Abstract
Bi2Se3 nanosheets were successfully synthesized by a microwave-assisted approach in the presence of polyvinylpyrroli done at a temperature of 180 °C for 2 h. The thin film was prepared on a silicon wafer via a self-deposition process in a Bi2Se3 nanosheet ink solution using the evaporation-induced self-assembly method. The structure and morphology of the obtained products were characterized by X-ray diffraction, scanning electron microscopy (SEM), x-ray photoelectron spectroscopy and Raman spectroscopy. The highly uniform Bi2Se3 particles could be formed by controlling the oxygen plasma treatment time. After the plasma pretreatment from 10 to 20 s, the surface of Bi2Se3 film evolved from the worm-like structure to particles. The highly uniform thin film was formed on further increasing the plasma treatment time, which is consistent with the observed SEM results. Several important processes can result in the morphological evolution of Bi2Se3 nanosheets: (1) formation of Bi2Se3 oxide layer; (2) self-assembly of oxide nanoparticles under the action of high-energy oxygen plasma; and (3) electrostatic interaction and etching mechanism.
Similar content being viewed by others
Introduction
Two-dimensional (2D) layered materials have attracted considerable attention in recent years because of their potential applications in electric and optoelectronic devices1,2,3,4. The morphology, thickness and microstructure of nanomaterials have important effects on their transport and optical properties. Layered bismuth selenide (Bi2Se3), an important narrow band gap semiconductor, possesses topologically protected chiral metallic surface states and an insulating bulk5,6,7,8,9,10. Bi2Se3 has a rhombohedral structure consisting of hexagonal close-packed atomic layers periodically arranged along the c-axis11. The neighboring quintuple layers are bound by the weak van der Waals forces. Compared with Bi2Se3 bulk material, its nanostructures remain relatively unexplored. A number of Bi2Se3 nanostructures have been successfully synthesized using various methods for different applications. These methods are usually termed as “top-down” and “bottom-up” methods12,13. Nanomaterial ink has emerged as a low-cost alternative method for the fabrication of many functional films14,15,16. Lin et al. report the use of 2D colloidal Bi2Se3 nanoplates as an ink material to obtain high-performance electronic thin films via a solution assembly method17. It is an easy and common technology to realize 2D nanoplates as a building block for Bi2Se3 thin films on any substrates for the application in electronic and optoelectronic devices.
Recently, the effect of surface oxide layers on Bi2Se3 electronic characteristics has been investigated18. In fact, the formation of surface oxide layer is inevitable during the preparation of materials and devices. The intentionally growing oxide insulator layers can significantly improve the photoelectric characteristics of solar cells19. Plasma post-treatment technology has been one of the central techniques for thinning the layered materials to a desired thickness and further improve their properties20. Precise control of the growth and clear understanding of the mechanism of morphological evolution are the key to obtain a high-quality Bi2Se3 nanostructure by post-treatment technology. A detailed study about the Bi2Se3 thin film treated with oxygen plasma helps understand the morphological evolution and thinning mechanism. Various experimental groups have investigated the morphological evolution of the thin film comprising nanosheets by oxygen plasma treatment17,20,21,22,23,24. The atomic-scale mechanisms of graphene etched by oxygen plasma were studied by first-principles molecular dynamics calculations combined with rare events sampling techniques25. Although a lot of work has been done on the 2D materials or device surfaces treated with oxygen plasma, the morphological evolution and mechanism of action remain relatively unexplored.
The present study reported the rapid synthesis of high-quality Bi2Se3 nanosheets by a microwave-assisted technology; these sheets are less toxic, biocompatible and environmentally friendly. Meanwhile, the study also investigated the use of solution assembly method to prepare Bi2Se3 thin films from as-prepared few-layer Bi2Se3 nanoplates dispersed in solvents. Finally, the impact of the plasma treatment on the morphological evolution of Bi2Se3 thin films thus synthesized was studied.
