduction of the Fgssn3 mutant was nutrient dependent. The reduction in growth was more significant on synthetic media than on media with natural substrates. In yeast, Ssn3 also inhibits yeast filamentous growth in rich medium by phosphorylation of Ste12 and decreasing its stability23.
Although it was reduced in growth, the number of conidia produced by the Fgssn3 mutant was not reduced. In fact, conidiophore development was de-repressed in juvenile CMC cultures. As early as 12 h after inoculation, clusters of conidiophores were observed in the mutant CMC cultures. The up-regulation of PCS1 and HIF1 expression34,35 may be related to conidiophore formation in juvenile cultures. Nevertheless, we noticed that conidia produced by the mutant had abnormal morphology. Unlike normal 5–7 celled conidia produced by the wild type, Fgssn3 conidia vary from 1 to 4 compartments. We also noticed that many of the conidium compartments contained more than one nucleus. Therefore, FgSSN3 must also play a role in mitosis and cytokinesis during conidium development.
Although SSN3 orthologs are well conserved in plant pathogenic fungi, none of them have been shown to be related to pathogenesis. Our data showed that FgSSN3 is critical for plant infection. The disease index of the Fgssn3 mutant was less than 1, showing that it was defective in both causing symptoms in the inoculated kernels and spreading via the rachis in infected wheat heads. One contributing factor to its defects in plant infection could be related to the reduction in growth rate. However, the Fgssn3 mutant also was reduced in hyphopodium formation and penetration of lemma epidermal cells. In addition, the Fgssn3 mutant was significantly reduced in DON production in diseased wheat kernels and DON is an important virulence factor in F. graminearum4.
Although FgSSN3 is dispensable for male fertility, it is essential for female fertility, indicating that it may regulate the expression of sub-sets of genes important for the formation of protoperithecia and other developmental processes related to female fertility. In F. graminearum, a number of genes have been reported to be essential for female fertility but dispensable for male fertility, including the MGV1 and FgHOG1 MAP kinase and ZIF1 and MYT1 transcription factor genes7,14,49,50. However, to our knowledge, no mutants are known to be normal in female fertility but defective in male fertility in F. graminearum. It is likely that female fertility involving the formation of protoperithecia requires many more genes than male fertility in this homothallic fungus. In the rice blast fungus Magnaporthe oryzae, a heterothallic fungus, the MCM1 transcription factor is essential for male fertility51.
No perithecium formation was observed in self-crosses in the Fgssn3 mutant. In S. cerevisiae, SSN3, also known as UME5, is important for meiosis and sporulation52. However, because deletion of FgSSN3 blocked perithecium formation on mating plates, it is impossible to conclude that FgSSN3 is important for meiosis and ascospore formation in F. graminearum. As an important transcriptional regulator, FgSSN3 may be involved in the regulation of hyphal fusion and other processes necessary for proto-perithecium development. Because F. graminearum is a homothallic fungus, it is also possible that FgSSN3 is important for switching from vegetative growth to sexual reproduction.
Interestingly, two transcripts of FgSSN3 were observed in this study with transcript A encoding a 13-aa shorter protein than transcript B due to the retention of an intron in its 5′-UTR. Although the 13 extra amino acid residues at the N-terminal region of FgSsn3B is 39-aa upstream from its kinase domains and unlikely to affect its kinase function, we noticed that transcript A was the predominant transcript of FgSSN3 in conidia and hyphae but transcript B had a twice more abundant than transcript A in perithecia. Therefore, it remains possible that transcript B plays a stage-specific role in gene expression regulation during sexual reproduction.
In S. cerevisiae, the highly conserved aspartic acid residue at position 290 and lysine residue at position 183, are essential for the kinase activity and function of the Srb10 protein44,45. In F. graminearum, the D191A or K71R mutations at the equivalent sites of FgSsn3 produced similar phenotypes as the FgSSN3 deletion mutant, indicating the importance of kinase activity of FgSsn3 in the function of the protein.
