Abstract
The design and development of an economic and highly active non-precious electrocatalyst for methanol electrooxidation is challenging due to expensiveness of the precursors as well as processes and non-ecofriendliness. In this study, a facile preparation of core-shell-like NiCo2O4 decorated MWCNTs based on a dry synthesis technique was proposed. The synthesized NiCo2O4/MWCNTs were characterized by infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, X-ray diffraction and selected area energy dispersive spectrum. The bimetal oxide nanoparticles with an average size of 6 ± 2 nm were homogeneously distributed onto the surface of the MWCNTs to form a core-shell-like nanostructure. The NiCo2O4/MWCNTs exhibited excellent electrocatalytic activity for the oxidation of methanol in an alkaline solution. The NiCo2O4/MWCNTs exhibited remarkably higher current density of 327 mA/cm2 and a lower onset potential of 0.128 V in 1.0 M KOH with as high as 5.0 M methanol. The impressive electrocatalytic activity of the NiCo2O4/MWCNTs is promising for development of direct methanol fuel cell based on non-Pt catalysts.
Similar content being viewed by others
Introduction
The ever growing demand for next-generation clean and high-efficiency energy has inspired considerable efforts in the development of advanced alternative energy conversion and storage devices with the features of low cost and more importantly, environmental friendliness. Among them direct alcohol fuel cells (DAFC) are attractive due to their high energy conversion efficiency. Methanol has higher energy density than hydrogen. Abundant inexpensive sources are available for production of methanol and more over the direct methanol fuel cell (DMFC) possess high energy conversion efficiency and stability1,2,3,4. Currently platinum (Pt)-based electrocatalysts exhibit higher energy conversion but non-preferable due to its high cost and catalytic poisoning which reduce the catalytic activity5. The successful implementation of the fuel cells mainly depends on the economy, activity and the durability of the electrocatalysts. Therefore, it is important to design highly efficient as well as economical electrocatalysts for practical applications. The development of non-Pt based electrocatalysts is still challenging mainly due to lack of high conductivity and excellent catalytic activity and stability of the catalyst. Most of the preceding researches report non-Pt based electrocatalysts using transition metal oxides, for instance, NiO6,7, Co3O48. It has been reported that a strategy to combine both NiO and Co3O4 resulted in the high electrocatalytic efficiency than their individual counterparts. For example, NiCo2O4 exhibits excellent electrochemical activity due to higher electronic conductivity than either NiO or Co3O49,10.
Several synthetic strategies, such as conventional hydrothermal11,12,13,14,15, solvothermal16,17,18, electrochemical synthesis19,20 were for the preparation of Ni and Co-based bimetal oxide electrocatalysts with different morphologies. However, most of them involve in use of toxic chemicals such as NH4F, high temperatures and other non-ecofriendly solvents. In this study, we have developed an economical, environmental friendly, dry synthesis method for facile preparation of core-shell-like NiCo2O4 decorated multiwall carbon nanotubes (MWCNT). The synthesized electrocatalyst demonstrated higher catalytic activity for electrooxidation of methanol. To the best of our knowledge NiCo2O4/MWCNT has not been synthesized via such a simple grinding method followed by low temperature annealing. Further this is the first study to demonstrate the use of NiCo2O4/MWCNT as an electrocatalyst for direct methanol fuel cell.
Experimental
Materials
MWCNT (>95% in purity, 20 ~ 25 μm in length, <20 nm in diameter) was kindly provided by nanosolution Co., Korea. Cobalt (II) acetate tetra hydrate (CoAc, 99.0% assay, Sigma-Aldrich) and nickel acetate tetra hydrate (NiAc, 99.0% assay, Sigma-Aldrich) were utilized without any further modification as precursors. Methanol, potassium hydroxide were purchased from Junsei Co. Ltd. (Japan). Millipore water (Milli-Q system) was used for the preparation of solutions.
Synthesis of Core/Shell-like NiCo2O4-Decorated MWCNTs
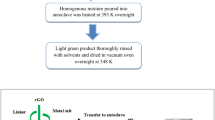
At first, 0.2 g of pure MWCNTs were treated with 3 M HNO3 and then refluxed for 48 h to remove metal impurities. After cooling to room temperature, the solution was diluted with 500 mL of deionized water and then vacuum-filtered through a filter paper with the pore size of 0.45 μm. The resultant purified MWCNTs were washed with deionized water until the pH became neutral and then dried in vacuum at 80 °C for 48 h.