Results
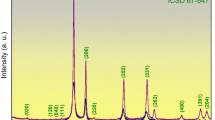
The X-ray diffraction (XRD) pattern of the as-prepared Bi2Se3 nanosheets is presented in Fig. 1(a). The reflection lines can be readily indexed with the rhombohedral geometry of Bi2Se3 (JCPDS: 33–0214). No extra peaks were observed for the impurities in the XRD spectrum, suggesting the high purity of the product. The morphology of the as-synthesized Bi2Se3 nanosheets was characterized using scanning electron microscopy (SEM). Figure 1(b) is a typical low-resolution SEM image of the Bi2Se3 nanosheets, revealing that the sample consisted of hexagonal nanosheets with an average diameter of 1–2 μm. The x-ray photoelectron spectroscopy (XPS) was used to characterize the surface composition of Bi2Se3 nanosheets. The Se3d spectrum (Fig. 1(c)) was broad and highly asymmetric, which could be deconvoluted into four peaks, one with a binding energy of 53.4 eV (corresponding to Se 3d5/2) and the other with a binding energy of 54.1 eV (corresponding to Se 3d3/2); the others could be ascribed to oxidation. A high-resolution XPS (Fig. 1d) showed peaks at about 441.9 and 465.7 eV, which corresponded to the reported values for the binding energies of Bi 4d5/2 and Bi 4d7/2, respectively.
The thin film was prepared on a silicon wafer via a self-deposition process in Bi2Se3 nanosheet ink solution. Figure 2(a) shows a schematic illustration for the formation of Bi2Se3 thin films by the evaporation-induced self-assembly process. 2D Bi2Se3 nanosheets thus synthesized could be well dispersed in an alcohol solution to form a stable colloidal ink (Fig. 2(b)). The alcohol gradually was evaporated at room temperature, resulting in the Bi2Se3 nanosheets tiled on the substrate. The effect of the oxygen plasma treatment time on the morphology of the Bi2Se3 nanosheets was analyzed by SEM images. The SEM images for the samples treated at different times of 0, 10, 20, 30 and 50 s are shown in Fig. 3(a–e), respectively. It was observed that the thin nanosheets were tiled on the silicon wafer one by one, rather than standing vertically on the substrate. The morphology of nanosheets evolved as a worm-like structure when the sample was treated after 10 s. As the treatment time was increased to 20 s, a number of nanoparticles were formed on the surface of nanosheets. It is interesting to note that the sheet was transparent to the electron beam with the increase in the oxygen plasma treatment time to 30 s, indicating that the sheet was very thin. A large number of holes appeared with the increase in time duration of etching to 50 s. This could be ascribed to the fact that the surface layer of Bi2Se3 nanosheets was oxidized, etched and then desorbed from the nanosheets. Figure 4(a) further illustrates the surface topography of oxidized Bi2Se3 nanoparticles. The thin films were acted upon by oxygen plasma for 20 s. It was clearly observed that oxidation led to the formation of well-defined nanostructures. The surface density of nanoparticles was approximately 3.0 × 105 cm−2. The particles were of an average size of 125 nm. The size of the nanoparticles was well described by Gaussian distributions (Fig. 4(b)), indicating that the formation of nanoparticles followed the assembly growth rule. The corresponding elemental compositions of the Bi2Se3 nanoparticles were determined by energy-dispersive spectroscopy (Fig. 4c,d). The appearance of characteristic peaks corresponded to Bi and Se and showed that the atomic percentage was slightly higher than the stoichiometry of Bi/Se (=2/3), indicating that Se ions were partly exchanged with O atoms. Hence, it can be concluded that the surface Se ions were replaced by O atoms under the action of oxygen plasma.
The characteristics of nanoparticles.
(a)SEM image of nanopaticles formed after 20 s oxygen plasma treatment. (b)The lateral size of the nanoparticles from SEM images (a) and the Gaussian fitting (blue line) are shown. (c)The corresponding elemental compositions of the Bi2Se3 nanoparticles were determined by energy dispersive spectroscopy, the nanoparticle is marked by the plus sign. (d) Corresponding EDS patterns are shown in (c).
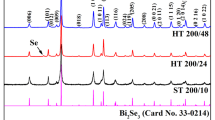
Figure 5 shows the XRD patterns of Bi2Se3 nanosheets treated with oxygen plasma radiation at different times of 10, 20 and 30 s, respectively. Some changes in the positions of peaks could be observed, for instance, the strength of the peaks located at the crystal orientation (006) and (015) changed. The diffraction peaks could be indexed with the paraguanajuatite phase of Bi2Se3 (PDF#33–0214). This indicated the changes in priority orientation. Compared with the XRD pattern for the Bi2Se3 powder (Fig. 1a), the observation of (0001) peaks suggested that the nanosheets preferentially lay on the substrate with the same orientation facing upward when self-deposited onto the substrate. As a result, only the plane parallel to the substrate, that is (0001) plane, could contribute to the XRD pattern. Together, the result demonstrated that Bi2Se3 nanosheets could be assembled into highly uniform thin films with a strong preference in orientation over the large-area substrate using the simple self-deposition process. Since no selenium phase was detected by XRD, it seems likely that the selenium released during the oxidation process left the surface layer of the Bi2Se3.