Interestingly, the Fgssn3 mutant was reduced in DON production but increased in aurofusarin biosynthesis under DON-inducing conditions. Therefore, deletion of FgSSN3 does not generally blocking secondary metabolism, which is consistent with the fact that SSN3 has both negative and positive regulatory roles in gene regulation in the budding yeast46. FgSSN3 likely has similar regulatory functions in F. graminearum. Our RNA-seq analysis results showed that the transcription of 1348 and 1491 genes was up- and down-regulated, respectively, in the Fgssn3 mutant. Some genes important for secondary metabolism appeared to be de-repressed in the CM cultures of the mutant, suggesting that FgSSN3 is involved in the repression of these genes during vegetative growth. To our surprise, two TRI genes had increased expression levels in CM cultures, although DON production and TRI gene expression were reduced in DON inducing cultures and infected wheat kernels in the Fgssn3 mutant. These results indicate that regulation of specific subsets of genes by FgSSN3 may depend on culture conditions, which is consistent with medium-dependent growth defects of Fgssn3 on different media.
In yeast, Ssn3 interacts with Ssn8 to form a kinase-cyclin pair that functions together with Srb8 and Srb9 as part of the kinase module of the mediator complex53. SSN8 is orthologous to the CID gene in F. graminearum47. Like the Fgssn3 mutant, the cid1 mutant was defective in conidium morphology and reduced in growth rate, DON production and virulence. We further showed that FgSsn3 physically interacted with Cid1. Therefore, FgSsn3 and Cid1 form a similar CDK-cyclin pair that is functionally related to the mediator to affect the transcription by the Pol II holoenzyme in F. graminearum.
For transcriptional regulation, Ssn3 can directly phosphorylate the Ser5 of the triple heptapeptide repeats in the CTD of the largest subunit of Pol II20. Sequence alignment analysis showed that these phosphorylation sites are conserved in the large subunit of Pol II in F. graminearum. Orthologs of many components of the yeast mediator complex also are conserved in F. graminearum and other filamentous ascomycetes. Our studies implicate the role of the mediator complex in plant infection, secondary metabolism and development in a fungal pathogen. In plants, the mediator complex has been shown to be involved in a variety of processes, including defense responses54. Considering the importance of the mediator complex in fungal-plant interactions, it will be important to identify and characterize different subsets of genes that are transcriptionally regulated by the FgSsn3-Cid1 CDK-cyclin pair during pathogenesis and the underlying mechanisms related to the mediator complex Pol II activity.
Materials and Methods
Strains and culture conditions
The F. graminearum wild-type strain PH-1 (NRRL 31084) and all the transformants generated in this study were routinely maintained on PDA plates at 25 °C. Conidiation in liquid CMC medium and growth rate on 5 × YEG, oatmeal agar and PDA plates were measured as described47. Mating on carrot agar plates were assayed as described9,19. Protoplast preparation and fungal transformation were performed as described14. Hygromycin B (Calbiochem, La Jolla, CA, USA) and G418 (Sigma, St. Louis, MO, USA) were added to the final concentration of 300 μg/ml and 400 μg/ml, respectively, for transformant selection. DNA and RNA were extracted from vegetative hyphae harvested from liquid YEPD (1% yeast extract, 2% peptone, 2% glucose) cultures.
Generation of the FgSSN3-GFP, FgSSN3D191A-GFP and FgSSN3K71R-GFP transformants
For generating Fgssn3/FgSSN3 complemented transformants, a 2.8-kb fragments of the FgSSN3 gene containing the 1.5-kb promoter region was amplified with primers FGSG_04484/F and FGSG_04484/R and co-transformed with XhoI-digested pFL2 vector (carrying geneticin resistance marker) into yeast strain XK1-25 as described55. The PFgSSN3-FgSSN3-GFP fusion construct was identified by PCR and confirmed by sequencing analysis. The D191A and K71R mutations were introduced into FgSSN3 by overlapping PCR using primers DA/1F and DA/4R and KR/1F and KR/4R, respectively. The same yeast gap repair approach was used to generate the PRP27-FgSSN3-GFP, PFgSSN3-FgSSN3D191A-GFP and PFgSSN3-FgSSN3K71R-GFP constructs. All the GFP fusion constructs were transformed into the protoplasts of the Fgssn3 mutant M9. The resulting transformants were analyzed by PCR and examined for GFP signals with an Olympus BX-51 epifluorescence microscope (Olympus, Tokyo, Japan).
