Abstract
The interaction between roots and bacterial communities in halophytic species is poorly understood. Here, we used Jerusalem artichoke cultivar Nanyu 1 (NY-1) to characterise root distribution patterns and determine diversity and abundance of bacteria in the rhizosphere soil under variable salinity. Root growth was not inhibited within the salinity range 1.2 to 1.9 g salt/kg, but roots were mainly confined to 0–20 cm soil layer vertically and 0–30 cm horizontally from the plant centre. Root concentrations of K+, Na+, Mg2+ and particularly Ca2+ were relatively high under salinity stress. High salinity stress decreased soil invertase and catalase activity. Using a next-generation, Illumina-based sequencing approach, we determined higher diversity of bacteria in the rhizosphere soil at high than low salinity. More than 15,500 valid reads were obtained, and Proteobacteria, Acidobacteria, Bacteroidetes and Actinobacteria predominated in all samples, accounting for >80% of the reads. On a genus level, 636 genera were common to the low and high salinity treatments at 0–5 cm and 5–10 cm depth. The abundance of Steroidobacter and Sphingomonas was significantly decreased by increasing salinity. Higher Shannon and Chao 1 indices with increasing severity of salt stress indicated that high salt stress increased diversity in the bacterial communities.
Similar content being viewed by others
Introduction
Soil salinity is one of the most important abiotic stresses limiting the growth and crop production worldwide1. It was estimated that about 20% (45 million ha) of irrigated land, producing 1/3 of the world food production, is salt affected2. In the past few decades, soil salinization reduced the world’s production of major crops by more than 50%. By 2050, it is estimated that 50% of the world’s arable land will be affected by salinity3,4. Thus, understanding salt-tolerance mechanisms and developing salt-tolerant crops are essential for maintaining the world’s food security5.
The root is an important plant organ in direct contact with the soil solution, thus being the first to encounter the saline medium1. The root distribution pattern in soil is reflection of the plant ecological adaptation, and may increase a chance of plant survival under stress6. However, root responses to soil salinity in halophytes and their relation with plant growth are poorly understood.
Jerusalem artichoke (Helianthus tuberosus L.) belongs to sunflower family; it is a perennial tuberous plant rich in inulin and a potential energy crop7. Distributed throughout the world, Jerusalem artichoke has showed wide ecological adaptability. It has a number of advantageous characteristics over traditional agricultural crops, including high growth rate, good tolerance to frost, drought and poor-fertility soil, strong resistance to pests and diseases, and the minimal-to-zero fertilizer requirements. Unlike grain crops, Jerusalem artichoke can grow well in barren, poor-quality land8. Jerusalem artichoke is a versatile plant, potentially achieving a high biomass yield of tubers and stalks9. The plant produces underground tubers that are rich in inulin10 (amounting to 10–20% of fresh tuber weight).
Plant growth is affected by soil enzyme activity and soil microorganisms. Enzymes are secreted by microorganisms, plants and soil animals, and they regulate many soil biological processes11. Soil microorganisms are recognized as a key factor influencing plant growth, but it is challenging to fully characterize soil microbial communities. In particular, there is little knowledge about the structure of microbial communities in the rhizosphere of halophyte plants growing in salt-affected soils.
In this paper, we characterized Jerusalem artichoke root distribution patterns and bacterial communities in the rhizosphere under different salinity stress conditions to shed light on the response mechanisms induced by salt stress in Jerusalem artichoke roots.
Methods
Plant material and growth conditions
Jerusalem artichoke (Helianthus tuberosus L.) was collected from Experimental Station “863” at Dafeng, 4 km from the Yellow Sea shore in Jiangsu Province of China (32°59′N, 120°49′E). The area is located in the subtropical monsoon climate zone; the annual precipitation is 1,058 mm, mainly concentrated in the June-to-August rainy season. Four fields with different salinity were selected (in g salt/kg soil: S1 = 1.2 ~ 1.9; S2 = 1.6 ~ 1.8; S3 = 2.1 ~ 2.6; S4 = 2.6 ~ 3.0), and each field included three replicate plots. As annual, Jerusalem artichoke was planted in March and sampled in August 2014. Jerusalem artichoke cultivar was Nanyu 1 (NY-1), and its growth period was about 230 d. Plant spacing was 60 cm inter-row, and the intra-row distance between plants was 50 cm.
