Abstract
To date, the effects of ocean acidification on toxic metals accumulation and the underlying molecular mechanism remains unknown in marine bivalve species. In the present study, the effects of the realistic future ocean pCO2 levels on the cadmium (Cd) accumulation in the gills, mantle and adductor muscles of three bivalve species, Mytilus edulis, Tegillarca granosa and Meretrix meretrix, were investigated. The results obtained suggested that all species tested accumulated significantly higher Cd (p < 0.05) in the CO2 acidified seawater during the 30 days experiment and the health risk of Cd (based on the estimated target hazard quotients, THQ) via consumption of M. meretrix at pH 7.8 and 7.4 significantly increased 1.21 and 1.32 times respectively, suggesting a potential threat to seafood safety. The ocean acidification-induced increase in Cd accumulation may have occurred due to (i) the ocean acidification increased the concentration of Cd and the Cd2+/Ca2+ in the seawater, which in turn increased the Cd influx through Ca channel; (ii) the acidified seawater may have brought about epithelia damage, resulting in easier Cd penetration; and (iii) ocean acidification hampered Cd exclusion.
Similar content being viewed by others
Introduction
Ocean acidification occurs as a result of pumping enormous amount of carbon dioxide into the atmosphere and it is changing seawater chemistry at an unprecedented rate. Many marine organisms are sensitive to these changes, with evidence suggesting that mass extinctions and ‘reef gaps’ were driven by ocean acidification during the Paleocene-Eocene Thermal Maximum1. The phenomenon has drawn much attention, with numerous effects on marine organisms have been reported2,3. Ocean acidification may negatively affect marine organisms by reducing the calcium carbonate (CaCO3) state4 and disturbing the acid-base physiology5 leading to reductions in the calcification rate of many shell-forming marine organisms3,4,6. In addition to calcification, dissolved CO2 may negatively affect marine organisms in numerous ways, including fertilization success7, embryonic development2, metabolism8, immune response9 and survival rate10.
Cadmium (Cd), which is obtained as a by-product of zinc, is a toxic metal used in a wide array of applications. A large employment of Cd in industrial and agricultural activities has led to substantial anthropogenic emissions of Cd into the marine environment. Compared with other toxic metals, Cd is relatively soluble and can be accumulated by organisms such as bivalves. These characteristics of Cd would result in toxic metal poisoning, therefore consequently, Cd is considered to be a significant environmental threat11. Numerous studies have shown that Cd pose adverse impacts on the immune12 and reproductive systems13 of various species, giving rise to chromosomal damage14.
Marine bivalves, as filter feeders, are able to concentrate pollutants to several orders of magnitude above ambient levels and accumulate toxic metals in their tissues in proportion to the amount of toxic metals in the environment. Therefore, marine bivalves are deemed to be suitable bioindicators for toxic metal pollution monitoring due to their readily interpretable biological consequences of contamination. On the contrary, many marine bivalves are traditional aquaculture species that are widely distributed throughout the world and provide approximately 1 × 108 tons seafood for consumers yearly15. Since toxic metals such as Cd can be accumulated by bivalves, there is an increasing concern of the bivalve safety as seafood. Nowadays, several approaches have been proposed to assess the potential health risks of toxic metals intake. Among which, the target hazard quotient (THQ, the ratio between the estimated dose of a contaminant and the reference dose) has been widely used in health risk assessment of toxic metals in food16. Surveys conducted in the major seafood markets of the Pearl River Delta, south China, presented an view of the Cd contamination in edible marine bivalves as a potential hazard to public health17. Hence, the analysis of Cd accumulation in marine bivalves can provide useful information for both marine environmental assessments and seafood safety.
Previous studies have demonstrated that changes in seawater pH and chemistry would affect the speciation, adsorption, toxicity and rates of redox processes of metals in seawater18. Unlike a decrease in water pH by adding a strong acid or acid rain, ocean acidification driven by increased pCO2 contains more dissolved CO2, HCO3− and CO32− at the same pH values; therefore, more physiological processes of the organisms would be affected11. For instance, the acidity manipulated by CO2 showed a stronger toxicity to the embryonic development of sea bream19 and coastal meiobenthic copepods11. Although the effects of increased acidity on metal accumulation has been well studied, especially in freshwater organisms20,21, to our knowledge, the impacts of CO2-driven ocean acidification on the toxic metal accumulation in marine bivalves and the underlying mechanism remains elusive. Limited comparable studies in molluscs were conducted with cephalopods, suggesting that the increase of seawater pCO2 enhanced the uptake of toxic metal during the early life stage of these species22,23,24.