Discussion
Although the Bi2Se3 surface treated with the oxygen plasma can help tune the morphology and size, it may induce plasma damage, including oxidation and etching. The disordering of the Bi2Se3 surface and oxygen doping may affect the electrical structure and optical properties of Bi2Se3 thin films. Raman spectra are an efficient tool to investigate the optical characteristics of nanomaterials. It is well known that layered Bi2Se3 has a rhombohedral structure with the space group of R3-m/D3d5 and is composed of hexagonal close-packed atomic layers periodically arranged along the c-axis. The atomic arrangement can be considered as repeating units, each consisting of five atomic Se1-Bi-Se2-Bi-Se1 layers called quintuple layers, weakly bound by van der Waals forces with a slightly covalent nature. The Raman spectra before and after oxygen plasma etching are shown in Fig. 6(a). It can be clearly seen that two main Raman characteristic peaks assigned to the vibration modes  and
and  , respectively. As the oxygen plasma treatment time was increased from 0–50 s, the peak position of the mode shifted to the low wave (denoted by the vertical dot line), indicating that the inner structure of Bi2Se3 was not changed by oxygen plasma and mainly the surface of Bi2Se3 film was oxidized by the oxygen plasma radiation. Se atoms exhibit two different chemical environments. The chemical bonding between Bi and Se2 is of a pure covalent nature, while it is slightly ionic but still covalent in nature between Bi and Se111,26,27,28,29,30. As for the Bi2Se3 compounds, O atoms preferentially replace Se at Se2 sites because O atoms at Se2 sites are more stable than the O atoms at Se1 sites, thus resulting in a measurable increase in the values of the lattice parameters of Bi2Se3. Raman shifting of Bi2Se3 nanosheets can be ascribed to the change in the center mass due to O occupying the Se site31,32. However, the crystalline structures and lattice dynamics of Bi2Se3 are changed, eventually leading to the high-frequency modes and a slight Raman shift and broadening under highly nonequilibrium conditions. This can be ascribed mainly to the rapid oxidation of the surface and morphological evolution. The Raman shifts of the vibrational modes with the increase in oxygen plasma treatment time are summarized in Fig. 6(b). The mode displayed a blue shift when the treatment time was increased. The vibrational mode is sensitive to defects because the interlayer van der Waals interactions influence the effective restoring forces acting on Bi and Se atoms. This effect can be more obvious on the out-of-plane mode because the defect causes a change in not only the restoring forces but also the thickness30.
, respectively. As the oxygen plasma treatment time was increased from 0–50 s, the peak position of the mode shifted to the low wave (denoted by the vertical dot line), indicating that the inner structure of Bi2Se3 was not changed by oxygen plasma and mainly the surface of Bi2Se3 film was oxidized by the oxygen plasma radiation. Se atoms exhibit two different chemical environments. The chemical bonding between Bi and Se2 is of a pure covalent nature, while it is slightly ionic but still covalent in nature between Bi and Se111,26,27,28,29,30. As for the Bi2Se3 compounds, O atoms preferentially replace Se at Se2 sites because O atoms at Se2 sites are more stable than the O atoms at Se1 sites, thus resulting in a measurable increase in the values of the lattice parameters of Bi2Se3. Raman shifting of Bi2Se3 nanosheets can be ascribed to the change in the center mass due to O occupying the Se site31,32. However, the crystalline structures and lattice dynamics of Bi2Se3 are changed, eventually leading to the high-frequency modes and a slight Raman shift and broadening under highly nonequilibrium conditions. This can be ascribed mainly to the rapid oxidation of the surface and morphological evolution. The Raman shifts of the vibrational modes with the increase in oxygen plasma treatment time are summarized in Fig. 6(b). The mode displayed a blue shift when the treatment time was increased. The vibrational mode is sensitive to defects because the interlayer van der Waals interactions influence the effective restoring forces acting on Bi and Se atoms. This effect can be more obvious on the out-of-plane mode because the defect causes a change in not only the restoring forces but also the thickness30.