Root sampling and analyses
Three uniform plants were selected in each salinity plot. Root samples were collected by soil layer (0–5, 5–10, 10–15 and 15–20 cm from the surface) using a sampling tube, and in different sections horizontally (0–10, 10–20 and 20–30 cm from the plant center in each layer). Samples were sealed in plastic bags and transported to the laboratory, where visible roots were collected from soil manually, followed by applying water to collect fine roots on nylon gauze (0.8-mm mesh). Root samples were stored in zip-lock bags and marked for further investigations. A detailed description of the root collection procedures was given in the previous reports12,13.
Roots in each soil layer and horizontal section were divided into three size classes based on root diameter measured using a vernier caliper (0.02-mm resolution): fine roots (<2 mm), medium roots (2–4 mm) and coarse roots (>4 mm). The root length density (RLD, root length per unit volume of soil, m·m−3) of each layer was determined as follows: RLDni = Lni/Vni, where L was the root length in each soil block, and Vni (Vni = π(r2ni-r2n(i−1))) was the volume of soil, “n” was horizontal section (n = 1,2,3), and “i” was the vertical stratification (i = 1,2,3,4). Root samples were oven-dried at 80 °C for at least 48 h and weighed. Concentrations of Ca2+, K+, Na+ and Mg2+ in roots were determined by Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES, Optima 2100DV, Pekin-Elmer, USA) after digesting14.
Soil sampling and analyses
Soil samples were collected from each soil layer (0–5, 5–10, 10–15 and 15–20 cm from the surface) using a cylindrical soil core. The soil samples were kept in zip-lock bags for transport to the laboratory. Soil water content was measured using a subsample of approximately 10 g before and after drying at 80 °C for at least 48 h. Remaining soil samples were sieved through a 1-mm sieve and air-dried at least one week for the following analyses. Soil pH and soluble salt content were measured using a 1:5 (soil: water) suspension. The total Ca2+, K+, Na+ and Mg2+ concentrations in the same supernatant were determined by ICP-AES15.
Tuber growth and biomass
In mid-December 2014, three tuber samples were collected from each of the four saline treatments in the same layers (vertically) and sections (horizontally) as for the root samples, then placed in plastics bags and transported to the laboratory. Tuber samples were oven-dried at 80 °C for at least 96 h and weighed.
Soil enzyme activity
Soil urease activity was detected using improved sodium phenate and sodium hypochlorite colorimetry16. Invertase activity was determined by the method that involved the colorimetric determination of reducing sugars that react with 3,5-dinitrosalicylic acid upon incubation of soil in buffered (0.17 M modified universal buffer, pH 5.5) sucrose solution and toluene at 37 °C for 24 h17. Catalase activity was based on the recovery rates of H2O2, and the residual H2O2 was determined by titration with KMnO4 in the presence of H2SO418,19.
Soil bacterial communities
Soil sampling
According to Riley and Barber20,21, whole plants were extracted from fields. The bulk soil was obtained by gently shaking roots. The rhizosphere soil was then collected as soil that adhered to roots. The rhizosphere soil samples were collected from two salinity treatments (S1 and S4) at 0–5 cm and 5–10 cm depth. The rhizosphere soil samples were transferred into DNA-free polythene bags, kept on dry ice for transport to the laboratory, and were then stored at −20 °C for biological and biochemical analyses. Bulk soil samples were kept in zip-lock bags, transferred to the laboratory, and air-dried at room temperature for biological and biochemical analyses22.
Soil DNA extraction
Three replicate samples were randomly picked from each treatment in the lab and used for DNA extraction. Soil DNA was extracted from 0.25 g of soil (after passing a 1-mm sieve) using a PowerSoil DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA), according to the manufacturer’s instructions. The extracted soil DNA was dissolved in 100 mL TE buffer (Tris-hydrochloride buffer, pH 8.0, containing 1.0 mM EDTA), quantified by ND1000 and stored at −80 °C before using23.