For the preparation of core-shell-like NiCo2O4-decorated MWCNTs, 0.2 g of purified MWCNT was ground using a mortar and pestle for 10 min. Then, 0.05 g of each CoAc and NiAc were added into the ground-MWCNTs and ground well. The homogeneous mixture of MWNTs and CoAc and NiAc was obtained in 15 min. Finally, the mixture was calcinated at 300 °C for 4 h in air atmosphere. The composition of the CoAc and NiAc were varied at a ratio of Ni:Co = 0.2 : 0.8, 0.4 : 0.6, 0.6 : 0.4, 0.2 : 0.8, 1.0 : 1.0 with fixing the total quantity as 0.1 g. The samples before calcination and after calcination were indicated by NiAc-CoAc/MWCNT and NiCo2O4/MWCNT, respectively.
Characterization
The morphologies of all the samples were observed under a JEOL JSM-5900 scanning electron microscopy (SEM) after sputtering the samples with platinum for 120 s. Energy dispersive X-ray measurements were conducted using the EDAX system attached to the same microscope. A field-emission scanning electron microscope (FE-SEM) was also used to observe the morphologies after sputtering the samples with osmium for 7 s. For TEM, the sample was prepared by dispersing in ethanol by sonication at a concentration of 0.1 mg/mL. 1 mL of sample was dropped on the Cu TEM grid and analyzed. The FT-IR of spectra of the samples was recorded using a Perkin Elmer instrument. X-ray diffraction (XRD) patterns were recorded on a Rikaku X-ray diffractometer (Cu Kα radiation). The surface of the samples was analyzed by X-ray photoelectron spectroscopy (XPS, ESCALAB250, Al Kα radiation). VersaSTAT4 (USA) electrochemical analyzer and a conventional three-electrode electrochemical cell were utilized to investigate the electrochemical measurements. Pt wire and Ag/AgCl (saturated KC) were used as the counter and reference electrodes, respectively. The glassy carbon electrode (GCE, 3 mm diameter; area 0.07 cm2) was used as the working electrode. The MWNT/NiCo2O4 powder (2 mg) was dispersed in 2-propanol (400 μL) and sonicated for 5 min. And then the mixture was added nafion solution (20 μL) and sonicated for 5 min. Then the solution was dropped on the surface of the GCE and dried at 60 °C for 15 min. Cyclic voltammetry (CV) and chronoamperometry (CA) measurements were performed to study the activity and stability of methanol oxidation reaction. The test solutions used in this study were 1.0 M KOH solution with and without addition of various methanol concentrations. All the experiments were performed at 298 ± 2 K. The electrochemical impedance spectroscopy (EIS) was recorded in the frequency range of 10 mHz to 100 kHz with a potential amplitude of 10 mV.
Results and Discussion
The chemical conversion of NiAc-CoAc/MWCNT into NiCo2O4/MWCNT was proved by means of FT-IR spectral changes before and after calcination (Fig. S1). The NiAc-CoAc/MWCNT exhibited characteristic peaks at 3128, 2885, 1522 and 1487 cm−1 corresponding to asymmetric stretching of C-H, symmetric stretching of C-H, O-C=O of acetate and C-H deformation. Disappearance of these unique peaks in the FT-IR spectrum of NiCo2O4/MWCNT after calcination indicated the successful synthesis.
The morphologies of the synthesized NiCo2O4/MWCNT were investigated by using FE-SEM and TEM measurements. Figure 1a,b showed the smooth surface morphologies of pristine MWCNT. On the other hand, the NiCo2O4/MWCNT exhibited a rough surface, suggesting the successful decoration of the MWCNTs with bimetal oxide nanoparticles (Fig. 1c–e). It was estimated that the thickness of the MWCNT increased from ~20 nm to 48 nm on average after decoration with NiCo2O4 nanoparticles. A close examination of the HR-TEM image of the NiCo2O4/MWCNT showed successful deposition of quasi-spherical NiCo2O4 nanoparticles on the MWCNTs, which is similar to the core (MWCNT)/shell (NiCo2O4) structure. The average size of the nanoparticles over the surface of MWCNTs was 6 ± 2 nm. Further, the SAED pattern (Fig. 1f) exhibited well-defined rings of (511), (220), (111), (311) and (400) suggesting the polycrystalline nature of the synthesized NiCo2O4/MWCNT (JCPDS no. 73–1702).