According to previous studies, the P-glycoproteins (PGP) have been closely linked to Cd exclusion. PGPs belong to ATP-binding cassette transporters that resist drugs and toxins by an ATP-consuming process25. Gene pgp-5 is induced upon exposure to toxic metals and is reported to function as an ATP-dependent efflux pump that protects animals by exporting exogenous toxins26.
The present study was therefore conducted to (i) determine the effects of ocean acidification on the cadmium accumulation in gills, mantle and adductor muscles of three aquiculture bivalves, blue mussel (Mytilus edulis), blood clam (Tegillarca granosa) and hard clam (Meretrix meretrix); (ii) investigate the effects of ocean acidification on the Cd2+/Ca2+ content in the water environment; (iii) evaluate the influences of ocean acidification on the expressions of Cd exclusion related pgp-5 gene and (iv) provide firsthand evidence estimating the effects of realistic future ocean acidification on seafood safety.
Results
At an experimental temperature of 25.5 ± 1.7 °C, the measured values of pHs for the control and two experimental trials were 8.07 ± 0.05, 7.79 ± 0.06 and 7.42 ± 0.03, respectively. Both the Cd2+ and Ca2+ concentrations in the seawater significantly differed among the three pCO2 trials (Table 1). The concentration of the cadmium and calcium increased and decreased markedly (p < 0.05) as the pH declined, respectively. Compared to control, significant higher Cd2+/Ca2+ ratios were found in the two CO2 acidified seawater samples at pH 7.79 and 7.42 (about 1.15 and 1.36 times of the control, respectively).
Toxic metal accumulation in the various tissues of the three bivalves
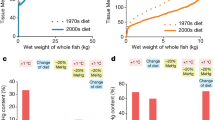
After raising in seawater containing 0.05 mg/L Cd at various pCO2 levels for 30 days, the Cd accumulations in the gills, mantle and adductor muscles of M. edulis, T. granosa and M. meretrix were shown in Fig. 1. Compared to control, CO2 acidified seawaters led to a significant higher Cd accumulation in the tissues of all three bivalves investigated. The highest Cd contents were found in the individuals from the pH 7.4 experimental groups followed by those from pH 7.8 and pH 8.1 groups. Furthermore, results obtained in the present study showed that different tissues accumulate Cd differently, with average concentrations of Cd detected were in the order of mantle > gills > adductor muscles (Fig. 1).
Gene expressions of pgp-5 in different pCO2 levels
After raising in CO2 acidified seawater (pH 7.4) for 30 days, pgp-5 expression was significantly lower than that of the control (p < 0.05). Gene expression of pgp-5 in pH 7.4 CO2 acidified seawater was decreased to approximately one seventh of the control (Fig. 2).
Cd THQs of bivalves in different pCO2 levels
The Cd EDIs and THQs via consuming M. meretrix under different pCO2 levels were shown in Table 2. The THQ values of Cd were significantly higher (p < 0.05) when the animals were exposed to pCO2 acidified seawater, which were about 1.21 and 1.32 times of that of the control for experimental groups at pH 7.8 and 7.4, respectively.
Discussion
Toxic metal accumulation was shown to increase with a decrease in pH upon manipulation with the addition of a strong acid20,27. Results obtained in the present study showed that ocean acidification exert a similar effect on Cd accumulation in bivalves, which might be explained by the following reasons (Fig. 3).
Effects of ocean acidification on cadmium uptake and exclusion.
(a) Acidified seawater has higher Cd concentration and Cd2+/Ca2+ ratio, which facilitate the entry of Cd2+ through Ca2+ channel. (b) Epithelia damage as a result of acidified seawater made it more penetrable to Cd. (c) Acidified seawater had an inhibitory effect on the gene expression of pgp-5, which reduced the exclusion of Cd. (d) Ocean acidification may cause stress on marine organisms and constrain the energy available for Cd exclusion. For more details, see the discussion text.