Bacterial 16S rRNA gene amplification and Illumina Sequencing
Primers 577F (5′-AYTGGGYDTAAAGNG-3′) and 926R (5′-CCGTCAATTCMTTTRAGT-3′) targeting the regions (V3-V4) of the 16S rRNA gene were used for PCR, because sequences in that regions provided the greatest diversity at the domain and bacterial phylum levels24. Amplification reactions were performed in 25-μL volume containing 12.5 μL Premix Ex TaqTM Hot Start Version (Takara Biotechnology Co. Ltd, Dalian, China.), 0.1 μM of each primer, and 20 ng of template. Amplification was initiated at 98 °C for 3 s, followed by 35 cycles of denaturation at 98 °C for 10 s, primer annealing at 54 °C for 30 s, extension at 72 °C for 45 s, and final extension for 10 min. Amplicon pyrosequencing was performed on an Illumina MiSeq platform at LC-Bio Technology Co., Ltd, Hangzhou, Zhejiang, China. The complete data sets were deposited in the NCBI, and the GenBank accession numbers are KT783673 - KT784803.
Pairs of reads were merged from the original DNA fragments by using FLASH (version1.2.8)25 that was designed to merge pairs of reads when the original DNA fragments were shorter than two times the reads length. Sequencing reads were distributed to each sample according to its unique barcode. QIIME (version 1.7.0)26 software package (Quantitative Insights Into Microbial Ecology) and the CD-HIT pipeline were used to analyze sequences. The reads were filtered by QIIME quality filters at first. Default settings for Illumina processing in QIIME was used (r = 3 p = 0.75 total read length; q = 3; n = 0).
(p) min_per_read_length: minimum number of consecutive high-qualitybase calls to retain read (as percentage of totalread length).
(r) max_bad_run_length: maximum number of consecutive low-quality base calls allowed before truncating a read.
(n) sequence_max_n: maximum number of ambiguous (N) characters allowed in a sequence.
(q) phred_quality_score: last quality score considered low quality.
The CD-HIT pipeline was used for picking operational taxonomic units (OTUs). Sequences were assigned to OTUs at 97% similarity. Representative sequences were chosen for each OTU and taxonomic data were then assigned to each representative sequence using the RDP (Ribosomal Database Project) classifier27. In order to estimate Alpha Diversity, the OTU table was rarified and four metrics were calculated: Chao1 metric to estimate the richness, the Observed OTUs metric as the count of unique OTUs found in the sample, Shannon index and Simpson index28,29.
Data analysis
All measurements were replicated thrice as mentioned in each section. The mean values of all parameters were taken from three replicates, and the standard error of the means was calculated. For statistical analyses, one-way or two-way ANOVA, Duncan post-hoc tests (p = 0.05) and Spearman’s rank correlations were used separately for each soil layer by SPSS Statistics 19.0 (IBM, Armonk, New York, USA). The community richness index, community diversity index, data preprocessing, operational taxonomic unit-based analysis and hypothesis tests were performed using QIIME 1.7.0. The histograms were created using Microsoft Excel 2010 and all tables were made with Microsoft Word 2010 (Microsoft, Redmond, Washington, USA). Individual means were compared using the least significant difference test α = 0.05 significance level.
Results
Root distribution
The Jerusalem artichoke (NY-1) roots were mainly distributed in the top soil layer (Fig. 1a), with less than 8.5% of total root length density being in the 15–20 cm soil layer.
Jerusalem artichoke (cv. NY-1) root length density distribution (a), percentage of root length density down the soil profile (b), and the changes in water content (c), soluble salt content (d), and pH (e) at different soil depths. Also shown is the percentage of total root length in different diameter class (f) of Jerusalem artichoke. Data are means + SE (n = 3). For graphs a-f, one-way ANOVA (main factor = salinity) followed by Duncan test (p = 0.05) was done for each soil layer separately.
The root length density in the vertical direction tended to decrease with the increased salt stress as well as with soil depth (Fig. 1a). However, the root length density in the 0–5 and 5–10 cm soil layers was not significantly different between S1 and S2, and there were no significant differences among the treatments between 10–15 and 15–20 cm soil layers (Fig. 1a).
The percentage of root length density in the 0–5 cm depth layer did not significantly differ among the salinity treatments, but there was a decreasing trend with an increase in severity of salt stress (Fig. 1b). The proportion of root length density was higher in the 0–5 cm depth layer compared with the other three layers in the treatments S1, S2 and S3, but in S4 there was no significant difference between 0–5 and 5–10 cm depth (Fig. 1b).
Horizontally, NY-1 root growth reached at least 30 cm from the plant center (Table 1), with H1, H2 and H3 representing the sections 0–10, 10–20 and 20–30 cm from the plant center, respectively. In H1 section, S2H1 had highest root length density; there was no significant difference compared with S1H1, but significant differences were found in comparison with S3H1 and S4H1. A similar trend was found in H2 and H3, which meant that root growth was not affected by the increased salt stress in S2.