In order to determine the weight percentage (wt%) of Ni and Co in NiCo2O4/MWCNT and to inspect their distribution, FE-SEM/EDS and their corresponding elemental mapping images were taken for NiCo2O4/MWCNT (Fig. 2). The wt% of the elements were C, 79.13%; O, 14.02%; Ni, 3.22%; Co, 3.63%. The homogeneous distribution of Ni and Co in NiCo2O4/MWCNT indicated that the proposed synthesize method is effective and successful.
Figure 3 shows the XRD patterns of pristine MWCNT and NiCo2O4/MWCNT. In the case of pristine MWCNTs, the diffraction peaks were observed at 26.5o, 42.4o and 64.7o, corresponding to the (002), (100) and (004), respectively, which is attributed to the hexagonal graphite structures of CNTs. On the other hand, the XRD profile of NiCo2O4/MWCNT exhibited peaks corresponding to the (111), (220), (311), (400), (422), (511) and (440) reflections of the spinel NiCo2O4 crystalline structure (JCPDS no. 73–1702).
In order to understand the chemical composition and the oxidation states of the metals in NiCo2O4/MWCNTs, the samples were subjected to XPS analyses and the results are shown in Fig. 4. The survey spectrum indicated the presence of elements Ni, Co, O and C as well as absence of any impurities. The Ni 2p spectrum best fitted with two spin-orbit doublets corresponding to the oxidation states of 2+ and 3+ of nickel along with two shake-up satellites (indicated as ‘Sat.) at the high binding energy side of the Ni 2p3/2 and 2p1/2 edge. Similarly, the spectrum of Co 2p also best fitted with two spin-orbit doublets that are corresponding to Co2+ and Co3+ and two shake-up satellites. Thus the results indicated that the NiCo2O4/MWCNT contains Ni2+/Ni3+ and Co2+/Co3+. The XPS results are in agreement with preceding literature for synthesis of NiCo2O421,22,23,24. Further the O 1 s spectrum revealed the presence of metal oxygen bonds by exhibiting a peak at 529.8 eV. The peak at 532.9 eV is ascribed to the chemisorbed oxygen. The peaks at 529.8 and 531.6 eV are due to O2− which indicates the formation of spinel NiCo2O423,24.
In general, the electrochemical performance of any material depends on the rate of electron transfer or charge transfer. The electron transfer kinetics of NiCo2O4/MWCNT was studied by the electrochemical impedance spectroscopy (EIS) in 1.0 M KOH with and without different concentrations of methanol (Fig. 5). The EIS curves exhibited a semicircle and a straight line. The semicircle is the characteristic behavior for charge transfer resistance of the material and the straight line is due to the reduction-oxidation behavior of nickel oxide and cobalt oxide25. It is noteworthy that the internal resistance of the material increased slightly in proportion to the increasing concentration of the methanol. Though the change in resistance between each solution is too small, it could be attributed to the effect of adsorption of the reactant and intermediate molecules on the catalyst.
It is noteworthy that surface activation of the NiCo2O4/MWCNT is important for better electrochemical activity26. Further, the composition ratio of the Ni and Co also influences the electrocatalytic activity. Therefore, the working electrode prepared with NiCo2O4/MWCNT having different Ni:Co ratio (0.2 : 0.8, 0.4 : 0.6, 0.6 : 0.4, 0.2 : 0.8, 1.0 : 1.0) were subjected to a pre-activation of 20 cycles in cyclic voltammetry at a scan rate of 100 mV/s (Fig. 6a–e). It is observed that all the CV curves consist of a pair of well-defined redox peaks. These peaks were attributed to the oxidation and reduction of Co(II) and Ni(III). It should be noted that the redox potentials of cobalt oxide and nickel oxide are close to each other. Therefore, the redox peaks of cobalt oxide and nickel oxide are not clearly distinguishable in the CV curve of NiCo2O4/MWCNTs (Fig. 6a–e). In the present study, NiCo2O4/MWCNT with Ni:Co=1.0 : 1.0 was a best performing material attributed to higher current density (32.72 mA/cm2) and lower oxidation peak potential (0.49 V) (Fig. 6f).