(a) Typical Raman spectra of Bi2Se3 treated by oxygen plasma at different times of 0, 10 s, 20 s, 30 s and 50 s, respectively. The dot vertical lines highlight the positions of the different vibration modes. (b) Positions of the Raman peaks (corresponding to panel (a)) as a function of the treatment time.
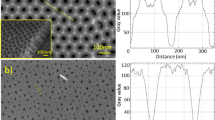
The atomic force microscopy images of the samples are shown in Fig. 7(a–d). This indicates that oxygen plasma can provide energy to change the morphology of Bi2Se3 nanomaterial. After the plasma pretreatment from 10–20 s, the surface of Bi2Se3 film evolved from the worm-like structure to particles. The highly uniform thin film was formed on further increasing the plasma treatment time, which is consistent with the observed SEM results. The root-mean-square (RMS) surface roughness values were extracted from AFM surface images. The RMS decreased from 77% (Fig. 7(a)) to 93% (Fig. 7(d)), indicating considerable improvement compared with the smoothness of self-deposition Bi2Se3 thin film without the plasma treatment.
To clearly understand the mechanism behind the evolution of the Bi2Se3 nanosheets acted upon by the oxygen plasma, the following important processes need to be considered:
Formation of Bi2Se3 oxide layer
A native bismuth oxide is quickly formed on the surface of Bi2Se3 thin film. During the oxygen plasma etching process under highly nonequilibrium conditions, O atoms preferentially replace Se atoms on the surface, since inner Se atomic sites are more stable than the surface sites of Se atoms, resulting in the formation of oxygen-doped Bi2Se3 surface layer.
Regrowth process
The Bi2Se3 nanosheets undergo a self-assembling regrowth process under the action of the high-energy oxygen plasma. The morphological evolution of Bi2Se3 nanosheets is the combined effect of heating and etching of oxygen plasma. The nanoparticle formation is mainly controlled through the migration of adatoms/nanoclusters induced by thermal energy and driving force from the strain between the Bi2Se3 thin film and the surface oxide layer. The Bi2Se3 thin film beneath the bismuth oxide layer plays the role of a wetting layer during the regrowth process. Under the continuous action of oxygen plasma, the atoms in the wetting layer or the surface nanoclusters can gain more energy to overcome the strain energy barrier to reach the nucleation centers and regrow. This can be ascribed to the increase in the size of nanoparticle. A previous study showed that the strain energy between the edge of nanoparticles and the wetting layer increases with the growth of nanoparticles, preventing the atoms from diffusing from the wetting layer to the surface of nanoparticles33.
The electrostatic interaction and etching mechanism
Terashima et al. showed that the enhancing local electrical field around the vicinity of the boron site on the surface of diamond appears to contribute to the formation of nanostructures during the oxygen plasma treatment process34. It is reasonable to imagine that under an energetic oxygen ion attack, the surface of nanosheets can be quickly enriched with electrons. An oxygen-doped Bi2Se3 surface can enhance the electron emission compared with the intrinsic topological insulator Bi2Se3. The resulting local electrical field around the surface of the oxygen sites on the Bi2Se3 surface appears to contribute to the formation of fine nanoparticles during the oxygen plasma etching. Once the nanoparticles are formed, the electrostatic repulsion between nanoparticles and the adjacent quintuple layers can be larger than the van der Waals forces, resulting in the separation of nanoparticles from the Bi2Se3 matrix. Generally, the topmost layer of Bi2Se3 sheets is oxidized quickly. The formation of oxides on the surface of nanosheets inhibits further oxidation in the inner layer of the Bi2Se3 sheets. The peeled-off Bi2Se3 layer can assemble again due to the action of the high-energy oxygen ion. The particles are formed under the continuous bombardment of the highly energetic oxygen ions. The final nanoparticles are separated from the Bi2Se3 matrix layer. Thus, in the present case, only the topmost layer was affected. The XRD results and Raman spectra prove this speculation. It opens the possibility of engineering Bi2Se3 nanostructure and thinning the 2D materials via oxygen plasma treatment.