Root distribution with gradients in soil moisture, salinity and pH
The soil moisture content increased with depth and reached maximum in the 10–15 cm layer, up to 23% (w/w) in the 10–15 cm of the treatment S3 (Fig. 1c). In the 0–20 cm soil layers in the S1 to S3 treatments, there were no significant differences in water moisture content, but water content was higher in the 0–15 cm soil layers in S4 than in the other three treatments (Fig. 1c). In contrast, soil soluble salt content tended to increase with soil depth, and was predictably higher with increasing severity of salt stress in the four salinity treatments (Fig. 1d). The soil pH increased with depth and with increasing soil salinity content (Fig. 1e).
Root length density had no significant correlation with soil water content (data not shown). Root length density (P = 0.005) had a significant negative correlation with soil soluble salt content. Root length density (P = 0.000) had a significant negative correlation with soil pH (data not shown).
Root diameter classes
Jerusalem artichoke fine roots (<2 mm) accounted for the largest proportion of the total root length, followed by medium (2–4 mm) and coarse (>4 mm) roots (Fig. 1f). Fine roots had a very significant positive correlation with medium roots and coarse roots (P = 0.000), and medium roots and coarse roots were also significantly positively correlated (P = 0.000) (data not shown).
Ion concentration in roots and soil
The concentrations of Ca2+ and Mg2+ in soil were obviously higher than those in roots. The K+ concentrations in soil and roots were similar, but Na+ concentration was lower in soil than roots (see Supplementary Fig. S1). The Na+ concentration was only about 3.6 mg/g in soil, and was about 3–4 times higher in roots. This result indicated that NY-1 roots can accumulate Na+, but not K+, Ca2+ and Mg2+ (see Supplementary Fig. S1).
Tuber biomass and distribution
Tubers could be found at 70-cm horizontal distance from the plant center in the 0–5 cm soil layer (Fig. 2). Tuber distribution horizontally decreased with increasing salinity (Fig. 2). Tuber biomass showed no significant difference between S1 and S2 or S3 and S4, but significant differences were observed in S1 and S2 compared with S3 and S4 (Table 2). Tuber water content at the highest salinity treatment (S4) was significantly lower than in the other treatments (Table 2).
Soil enzyme activity
Soil urease activity gradually decreased with increasing soil depth, but catalase and invertase activities did not show such a trend (data not shown). Urease activity and soil soluble salt content had no significant correlation (Table 3). Invertase (P = 0.006) or catalase activity (P = 0.002) was negatively correlated with soil soluble salt content. Urease (P = 0.049), invertase (P = 0.027) or catalase activity (P = 0.048) was negatively correlated with soil pH. Root length density had a very significant correlation with urease (P = 0.004) and catalase activity (P = 0.003).
Soil microbial abundance
Richness
Through a sequence optimization process, more than 15,500 valid reads were obtained for each replicate; after quality filtering, median sequence length of each read was 100 bp. In the 0–5 cm and 5–10 cm depth layers in the S4 treatment, more than 1600 additional OTUs were observed compared with the S1 treatment (Fig. 3a).
(a) Rarefaction curves showing the observed OTU richness (at 97% identity) of the 16S rRNA gene with increasing sequencing depth. Mean values (n = 3) were shown for the two salinity treatments (S1 and S4) and two soil depths. (b) Comparison of the bacterial communities at the phylum level. Relative read abundance of different bacterial phyla in bacterial communities. Sequences that could not be classified into any known group were labeled “others”.
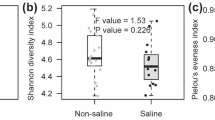
The richness indices of the bacterial communities were computed (Table 4). Higher Shannon and Chao 1 indices in S4 than S1 indicated that high severity of salt stress increased diversity in the bacterial communities (Table 4). The Simpson index is reverse of diversity (the lower the index, the greater biodiversity). Hence, lower Simpson index in S4 than S1 (Table 4) would indicate greater diversity, thus confirming the results derived from the Shannon and Chao 1 indices.