Figure 7 shows the electrochemical oxidation of MeOH by NiCo2O4/MWCNT with Ni:Co=1.0 : 1.0 in 1.0 M KOH at a scan rate of 50 mV/s. It is noteworthy that the addition of even 0.5 M MeOH increased the anodic peak current density to ~3 times (97.53 mA/cm2) with a peak potential of 0.553 V. It should be noted that the heterogeneous catalysis reaction involves in adsorption of the reactant on the catalyst followed by the formation of intermediate species and then products. Thus, the anodic peak observed in the curve of forward sweep was corresponding to the oxidation of the MeOH, whereas, the peak observed in the curve of reverse sweep was attributed to the oxidation of adsorbed intermediate species produced in the forward sweep.
The mechanism for electro-oxidation of methanol by NiCo2O4/MWCNT is supposed to be similar to nickel oxide and cobalt oxide catalysts as given below (Eq. 1–3)27,28,29,30.



The concentration of alcohol is one of the important parameter to determine the performance of the fuel cell. Generally, DAFCs working with highly concentrated alcohol solution is preferable, since it could dramatically decrease the size of the fuel cell and simultaneously increase the power density. Further, it is impossible to use absolute methanol as the anodic reaction (Eq. 1) because it requires water. Unfortunately, most of the preceding DMAC studies involving NiCo2O4 based catalysts have been reported to use lower concentrations of MeOH such as 0.5 M to 3.0 M MeOH, which do not show any performance beyond this concentration threshold. Interestingly, in the present study, NiCo2O4/MWCNT exhibited electrocatalytic performance up to 5.0 M MeOH with a high current density of 327 mA/cm2 and peak potential of 0.675 V (Table 1). For 6.0 M MeOH, the current density slightly decreases, which indicate that the concentration threshold in this study is 5.0 M MeOH. To the best of our knowledge, this is the highest alcohol concentration ever to be reported, especially for NiCo2O4 -based catalysts for methanol electro-oxidation.
Onset potential is the other important parameter to demonstrate the electrocatalytic activity of the catalyst since it is the indicative measure for over potential. Pt-based catalysts perform better than non-precious electrocatalysts due to their smaller onset potential. In the present study, the onset potentials range from 0.128 to 0.262 V. The small organic molecules that were adsorbed on the catalyst and the molecules which are not fully oxidized are ascribed for the slight increase in the onset potentials. However, the onset potential reported in this study is one of the lowest values reported in the literature so far. Further to study the stability of the electrocatalytic activity, the NiCo2O4/MWCNT was subjected to longer CV cycles in 1.0 M KOH with 5.0 M MeOH. Figure 8 displays the good stability of NiCo2O4/MWCNT even after 250 cycles with retaining current density over 76%. Table 2 shows the anodic peak potentials of different Ni and Co based electrocatalysts for methanol oxidation. It is noteworthy that the high performance of the synthesized core-shell-like NiCo2O4/MWCNT was due to the fine size of the metal oxide nanoparticles and their homogeneous distribution on MWCNTs.
Conclusions
In this work, we report a new and easy synthetic route to prepare core-shell-like NiCo2O4/MWNTs via a dry synthesis method. The efficient electrocatalytic oxidation of methanol on NiCo2O4/MWNTs was studied by cyclic voltammetry in 1.0 M KOH in the presence and absence of methanol. The NiCo2O4/MWNTs exhibited an impressively high electrocatalytic activity for methanol oxidation as the corresponding current increased with increasing the methanol concentration in the alkaline medium. This electrocatalyst is active for up to 5.0 to 6.0 M methanol. Remarkably this catalyst revealed a small onset potential lesser than 0.128 V vs Ag/AgCl, which is one of the superior value among the reported non-precious electrocatalyst. Overall, this work opens up opportunities for facile preparation of non-precious metal electrocatalysts via an economical, simple and eco-friendly synthetic route with high catalytic activity.
Additional Information
How to cite this article: Ko, T.-H. et al. Facile Synthesis of Core/Shell-like NiCo2O4-Decorated MWCNTs and its Excellent Electrocatalytic Activity for Methanol Oxidation. Sci. Rep. 6, 20313; doi: 10.1038/srep20313 (2016).