In conclusion, in this study, Bi2Se3 nanosheets were successfully synthesized by a microwave-assisted approach in the presence of polyvinylpyrrolidone at a temperature of 180 °C for 2 h. The structure and morphology of the obtained products were characterized by XRD, SEM, XPS and Raman spectroscopy. The thin film was prepared on a silicon wafer via a self-deposition process in Bi2Se3 nanosheet ink solution according to the evaporation-induced self-assembly method. The highly uniform Bi2Se3 nanoparticles could be formed by controlling the oxygen plasma treatment time. Several important processes can result in the morphological evolution and thinning of Bi2Se3 nanosheets: (1) formation of Bi2Se3 oxide layer; (2) self-assembly of oxide nanoparticles under the action of the high-energy oxygen plasma; and (3) electrostatic interaction and etching mechanism.
Methods
The high-quality nanosheets were successfully synthesized by a microwave-assisted approach in the presence of polyvinylpyrrolidone at a temperature of 180 °C for 2 h. 2D Bi2Se3 nanosheets thus synthesized could be well dispersed in an alcohol solution to form a stable colloidal ink. The thin film was prepared on a silicon wafer via a self-deposition process in a Bi2Se3 nanosheet ink solution using the evaporation-induced self-assembly method. The alcohol gradually was evaporated at room temperature, resulting in the Bi2Se3 nanosheets tiled on the substrate. The highly uniform Bi2Se3 particles could be formed by controlling the oxygen plasma treatment time. After the plasma pretreatment from 10–20 s, the surface of Bi2Se3 film evolved from the worm-like structure to particles. The highly uniform thin film was formed on further increasing the plasma treatment time. The effect of the oxygen plasma treatment time on the morphology of the Bi2Se3 nanosheets was analyzed by SEM images.
Synthesizing of Bi2Se3 nanosheets and preparing Bi2Se3 films
All chemicals were analytical grade and used without further purification. In a typical synthesis of Bi2Se3 nanosheets, 0.2 mmol Bi(NO3)3·5H2O (0.0970 g), 0.3 mmol NaSeO3 (0.0519 g) and 2 mmol PVP (0.2223 g) were dissolved in 20 mL ethylene glycol. The mixture was stirred for 10 min and then heated to 180 oC in a 25 mL three-neck flask equipped with thermal couple and reflux condenser in a heating mantle. After 2 hr, the heating mantle was removed and the mixture was allowed to cool down to room temperature naturally. The mixture was then centrifuged at 10 K rpm for 10 min after the addition of 20 mL isopropanol and 10 mL acetone. The washing steps were repeated for three more times. The supernatant was discarded and the solid was dispersed in another 40 mL alcohol. Using the colloidal Bi2Se3 nanoplates ink, a uniform thin film of Bi2Se3 can be readily prepared on the silicon substrate by evaporating self assembling process. The (100) intrinsic silicon has been carefully cleaned in a sonicating bath warm acetone (50 °C) for an hour to remove the organic pollutant. The surface oxide layer in the silicon does not remove.
Plasma treatment
Plasma surface treatment of Bi2Se3 films was carried out in a plasma cleaner system (Y2008-5C, China). Control samples were not plasma treated. Plasma was created with an inductively coupled RF generator operating at a frequency of 40 kHz with a power 150 W. Flow rates of oxygen were independently controlled and are fixed as 20 sccm. Oxygen pressure was fixed as 5 mTorr. The treatment, temperature of the sample in 273 K.
Characterization
Characterizations were carried out using scanning electron microscopy (SEM, JEOL JSM-6700F FE-SEM) with energy dispersive spectroscopy (EDAX), X-ray diffraction (XRD, Pan alytical X’Pert Pro X-ray Powder Diffractometer), atomic force microscopy (AFM, Micronano Scanning Probe Microscope), X-Ray Photoelectron Spectroscopy (XPS, PHI 5300), UV-Vis-NIR spectroscopy (Shimadzu 3100 PC). Raman spectroscopy is performed under excitation of a 632 nm laser (15 mW) and a scanning wave number range from 300 cm−1 to 100 cm−1 in the backscattering configuration.
Additional Information
How to cite this article: Jia, G. et al. Morphological evolution of self-deposition Bi2Se3 nanosheets by oxygen plasma treatment. Sci. Rep. 6, 22191; doi: 10.1038/srep22191 (2016).