Taxonomic coverage
All of the sequences were classified into 32 phyla or groups by RDP (Ribosomal Database Project: http://rdp.cme.msu.edu/index.jsp). The overall bacterial composition of different samples was similar, but the distribution of each phylum in group varied (Fig. 3b). In all samples, Proteobacteria, Acidobacteria, Bacteroidetes, and Actinobacteria were the four most dominant phyla, accounting for >80% of the reads. Compared with the high salt treatment (S4) averaged over two depths, the low salt treatment (S1) had a significantly higher percentage of Proteobacteria (1.3-fold), Acidobacteria (1.4-fold), Chloroflexi (1.4-fold) and Gemmatimonadetes (1.9-fold), and a lower percentage of Firmicutes (1.1-fold) and Verrucomicrobia (1.2-fold). The percentages of Bacteroidetes, Actinobacteria and Planctomycetes were similar in S1 and S4.
On a genus level, all 643 detected genera were found in all the samples, except for Loktanella, Salinimicrobium, Kordiimonas, and Muricauda that were not detected in S1, and Aquabacterium, Gp13 and Klebsiella that were not detected in S4.
The 72 genera showing significant differences among the samples were listed in Table 5. Steroidobacter, Sphingomonas, Kofleria, Pseudolabrys, Desertibacter, Gaiella, Dongia, Iamia, Flavobacterium, Tistlia, Janthinobacterium, Blastobacter, Aminobacter and Pseudoxanthomonas were significantly higher in S1 than S4. In contrast, Pelagibius, Rhodoligotrophos, Thiohalomonas, Limimonas, Thermoleophilum, Roseicyclus, Azoarcus, Euzebya, Fulvivirga, Haliea, Rubribacterium and Thioalkalispira were higher in S4 than S1.
Discussion
Jerusalem artichoke has strong salt tolerance that is largely related to the ecological and biological characteristics of the root system30. The root architecture is important for plants to access soil resources, and morphological and physiological adaptation of the root system under stress conditions may result in continuation of nutrient absorption and utilization31. Roots of Jerusalem artichoke (NY-1) were mainly distributed in the 0–15 cm depth layer (Fig. 1a), indicating root growth in the upper soil layers to potentially avoid salinity stress. Indeed, compared with shallow layers, the 15–20 cm depth layer had higher salinity, lower water content and higher pH in each salt treatment, resulting in poor root growth in that layer.
In the present study under high salt stress (S4), roots extended horizontally more than into depth (Table 1, Fig. 1a), suggesting that NY-1 escaped from salt stress by preferentially elongating roots horizontally rather than going deep. Root extension in horizontal direction was beyond 30 cm, suggesting that horizontal expansion in the absence of deep root growth might have been caused by the need for resource acquisition in a stress environment.
Soil salinity increased from the treatment S1 to S4. Root growth in the 0–10 cm soil depth layer was significantly inhibited in S3 and S4 (Fig. 1a). In addition to salinity, there might have been other factors affecting the root system distribution, such as soil fertility32.
Concentrations of K+, Ca2+, Na+ and Mg2+ in roots were relatively high, but did not result in root death. One potential reason is that Ca2+ concentration was higher than that of other ions, potentially reducing the damage33. So, one of the salt resistance mechanisms of Jerusalem artichoke NY-1 may be maintenance of relatively high Ca2+ concentration in roots (see Supplementary Fig. S1).
Tubers were distributed only in the surface soil, and were found up to 70 cm horizontally away from the plant center. Tuber distribution became restricted with increasing salinity, suggesting that roots extending in saline soils may the capacity to grow tubers impaired with increasing salinity stress.
Soil enzymes are involved in biological cycling and soil fertility, so they are crucial indicators of soil biochemistry34. With the soil soluble salt content increasing, invertase and catalase activities were both reduced significantly (Table 3), suggesting impaired ecosystem functions. Catalase is present in almost all aerobic microorganisms35,36. Because of a decrease in abundance of certain groups of microbes at high salinity (Tables 4 and 5), the activity of soil catalase might have decreased. For example, Sphingomonas is obligate aerobic and produces redox mediators37. Some species of genus Steroidobacter were strictly aerobic, and had a positive relationship with soil catalase activity38.