Plant infection assays
Conidia harvested from 5-day-old CMC cultures were resuspended to 2.0 × 105 conidia/ml in sterile water for plant infection assay. Flowering wheat head of cultivar Xiaoyang 22 were inoculated with 10 μl conidial suspension at the fifth spikelet from the base of the wheat head as described55. Spikelets with typical head blight disease symptoms were examined 14 dpi and disease indexes were calculated55. Infection assays with corn stalks and silks of cultivar 2375 were performed as described56,32. Stalk rot symptoms and discoloration of infected corn silks were examined 14 and 5 dpi, respectively.
Assays for DON production
The inoculated wheat kernels with typical head blight symptoms were harvested for DON assays as described56. DON production in rice cultures40 was assayed as described56,57.
Assays for penetration and infectious growth
Lemmas were collected from inoculated spikelets at 24 and 48 hpi. After fixation with 4% (vol/vol) glutaraldehyde in 0.1 M phosphate buffer (pH 6.8) overnight at 4 °C, samples were dehydrated in a series of acetone (30, 50, 70, 80, 90 and 100% [vol/vol]). The dehydrated samples were then sputter coated with gold-palladium and examined for penetration structures with a JEOL 6360 scanning electron microscope (Jeol Ltd., Tokyo, Japan). For light microscopy observation, infected lemma and rachis were fixed, dehydrated and embedded in Spurr resin as described35. Thick sections (1 μm) were stained with 0.5% (wt/vol) toluidine blue and examined with an Olympus BX-53 microscope. At least three independent biological replicates were examined for the wild-type and Fgssn3 mutant strains.
qRT-PCR analysis
RNA samples were isolated with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from conidia, germlings and perithecia for assaying FgSSN3 expression, from 12 h YEPD cultures for assaying the expression levels of conidation related genes and from 6 d DON-inducing cultures containing 5 mM arginine for assaying the expression of TRI genes and aurofusarin biosynthesis pathway genes. cDNA was synthesized with the Fermentas First cDNA synthesis kit (Hanover, MD, USA) following the instructions provided by the manufacturer. The beta-tubulin gene FgTUB2 was used as internal control56. Relative expression level of each gene were calculated by the 2−△△Ct method58. For each gene, qRT-PCR data from three biological replicates were used to calculate the mean and standard deviation.
Yeast two-hybrid assays
Protein-protein interactions were assayed with the Matchmaker yeast two-hybrid system (Clontech, Mountain View, CA, USA). ORFs of the FgSSN3, FgMED8 and CID1 genes were amplified from the cDNA of PH-1 and cloned into pGADT7 and pGBK7 (Clontech) as the prey and bait constructs. The resulting bait and prey vectors were co-transformed in pairs into yeast strain AH109 (Clontech). The Leu + and Trp + transformants were isolated and assayed for growth on SD-Trp-Leu-His medium and galactosidase activities with filter lift assays51. The positive and negative controls were provided in the Matchmaker library construction kit (Clontech).
RNA-seq analysis
Vegetative hyphae of PH-1 and Fgssn3 mutant M9 were harvested from 36 h liquid CM cultures. For each strain, two biological replicates were used. Total RNAs were extracted with the Qiagen RNeasy Micro kit and treated with RNase-free DNase I. Complementary DNA libraries with the average insert size of 330 bp were constructed with the Illumina TruSeq RNA Sample Preparation Kit and sequenced with Illumina HiSeq 2000 at the Novogene Bioinformatics Institute (Beijing, China). For each sample, at least 18 Mb paired-end reads were obtained. The resulting RNA-seq reads were mapped onto the reference genome of F. graminearum strain PH-1 with Tophat 2.0.1259. The number of reads (counts) aligned to each predicted transcript was calculated by FeatureCounts60. Differential expression analysis of genes was performed with the edgeRun package61 using the UCexactTest function with the Benjamini and Hochberg’s algorithm to control the false discovery rate (FDR). To filter out weakly expressed genes, only genes with a minimum expression level of 1 count per million in at least two samples were included in the analysis. Genes with a FDR of below 0.05 were considered differentially expressed between Fgssn3 mutant and PH-1. The RNA-Seq data have been deposited in the NCBI Sequence Read Archive database with accession code PRJNA289285.