References
Castro Neto, A. H., Guinea, F., Peres, N. M. R., Novoselov, K. S. & Geim, A. K. The electronic properties of graphene. Rev. Mod. Phys. 81, 109–162, doi: 10.1103/RevModPhys.81.109 (2009).
Rao, C. N. R., Sood, A. K., Subrahmanyam, K. S. & Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 48, 7752–7777, doi: 10.1002/anie.200901678 (2009).
Coleman, J. N. et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 331, 568–571, doi: 10.1126/science.1194975 (2011).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat. Nanotech. 6, 147–150, doi: 10.1038/nnano.2010.279 (2011).
Chang, C.-Z. et al. Chemical-Potential-Dependent Gap Opening at the Dirac Surface States of Bi2Se3 Induced by Aggregated Substitutional Cr Atoms. Phys. Rev. Lett. 112, doi: 10.1103/PhysRevLett.112.056801 (2014).
Liu, Y. et al. Tuning Dirac states by strain in the topological insulator Bi2Se3 . Nat. Phys. 10, 294–299, doi: 10.1038/nphys2898 (2014).
Yan, Y. et al. Topological Surface State Enhanced Photothermoelectric Effect in Bi2Se3 Nanoribbons. Nano Lett. 14, 4389–4394, doi: 10.1021/nl501276e (2014).
Zareapour, P. et al. Proximity-induced high-temperature superconductivity in the topological insulators Bi2Se3 and Bi2Te3 . Nat. Commun. 3, doi: 10.1038/ncomms2042 (2012).
Zhang, H. et al. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat. Phys. 5, 438–442, doi: 10.1038/nphys1270 (2009).
Zhu, Z. H. et al. Layer-By-Layer Entangled Spin-Orbital Texture of the Topological Surface State in Bi2Se3 . Phys. Rev. Lett. 110, doi: 10.1103/PhysRevLett.110.216401 (2013).
Zhang, J. et al. Raman Spectroscopy of Few-Quintuple Layer Topological Insulator Bi2Se3 Nanoplatelets. Nano Lett. 11, 2407–2414, doi: 10.1021/nl200773n (2011).
Kong, D. & Cui, Y. Opportunities in chemistry and materials science for topological insulators and their nanostructures. Nat. Chem. 3, 845–849, doi: 10.1038/nchem.1171 (2011).
Jia, G. Z. et al. Excellent photothermal conversion of core/shell CdSe/Bi2Se3 quantum dots. Nano Res. 8, 1443–1453, doi: 10.1007/s12274-014-0629-2 (2015).
Dang, R. et al. Synthesis and Self-Assembly of Large-Area Cu Nanosheets and Their Application as an Aqueous Conductive Ink on Flexible Electronics. ACS Appl Mater Interfaces 6, 622–629, doi: 10.1021/am404708z (2014).
Wang, Y.-C. et al. FeS2 Nanocrystal Ink as a Catalytic Electrode for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 52, 6694–6698, doi: 10.1002/anie.201300401 (2013).
Mensinger, Z. L. et al. Synthesis of Heterometallic Group 13 Nanoclusters and Inks for Oxide Thin-Film Transistors. Angew. Chem. Int. Ed. 47, 9484–9486, doi: 10.1002/anie.200803514 (2008).
Lin, Z. et al. Solution Processable Colloidal Nanoplates as Building Blocks for High-Performance Electronic Thin Films on Flexible Substrates. Nano Lett. 14, 6547–6553, doi: 10.1021/nl503140c (2014).
Kong, D. et al. Rapid Surface Oxidation as a Source of Surface Degradation Factor for Bi2Se3 . Acs Nano 5, 4698–4703, doi: 10.1021/nn200556h (2011).
Ivanov, V. I. et al. Surface structure of unoxidized and oxidized Bi2Se3 crystals. Inorg. Mater. 46, 1296–1298, doi: 10.1134/s0020168510120046 (2010).
Xiao, N. et al. Enhanced Thermopower of Graphene Films with Oxygen Plasma Treatment. Acs Nano 5, 2749–2755, doi: 10.1021/nn2001849 (2011).
Zheng, W. et al. Patterning two-dimensional chalcogenide crystals of Bi2Se3 and In2Se3 and efficient photodetectors. Nat. Commun. 6, doi: 10.1038/ncomms7972 (2015).