The 16S rRNA gene sequencing results indicated a positive relationship between increasing salinity and enhancing biodiversity. This might be attributed to proliferation of halophylic bacteria in soil. Higher percentages of Proteobacteria, Acidobacteria, Chloroflexi and Gemmatimonadetes and lower percentages of Firmicutes and Verrucomicrobia were observed in low-salt than high-salt soil. The phylum Verrucomicrobia is widespread, but a poorly characterized group of bacteria that occur in a wide range of habitats including soils, aquatic systems, marine sediments, and hot springs; some even occur as endosymbionts39. In our study, the Verrucomicrobia abundance decreased from 1.11% to 0.64% with the salinity increasing from the treatment S1 to S4. More research is required to determine whether this phylum has specific roles in the rhizosphere soil.
In this study, Loktanella and Kordiimonas were only found at high soil salinity. Loktanella is a genus of the Rhodobacteraceae that have been reported to be halophilic and found in seawater40. Kordiimonas was isolated from the marine environment and could survive in oligotrophic environment41.
In this study, Lysobacter was among dominant populations in the Jerusalem artichoke (NY-1) rhizosphere soil. In other studies, Lysobacter was found in soil and water and had biolytic activity against a variety of pathogenic fungi, bacteria, and nematodes42. Lysobacter not only colonized the rhizosphere of various plants43, but also secreted a variety of antibiotics44,45, exocellular enzymes and biologically-active material46,47 to inhibit bacterial growth, thus controlling plant diseases48. Unfortunately, the understanding related to rhizosphere populations of Lysobacter is still in its infancy.
Sphingomonas belongs to a group of Gram-negative, rod-shaped, chemoheterotrophic, strictly aerobic bacteria that are widely distributed in nature, having been isolated from many different land and water habitats, as well as from plant root systems, clinical specimens, and other sources; they have the capacity to survive at low nutrient concentrations, as well as to metabolize a wide variety of carbon sources49. Sphingomonas was one of the most effective microbial groups to clean up the toxic substances in soil50. Some Sphingomonas strains showed characteristics of nitrogen fixation and denitrification, suggesting they played an important role in the nitrogen cycle49. In the present study, greater abundance of Sphingomonas was found at low compared with high soil salinity, indicating their potential low salt tolerance.
Additional Information
How to cite this article: Yang, H. et al. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci. Rep. 6, 20687; doi: 10.1038/srep20687 (2016).
References
Bernstein, N. & Kafkafi, U. Root growth under salinity stress. Plant roots: The hidden half. 3, 787–805 (2002).
Abogadallah, G. M. Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav. 5(4), 369–374 (2010).
Bartels, D. & Sunkar, R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 24(1), 23–58 (2005).
Wang, W., Vinocur, B. & Altman, A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 218(1), 1–14 (2003).
Foolad, M. R. Recent advances in genetics of salt tolerance in tomato. Plant Cell Tiss Org. 76(2), 101–119 (2004).
Hartlea, R. T., Fernandez, G. C. J. & Nowaka, R. S. Horizontal and vertical zones of influence for root systems of four Mojave Desert shrubs. J Arid Environ. 64(4), 586–603 (2006).
Lachman, J., “Kays, S. J. & Nottingham, S. F. Biology and chemistry of Jerusalem artichoke Helianthus tuberosus L.” Biol Plantarum. 52(3), 492–492 (2008).
Li, L. L., Li, L., Wang, Y., Du, Y. G. & Qin, S. Biorefinery products from the inulin-containing crop Jerusalem artichoke. Biotechnol Lett. 35(4), 471–477 (2013).
Slimestad, R., Seljaasen, R., Meijer, K. & Skar, S. L. Norwegian-grown Jerusalem artichoke (Helianthus tuberosus L.): morphology and content of sugars and fructo-oligosaccharides in stems and tubers. J Sci Food Agr. 90(6), 956–964 (2010).
Gunnarsson, I. B., Svensson, S. E., Johansson, E., Karakashev, D. & Angelidaki, I. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as a biorefinery crop. Ind Crop Prod. 56, 231–240 (2014).
Marx, M. C., Wood, M. & Jarvis, S. C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem. 33(12), 1633–1640 (2001).
Imada, S., Yamanaka, N. & Tamai, S. Water table depth affects Populus alba fine root growth and whole plant biomass. Funct Ecol. 22(6), 1018–1026 (2008).
Cankur, O., Yathavakilla, S. K. V. & Caruso, J. A. Selenium speciation in dill (Anethum graveolens L.) by ion pairing reversed phase and cation exchange HPLC with ICP-MS detection. Talanta. 70(4), 784–790 (2006).