References
Guo, Y. G., Hu, J. S. & Wan, L. J. Nanostructured Materials for Electrochemical Energy Conversion and Storage Devices. Adv. Mater. 20, 2878–2887 (2008).
Tian, Z. Q., Jiang, S. P., Liang, Y. M. & Shen, P. K. Synthesis and Characterization of Platinum Catalysts on Multiwalled Carbon Nanotubes by Intermittent Microwave Irradiation for Fuel Cell Applications. J. Phys. Chem. B 110, 5343–5350 (2006).
Shen, J. et al. Pt-Co supported on single-walled carbon nanotubes as an anode catalyst for direct methanol fuel cells. Electrochim. Acta. 53, 7276–7280 (2008).
Shao,Y., Sui, J., Yin, G. Y. & Gao, Y. Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl. Catal. B 79, 89–99 (2008).
Geng, D. & Lu, G. Dependence of Onset Potential for Methanol Electrocatalytic Oxidation on Steric Location of Active Center in Multicomponent Electrocatalysts. J. Phys. Chem. C 111, 11897–11902 (2007).
Tong, X. L. et al. Enhanced Catalytic Activity for Methanol Electrooxdiation of Uniformly Dispersed Nickel Oxide Nanoparticles-Carbon Nanotube Hybrid Materials. Small 8, 3390–3395 (2012).
Amin, R. S. et al. Pt-NiO/C anode electrocatalysts for direct methanol fuel cells. Electrochim. Acta 59, 499–508 (2012).
Wu, J. B., Li, Z. G., Huang, X. H. & Lin, Y. Porous Co3O4/NiO core/shell nanowire array with enhanced catalytic activity for methanol electro-oxidation. J. Power Sources 224, 1–5 (2013).
Li, Y., Hasin, P. & Wu, Y. NixCo3−xO4 Nanowire Arrays for Electrocatalytic Oxygen Evolution. Adv. Mater. 22, 1926–1929 (2010).
Lu, B. et al. Oxygen evolution reaction on Ni-substituted Co3O4 nanowire array electrodes. Int. J. Hydrogen Energy 36, 72–78 (2011).
Windisch, Jr, C. F. et al. Infrared transparent spinel films with p-type conductivity. Thin Solid Films 398–399, 45–52 (2001).
Ding, R., Qi, L., Jia, M. & Wang, H. Simple hydrothermal synthesis of mesoporous spinel NiCo2O4 nanoparticles and their catalytic behavior in CH3OH electro-oxidation and H2O2 electro-reduction. Catal. Sci. Technol. 3, 3207–3215 (2013).
Zhan, J., Cai, M., Zhang, C. & Wang, C. Synthesis of mesoporous NiCo2O4 fibers and their electrocatalytic activity on direct oxidation of ethanol in alkaline media. Electrochim. Acta 154, 70–76 (2015).
Ding, R., Qi, L., Jia, M. & Wang, H. Sodium dodecyl sulfate-assisted hydrothermal synthesis of mesoporous nickel cobaltite nanoparticles with enhanced catalytic activity for methanol electrooxidation. J. Power Sources 251, 287–295 (2014).
Cai, F. et al. Hierarchical CNT@NiCo2O4 core-shell hybrid nanostructure for high-performance supercapacitors. J. Mater. Chem. A 2, 11509–11511 (2014).
Ding, R., Qi, L., Jia, M. & Wanga, H. Porous NiCo2O4nanostructures as bi-functional electrocatalysts for CH3OH oxidation reaction and H2O2 reduction reaction. Electrochim. Acta 113, 290– 301 (2013).
Wang, W. et al. Nickel foam supported mesoporous NiCo2O4 arrays with excellent methanol electro-oxidation performance. New J. Chem. 39, 6491–6497 (2015).
Yu, X. Y. et al. Facile Synthesis of Urchin-like NiCo2O4 Hollow Microspheres with Enhanced Electrochemical Properties in Energy and Environmentally Related Applications. ACS Appl. Mater. Interfaces 6, 3689−3695 (2014).
Das, A. K. et al. Reduced graphene oxide (RGO)-supported NiCo2O4 nanoparticles: an electrocatalyst for methanol oxidation. Nanoscale 6, 10657–10665 (2014).