Additional Information
How to cite this article: Cao, S. et al. FgSsn3 kinase, a component of the mediator complex, is important for sexual reproduction and pathogenesis in Fusarium graminearum. Sci. Rep. 6, 22333; doi: 10.1038/srep22333 (2016).
References
Goswami, R. S. & Kistler, H. C. Heading for disaster: Fusarium graminearum on cereal crops. Molecular plant pathology 5, 515–525 (2004).
Bai, G. & Shaner, G. Management and resistance in wheat and barley to fusarium head blight. Annual review of phytopathology 42, 135–161 (2004).
Proctor, R. H., Hohn, T. M. & McCormick, S. P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Molecular plant-microbe interactions : MPMI 8, 593–601 (1995).
Desjardins, A. E., Bai, G., Plattner, R. D. & Proctor, R. H. Analysis of aberrant virulence of Gibberella zeae following transformation-mediated complementation of a trichothecene-deficient (Tri5) mutant. Microbiology 146 (Pt 8), 2059–2068 (2000).
Boenisch, M. J. & Schafer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 11, doi: 11010.1186/1471-2229-11-110 (2011).
Urban, M., Mott, E., Farley, T. & Hammond-Kosack, K. The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Molecular plant pathology 4, 347–359 (2003).
Zheng, D. et al. The FgHOG1 pathway regulates hyphal growth, stress responses and plant infection in Fusarium graminearum. PloS one 7, e49495 (2012).
Yu, H. Y. et al. Functional analyses of heterotrimeric G protein G alpha and G beta subunits in Gibberella zeae. Microbiology 154, 392–401 (2008).
Zheng, Q. et al. The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One 8, e66980 (2013).
Hu, S. et al. The cAMP-PKA pathway regulates growth, sexual and asexual differentiation and pathogenesis in Fusarium graminearum. Molecular plant-microbe interactions: MPMI 27, 557–566 (2014).
Song, X. S. et al. Trehalose 6-phosphate phosphatase is required for development, virulence and mycotoxin biosynthesis apart from trehalose biosynthesis in Fusarium graminearum. Fungal Genet Biol 63, 24–41 (2014).
Menke, J., Dong, Y. & Kistler, H. C. Fusarium graminearum Tri12p influences virulence to wheat and trichothecene accumulation. Molecular plant-microbe interactions: MPMI 25, 1408–1418 (2012).
Maier, F. J. et al. Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Molecular plant pathology 7, 449–461 (2006).
Hou, Z. M. et al. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation and plant infection. Molecular plant-microbe interactions: MPMI 15, 1119–1127 (2002).
Jenczmionka, N. J., Maier, F. J., Losch, A. P. & Schafer, W. Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head-blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr Genet 43, 87–95 (2003).
Kim, H. K., Cho, E. J., Lee, S., Lee, Y. S. & Yun, S. H. Functional analyses of individual mating-type transcripts at MAT loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiol Lett 337, 89–96 (2012).
Lee, S. H. et al. GzSNF1 is required for normal sexual and asexual development in the ascomycete Gibberella zeae. Eukaryot Cell 8, 116–127 (2009).
Qin, J., Wang, G., Jiang, C., Xu, J. R. & Wang, C. Fgk3 glycogen synthase kinase is important for development, pathogenesis and stress responses in Fusarium graminearum. Scientific Reports 5, 8504, doi: 10.1038/srep08504 (2015).
Wang, C. et al. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathogens 7, e1002460 (2011).
Hengartner, C. J. et al. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Molecular cell 2, 43–53 (1998).
Tsai, K. L. et al. A conserved Mediator-CDK8 kinase module association regulates Mediator-RNA polymerase II interaction. Nat Struct Mol Biol 20, 611–619 (2013).
Conaway, R. C. & Conaway, J. W. Function and regulation of the Mediator complex. Current Opinion in Genetics & Development 21, 225–230 (2011).
Nelson, C., Goto, S., Lund, K., Hung, W. & Sadowski, I. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 421, 187–190 (2003).