Huang, C. Y. L. & Schulte, E. E. Digestion of plant tissue for analysis by ICP emission spectroscopy. Commun Soil Sci Plant. 16(9), 943–958 (1985).
Sandroni, V. & Smith, C. M. M. Microwave digestion of sludge, soil and sediment samples for metal analysis by inductively coupled plasma–atomic emission spectrometry. Anal Chim Acta. 468(2), 335–344 (2002).
Vlek, P. L. G., Stumpe, J. M. & Byrnes, B. H. Urease activity and inhibition in flooded soil systems. Nutr Cycl Agroecosys. 1(3), 191–202 (1980).
Frankeberger, W. T. Jr & Johanson, J. B. Method of measuring invertase activity in soils. Plant Soil. 74(3), 301–311 (1983).
Johnson, J. L. & Temple, K. L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci Soc Am J. 28(2), 207–209 (1964).
Roberge, M. R. Methodology of soil enzymes measurement and extraction (Academic Press, London, UK, 1978).
Riley, D. & Barber, S. A. Bicarbonate accumulation and pH changes at the Soybean (Glycine max (L.) Merr) root-soil interface. Soil Sci Soc Am J. 33(6), 905–908 (1969).
Riley, D. & Barber, S. A. Salt accumulation at the soybean (Glycine max. (L.) Merr.) root-soil interface. Pro Soil Sci Soc Am J. 154–155 (1970).
Zhang, H., Wu, X., Li, G. & Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol Fert Soils. 47(5), 543–554 (2011).
Rodrigues, J. L. M. et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. P Natl Acad Sci USA 110(3), 988–993 (2013).
Peiffer, J. A. et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. P Natl Acad Sci USA 110(16), 6548–6553 (2013).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7, 335–336 (2010).
Wang, Q., Garrity, G. M., Tiedje J. M., Tiedje, M. J. & Cole, J. R. Na¨ıve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb. 73(16), 5261–5267 (2007).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. P Natl Acad Sci USA 108 (Supplement 1), 4516–4522 (2011).
Vishnivetskaya, T. A. et al. Mercury and other heavy metals influence bacterial community structure in contaminated Tennessee streams. Appl Environ Microb. 77(1), 302–311 (2011).
Kuffner, M. et al. Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiol Ecol. 82(3), 551–562 (2012).
Sun. X. & Yu, Z. A study on root system of Nitraria tangutorum . J Desert Research. 12(4), 50–54 (1992). (in chinese).
Li, S. Y., Gao, J. X., Zhu, Q. S., Zeng, L. Q. & Liu, J. A dynamic root simulation model in response to soil moisture heterogeneity. Math Comput Simulat. 113, 40–50 (2015).
Fransen, B., Kroon, H. D. & Berendse, F. Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia. 115(3), 351–358 (1998).
Jianglin, G. Effects of Ca2+ on seeds germination of two kinds of cucumber varieties under NaCl stress. J Anhui Agri Sci. 35(28), 8809–8810 (2008). (in chinese).
Alkorta, I. et al. Soil enzyme activities as biological indicators of soil health. Rev Environ Health. 18(1), 65–73 (2003).
Cowell, D. C., Dowman, A. A., Lewis, R. J., Pirzad, R. & Watkins, S. D. The rapid potentiometric detection of catalase positive microorganisms. Biosens Bioelectron. 9(2), 131–138 (1994).
Ying, Z. et al. Characterization of biofilm and corrosion of cast iron pipes in drinking water distribution system with UV/Cl2 disinfection. Water Res. 60, 174–181 (2014).
Keck, A. et al. Identification of quinoide redox mediators that are formed during the degradation of naphthalene-2-sulfonate by Sphingomonas xenophaga BN6. Appl Environ Microb. 68(9), 4341–4349 (2002).
Sakai, M., Hosoda, A., Ogura, K. & Ikenaga, M. The growth of Steroidobacter agariperforans sp. nov., a novel agar-degrading bacterium isolated from soil, is enhanced by the diffusible metabolites produced by bacteria belonging to rhizobiales. Microbes Environ. 29(1), 89–95 (2014).
Wagner, M. & Horn, M. The Planctomycetes, Verrucomicrobia, Chlamydiae and sister phyla comprise a superphylum with biotechnological and medical relevance. Curr Opin Biotech. 17(3), 241–249 (2006).