Zhang, H. X. & Feng, P. X. Controlling Bandgap of Rippled Hexagonal Boron Nitride Membranes via Plasma Treatment. ACS Appl. Mater. Interfaces 4, 30–33, doi: 10.1021/am201435z (2012).
Faber, H. et al. Impact of Oxygen Plasma Treatment on the Device Performance of Zinc Oxide Nanoparticle-Based Thin-Film Transistors. ACS Appl. Mater. Interfaces 4, 1693–1696, doi: 10.1021/am2018223 (2012).
Nan, H. et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. Acs Nano 8, 5738–5745, doi: 10.1021/nn500532f (2014).
Koizumi, K., Boero, M., Shigeta, Y. & Oshiyama, A. Atom-Scale Reaction Pathways and Free-Energy Landscapes in Oxygen Plasma Etching of Graphene. J. Phys. Chem. Lett. 4, 1592–1596, doi: 10.1021/jz400666h (2013).
Chis, V. et al. Vibrations in binary and ternary topological insulators: First-principles calculations and Raman spectroscopy measurements. Phys. Rev. B 86, doi: 10.1103/PhysRevB.86.174304 (2012).
He, R. et al. Observation of infrared-active modes in Raman scattering from topological insulator nanoplates. Nanotechnology 23, doi: 10.1088/0957-4484/23/45/455703 (2012).
Li, H., Peng, H., Dang, W., Yu, L. & Liu, Z. Topological insulator nanostructures: Materials synthesis, Raman spectroscopy and transport properties. Front. Phys. 7, 208–217, doi: 10.1007/s11467-011-0199-7 (2012).
Shahil, K. M. F., Hossain, M. Z., Goyal, V. & Balandin, A. A. Micro-Raman spectroscopy of mechanically exfoliated few-quintuple layers of Bi2Te3, Bi2Se3 and Sb2Te3 materials. J. Appl. Phys. 111, doi: 10.1063/1.3690913 (2012).
Jia, G., Wang, X., Li, Q. & Yao, J. Bi-rich grow topological insulator Bi2Se3 nanodomains structures. Superlattices Microstruct. 66, 33–38, doi: 10.1016/j.spmi.2013.11.021 (2014).
Xu, H. et al. Electrical transport properties of microwave-synthesized Bi2Se3-xTex nanosheet. Crystengcomm. 15, 5626–5632, doi: 10.1039/c3ce40296g (2013).
Soni, A. et al. Enhanced Thermoelectric Properties of Solution Grown Bi2Te3-xSex Nanoplatelet Composites. Nano Lett. 12, 1203–1209, doi: 10.1021/nl2034859 (2012).
Jia, G.-z., Yao, J.-h., Shu, Y.-c., Xing, X.-d. & Pi, B. Quantum rings formed in InAs QDs annealing process. Appl. Surf. Sci. 255, 4452–4455, doi: 10.1016/j.apsusc.2008.11.042 (2009).
Terashima, C. et al. Fabrication of Vertically Aligned Diamond Whiskers from Highly Boron-Doped Diamond by Oxygen Plasma Etching. ACS Appl. Mater. Interfaces 3, 177–182, doi: 10.1021/am1007722 (2011).
Acknowledgements
This work has been partly supported by the National Key Basic Research Program of China (2011CB922200), the National Natural Science Foundation of China (11247025), Tianjin Natural Science Foundation(14JCTPJC00480).
Author information
Authors and Affiliations
Contributions
J.G. wrote the main manuscript text, experimental work and data analysis. Z.W. and P.W. experimental work. Y.J. data analysis. K.C. project planning, data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jia, G., Wu, Z., Wang, P. et al. Morphological evolution of self-deposition Bi2Se3 nanosheets by oxygen plasma treatment. Sci Rep 6, 22191 (2016). https://doi.org/10.1038/srep22191
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22191
This article is cited by
-
In-situ synthesized and pattern Ag/Bi2Se3 composite structure by LDW and photothermal conversion
Scientific Reports (2019)
-
Microwave-assisted synthesis and photothermal conversion of Cu2 − xSe hollow structure
Journal of Nanoparticle Research (2019)
-
Materials analysis and focused ion beam nanofabrication of topological insulator Bi2Se3
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.