Szilagyi, Z., Banyai, G., Lopez, M. D., McInerny, C. J. & Gustafsson, C. M. Cyclin-dependent kinase 8 regulates mitotic commitment in fission yeast. Molecular and cellular biology 32, 2099–2109 (2012).
Szilagyi, Z. & Gustafsson, C. M. Emerging roles of Cdk8 in cell cycle control. Biochim Biophys Acta 1829, 916–920 (2013).
Tsutsui, T. et al. Mediator complex recruits epigenetic regulators via its two cyclin-dependent kinase subunits to repress transcription of immune response genes. J Biol Chem 288, 20955–20965 (2013).
Donner, A. J., Ebmeier, C. C., Taatjes, D. J. & Espinosa, J. M. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat Struct Mol Biol 17, 194–201 (2010).
Nonet, M. L. & Young, R. A. Intragenic and extragenic suppressors of mutations in the heptapeptide repeat domain of Saccharomyces cerevisiae RNA polymerase II. Genetics 123, 715–724 (1989).
Aristizabal, M. J. et al. High-throughput genetic and gene expression analysis of the RNAPII-CTD reveals unexpected connections to SRB10/CDK8. PLoS genetics 9, e1003758 (2013).
Borggrefe, T., Davis, R., Erdjument-Bromage, H., Tempst, P. & Kornberg, R. D. A complex of the Srb8, −9, −10 and −11 transcriptional regulatory proteins from yeast. J Biol Chem 277, 44202–44207 (2002).
Bose, S., Dutko, J. A. & Zitomer, R. S. Genetic factors that regulate the attenuation of the general stress response of yeast. Genetics 169, 1215–1226 (2005).
Yang, C. et al. The MADS-box transcription factor FgMcm1 regulates cell identity and fungal development in Fusarium graminearum. Environmental microbiology, doi: 10.1111/1462-2920.12747 (2015).
Lysøe, E., Pasquali, M., Breakspear, A. & Kistler, H. C. The transcription factor FgStuAp influences spore development, pathogenicity and secondary metabolism in Fusarium graminearum. Molecular plant-microbe interactions : MPMI 24, 54–67 (2011).
Jung, B. et al. A putative transcription factor pcs1 positively regulates both conidiation and sexual reproduction in the cereal pathogen Fusarium graminearum. The plant pathology journal 30, 236–244 (2014).
Zheng, W. et al. A conserved homeobox transcription factor Htf1 is required for phialide development and conidiogenesis in Fusarium species. PLoS One 7, e45432 (2012).
Yang, J. et al. A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae. Molecular plant-microbe interactions: MPMI 23, 112–123 (2010).
Zhou, Z., Li, G., Lin, C. & He, C. Conidiophore stalk-less1 encodes a putative zinc-finger protein involved in the early stage of conidiation and mycelial infection in Magnaporthe oryzae. Molecular plant-microbe interactions: MPMI 22, 402–410 (2009).
Chen, J. et al. Rac1 is required for pathogenicity and Chm1-dependent conidiogenesis in rice fungal pathogen Magnaporthe grisea. PLoS Pathog 4, e1000202 (2008).
Shi, Z. X., Christian, D. & Leung, H. Interactions between spore morphogenetic mutations affect cell types, sporulation and pathogenesis in Magnaporthe grisea. Mol Plant Microbe In 11, 199–207 (1998).
Seo, J. A., Kim, J. C., Lee, D. H. & Lee, Y. W. Variation in 8-ketotrichothecenes and zearalenone production by Fusarium graminearum isolates from corn and barley in Korea. Mycopathologia 134, 31–37 (1996).
Kim, J. E. et al. GIP2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl Environ Microbiol 72, 1645–1652 (2006).
Bruno, K. S., Tenjo, F., Li, L., Hamer, J. E. & Xu, J. R. Cellular localization and role of kinase activity of PMK1 in Magnaporthe grisea. Eukaryot cell 3, 1525–1532 (2004).
Jiang, C. et al. FgSKN7 and FgATF1 have overlapping functions in ascosporogenesis, pathogenesis and stress responses in Fusarium graminearum. Environ Microbiol 17(4), 1245–60 (2015).
Kuchin, S. & Carlson, M. Functional relationships of Srb10-Srb11 kinase, carboxy-terminal domain kinase CTDK-I and transcriptional corepressor Ssn6-Tup1. Molecular and cellular biology 18, 1163–1171 (1998).