Yoon, J. H., Kang, S. S. & Oh, T. K. Loktanella maricola sp. nov., isolated from seawater of the East Sea in Korea. Int J Syst Evol Micr. 57(8), 1799–1802 (2007).
Kwon, K. K., Lee, H. S., Yang, S. H. & Kim, S. J. Kordiimonas gwangyangensis gen. nov, sp. nov., a marine bacterium isolated from marine sediments that forms a distinct phyletic lineage (Kordiimonadales ord. nov.) in the ‘Alphaproteobacteria’. Int J Syst Evol Micr. 55(5), 2033–2037 (2005).
Jiang, Y. H., Bai-Shi, H. U. & Liu, F. Q. Selection and identification of antagonistic bacteria against soil-borne plant pathogens. Chinese J Biol Control. 21(4), 260–264 (2005). (in Chinese).
Park, J. H., Kim, R., Aslam, Z., Jeon, C. O. & Chung, Y. R. Lysobacter capsici sp. Nov, with antimicrobial activity, isolated from the rhizosphere of pepper, and amended description of the genus Lysobacter. Int J Syst Evol Micr. 58, 387–392 (2008).
Blackburn, R. K. & Van Breemen, R. B. Application of an immobilized digestive enzyme assay to measure chemical and enzymatic hydrolysis of the cyclic peptide antibiotic lysobactin. Drug Metab Dispos. 21(4), 573–579 (1993).
Kobayashi, D. Y. & Yuen, G. Y. The role of clp-regulated factors in antagonism against Magnaporthe poae and biological control of summer patch disease of Kentucky bluegrass by Lysobacter enzymogenes C3. Can J Microbiol. 51(8), 719–723(5) (2005).
Zhang, Z. & Gy, Y. Effects of culture fluids and preinduction of chitinase production on biocontrol of bipolaris leaf spot by Stenotrophomonas maltophilia C3. Biol Control. 18(3), 277–286 (2000).
Kilic-Ekici, O. & Yuen, G. Y. Comparison of strains of Lysobacter enzymogenes and PGPR for induction of resistance against Bipolaris sorokiniana in tall fescue. Biol Control. 30(2), 446–455 (2004).
Muhamed, T. I., Yasuyuki, H., Abhinandan, D., Toshiaki, I. & Satoshi, T. Suppression of damping-off disease in host plants by the rhizoplane bacterium lysobacter sp. strain SB-K88 is linked to plant colonization and antibiosis against soilborne peronosporomycetes. Appl Environ Microb. 71(7), 3786–3796 (2005).
Hu, J., He, X. H., Li, D. P. & Liu, Q. Progress in research of Sphingomonas. Chinese J Appl Environ Biol. 13(3), 431–437 (2007). (in chinese).
Takeuchi, M. et al. Taxonomic study of bacteria isolated from plants: Proposal of Sphingomonas rosa sp. nov., Sphingomonas pruni sp. nov., Sphingomonas asaccharolytica sp. nov., and Sphingomonas mali sp. nov . Int J Syst Bacteriol. 45(2), 334–341 (1995).
Acknowledgements
The authors are grateful for the financial support of National Natural Science Foundation of China (No. 31201692), Jiangsu Agricultural Science and Technology Independent Innovation Fund Project (No. CX(15)1005) and the National Key Projects of Scientific, Technical Support Programs funded by the Ministry of Science and Technology of China (No. 2011BAD13B09).
Author information
Authors and Affiliations
Contributions
H.Y. and X.L. designed the experiments. J.H. and H.Y. performed the experiments, H.Y., X.L., Z. L. and J.H. analyzed the data, H.Y., J.H. and Z.R. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, H., Hu, J., Long, X. et al. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci Rep 6, 20687 (2016). https://doi.org/10.1038/srep20687
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20687
This article is cited by
-
Genetic Mapping of the Root Mycobiota in Rice and its Role in Drought Tolerance
Rice (2023)
-
Structural Stability and Organic Matter Stabilization in Soils: Differential Impacts of Soil Salinity and Sodicity
Journal of Soil Science and Plant Nutrition (2023)
-
Biochar-induced changes in the soil diazotroph community abundance and structure in a peanut field trial
Biochar (2022)
-
Effects of Salt Stress on the Structure and Function of Oil-Contaminated Soil Bacteria
Water, Air, & Soil Pollution (2022)
-
Potato tillage method is associated with soil microbial communities, soil chemical properties, and potato yield
Journal of Microbiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.