Li, X. et al. There-Dimensional Hierarchical Self-Supported NiCo2O4/Carbon Nanotube Core-Shell Networks as High Performance Supercapacitor Electrodes. RSC Adv. 5, 7976–7985 (2015).
Thissen, A. et al. Photoelectron Spectroscopic Study of the Reduction of Li and Na with NiCo2O4 . Chem. Mater. 17, 5202–5208 (2005).
Kim, J. G., Pugmire, D. L., Battaglia, D. & Langell, M. A. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 165, 70–84 (2000).
Marco, J. F. et al. Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS and XPS Study. J. Solid State Chem. 153, 74–81 (2000).
Mo, Y. et al. 3-dimensional porous NiCo2O4 nanocomposite as a high-rate capacity anode for lithium-ion batteries. Electrochim. Acta 176, 575–585 (2015).
Mitchell, E. et al. Ultrathin porous hierarchically textured NiCo2O4–graphene oxide flexible nanosheets for high-performance supercapacitors. New J. Chem. 39, 2181–2187 (2015).
Qian, L. et al. Direct growth of NiCo2O4 nanostructures on conductive substrates with enhanced electrocatalytic activity and stability for methanol oxidation. Nanoscale 5, 7388–7396 (2013).
Nasser, A. M. B. et al. NixCo1−x alloy nanoparticle-doped carbon nanofibers as effective non-precious catalyst for ethanol oxidation. Int. J. Hydrogen Energy 39, 305–316 (2014).
Heli, H. & Yadegari, H. Nanoflakes of the cobaltous oxide, CoO: synthesis and characterization. Electrochim. Acta 55, 2139–2148 (2010).
Spinner, N. & Mustain, W. E. Effect of nickel oxide synthesis conditions on its physical properties and electrocatalytic oxidation of methanol. Electrochim. Acta 56, 5656–5666 (2011).
Asgari, M., Maragheh, M. G., Davarkhah, R. & Lohrasbi, E. Methanol electrooxidation on the nickel oxide nanoparticles/multi-walled carbon nanotubes modified glassy carbon electrode prepared using pulsed electrodeposition. J. Electrochem. Soc. 158, K225–K229 (2011).
Asgari, M. et al. Electrocatalytic oxidation of methanol on the nickel-cobalt modified glassy carbon electrode in alkaline medium. Electrochim. Acta 59, 284–289 (2012).
Prathap, M. U. A. & Srivastava, R. Synthesis of NiCo2O4 and its application in the electrocatalytic oxidation of methanol. Nano Energy 2, 1046–1053 (2013).
Acknowledgements
This work was supported by the Basic Research Laboratory Program (2014R1A4A1008140) through the Ministry of Science, ICT & Future Planning.
Author information
Authors and Affiliations
Contributions
K.D. and B.S.K. wrote the main manuscript text and analyzed the data; T.H.K. carried out the research and prepared the data; B.S.K., S.M.K. and H.Y.K. supervised this project. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ko, TH., Devarayan, K., Seo, MK. et al. Facile Synthesis of Core/Shell-like NiCo2O4-Decorated MWCNTs and its Excellent Electrocatalytic Activity for Methanol Oxidation. Sci Rep 6, 20313 (2016). https://doi.org/10.1038/srep20313
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20313
This article is cited by
-
Novel Electrochemical Sensor Based on PDA/MXene/MWCNTs/NiCo2O4 Nanocomposites for Rapid, Sensitive, and Selective Detection of Aflatoxin B1
Food Analytical Methods (2023)
-
Sea-Island-Like Morphology of CuNi Bimetallic Nanoparticles Uniformly Anchored on Single Layer Graphene Oxide as a Highly Efficient and Noble-Metal-Free Catalyst for Cyanation of Aryl Halides
Scientific Reports (2020)
-
Supercapacitor performance of MnO2/NiCo2O4@N-MWCNT hybrid nanocomposite electrodes
Journal of Sol-Gel Science and Technology (2019)
-
Reduced graphene oxide (rGO): supported NiO, Co3O4 and NiCo2O4 hybrid composite on carbon cloth (CC)—bi-functional electrode/catalyst for energy storage and conversion devices
Journal of Materials Science: Materials in Electronics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.