Surosky, R. T., Strich, R. & Esposito, R. E. The yeast UME5 gene regulates the stability of meiotic mRNAs in response to glucose. Molecular and cellular biology 14, 3446–3458 (1994).
Takagi, Y. & Kornberg, R. D. Mediator as a general transcription factor. J Biol Chem 281, 80–89 (2006).
Zhou, X., Heyer, C., Choi, Y. E., Mehrabi, R. & Xu, J. R. The CID1 cyclin C-like gene is important for plant infection in Fusarium graminearum. Fungal Genet Biol 47, 143–151 (2010).
Hansen, F. T. et al. An update to polyketide synthase and non-ribosomal synthetase genes and nomenclature in Fusarium. Fungal Genet Biol 75, 20–29 (2015).
Wang, Y. et al. A Novel Transcriptional Factor Important for Pathogenesis and Ascosporogenesis in Fusarium graminearum. Molecular Plant-Microbe Interactions: MPMI 24, 118–128 (2010).
Lin, Y. et al. A putative transcription factor MYT1 is required for female fertility in the ascomycete Gibberella zeae. PloS one 6, e25586, doi: 10.1371/journal.pone.0025586 (2011).
Zhou, X. et al. A MADS-box transcription factor MoMcm1 is required for male fertility, microconidium production and virulence in Magnaporthe oryzae. Mol Microbiol 80, 33–53 (2011).
Ohkuni, K. & Yamashita, I. A transcriptional autoregulatory loop for KIN28-CCL1 and SRB10-SRB11, each encoding RNA polymerase II CTD kinase-cyclin pair, stimulates the meiotic development of S. cerevisiae. Yeast 16, 829–846 (2000).
Kuchin, S., Yeghiayan, P. & Carlson, M. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc Natl Acad Sci USA 92, 4006–4010 (1995).
Zhu, Y. et al. CYCLIN-DEPENDENT KINASE8 differentially regulates plant immunity to fungal pathogens through kinase-dependent and -independent functions in Arabidopsis. Plant Cell 26, 4149–4170 (2014).
Chen, D., Wang, Y., Zhou, X. & Xu, J. R. The Sch9 kinase regulates conidium size, stress responses and pathogenesis in Fusarium graminearum. PLoS One 9, e105811 (2014).
Bluhm, B. H., Zhao, X., Flaherty, J. E., Xu, J. R. & Dunkle, L. D. RAS2 regulates growth and pathogenesis in Fusarium graminearum. Molecular plant-microbe interactions: MPMI 20, 627–636 (2007).
Seong, K. Y. et al. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol Microbiol 72, 354–367 (2009).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14, R36, doi: 10.1186/gb-2013-14-4-r36 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Dimont, E., Shi, J., Kirchner, R. & Hide, W. edgeRun: an R package for sensitive, functionally relevant differential expression discovery using an unconditional exact test. Bioinformatics, doi: 10.1093/bioinformatics/btv209 (2015).
Acknowledgements
We sincerely thank Xuli Gao, Huili Zhang and Tao Yin for their assistances with qRT-PCR and DON production assays. We also thank Dr. Chenfang Wang for fruitful discussions. This work was supported by the National Major Project of Breeding for New Transgenic Organisms (2012ZX08009003) and the National Basic Research Program of China (2013CB127702).
Author information
Authors and Affiliations
Contributions
S.C., C.H., S.Z. and H.L. performed the experiments and analyzed the data. J.X. and Q.J. designed the experiments and prepared the manuscript. S.C. and Q.J. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cao, S., Zhang, S., Hao, C. et al. FgSsn3 kinase, a component of the mediator complex, is important for sexual reproduction and pathogenesis in Fusarium graminearum. Sci Rep 6, 22333 (2016). https://doi.org/10.1038/srep22333
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22333
This article is cited by
-
Cereal diseases caused by Fusarium graminearum: from biology of the pathogen to oxidative burst-related host defense responses
European Journal of Plant Pathology (2018)
-
RNA editing of the AMD1 gene is important for ascus maturation and ascospore discharge in Fusarium graminearum
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.