First, seawater acidification driven by CO2 may change the chemistry of toxic metal compounds and subsequently lead to an increase in the toxic metal accumulation. In the present study, the concentration of the cadmium increased markedly (p < 0.05) in the acidified seawater (Table 1), a finding consistent with previous studies28. A higher environmental Cd2+ contamination would be expected to facilitate Cd entry into bivalves. It was found that Cd enters the cells of mammalian29 and marine organisms30,31 primarily through voltage-sensitive calcium channels since the Ca2+ channels may fail to distinguish between Cd2+ and Ca2+ ions due to the same charge and comparable sizes. In support of this view, decreased Cd2+ influx was observed with the application of Ca2+ channel blockers (nimodipine and verapamil) in a mangrove crab Ucides cordatus32 and freshwater teleost Oncorhynchus mykiss33. Similar results were observed in Crassostrea virginica34 and M. edulis35 as well, conforming that the Ca2+ channel is the uptake route for Cd in marine bivalves. In addition, probably due to the competition for the same channels, Ca2+ was found to protect against Cd2+ uptake in rainbow trout (O. mykiss)36, molluscs (Littorina littorea)37 and crabs (Carcinus maenas)38. In the present study, the Ca2+ concentration was found to be significantly lower in the CO2 acidified seawater, where the concentration of Cd2+ and Cd2+/Ca2+ ratio were significantly higher (Table 1). Therefore, an increased extracellular Cd2+ concentration and a reduced inhibitory effect of Ca2+, have provided a favourable environment for Cd2+ uptake into the body of bivalves in the CO2 acidified seawater.
Second, acidified seawater may bring about direct damages to the bivalves tissues and subsequently affect the accumulation of toxic metals. Entry through an apical epithelial membrane is the first step in toxic metal absorption, therefore damaged epithelia will increase the penetration of metals into cells39. In addition, it has been shown in M. edulis that the decrease in the pH of seawater significantly reduced the lysosome health, as measured by the Neutral Red Retention assay9. Therefore, the reduction in lysosome health caused by acidification would disrupt cellular pathways and increase membrane fragility and this may subsequently lead to the increase in Cd uptake due to a weakened defence system40.
Third, the changes in toxic metal exclusion could also have contributed to the higher Cd accumulation from the CO2 acidified seawater. The gene pgp-5, which belongs to the ATP-binding cassette transporters, was reported to be evoked by toxic metal stress25,26. Kurz found that pgp-5 was induced to at least threefold by the exposure to cadmium in Caenorhabditis elegans26, which suggested that pgp-5 was essential for a substantial resistance to Cd. As a result, a down-regulation of pgp-5 would lead to a reduced ability to export Cd.
The exclusion of intracellular Cd by pgp-5 is an energy consuming process and therefore is subjected to energy availability. A compensation hypothesis suggests that animals would alter energetic trade-offs among different aspects of the physiological maintenance budget to meet the increased energetic demands under stressful conditions41. For example, it had been shown that M. edulis was able to protect their tissues against seawater acidification in energetic costs, which led to a reduction in the energy budget for growth, shell formation and toxicant metabolism42. Previous study has also shown that acidified seawater would suppress the expression of genes related to the tricarboxylic acid cycle, electron transport chain and oxidative phosphorylation triggering a decrease in the production of ATP43. Therefore, the reduction in energy availability for Cd exportation may constrain the exclusion of intracellular Cd as well.
The potential impacts of climate change on the different aspects of human and animal health and welfare are widely debated topics. It was suggested that climate change will affect all four pillars of food security, namely, food availability, access to food, stability of food supplies and food utilization44. However, the potential consequences of ocean acidification on food safety of marine bivalves were largely overlooked. Marine bivalves provide an important and economical protein source for human consumption and are a primary protein source for over one billion of the poorest people in the world15. Moreover, marine bivalves are important food sources for supplying essential elements and are rich sources of certain vitamins, such as vitamins B6 and B12. However, since marine bivalves is capable of accumulating a large amount of pollutants, such as toxic metals in their tissues, in extreme conditions, these contaminants in the edible parts of marine bivalves can pose a severe threat to human health.
According to the data obtained in the present study, ocean acidification would increase Cd accumulation in bivalves through increased uptake and reduced exclusion. Since the intake of Cd via consuming bivalves is only part of the total oral Cd intake, a significant increase (p < 0.05, ANOVA) of the THQ value from less than 1/5 to about 1/4 indicated a higher risk for consumers, although all the THQ values obtained in the present study were less than the critical value of 1 implying a low risk of non-carcinogenic effects. In particular, past studies have suggested that the health risk regarding Cd contamination is increasing due to consumption of other food, such as vegetables and fruits16. In addition, since cultured bivalves in farms are exposed to the environmental Cd contamination for a markedly longer period (at least a year for the three species investigated) than the 30 days duration of the present study, the health risk posed by marine bivalves consumptions under future ocean acidification scenario is expected to be more severe. Furthermore, with the increased risk of toxic metal contamination brought about by ocean acidification, it is highly likely that the sea areas suitable for bivalve aquaculture and capture will shrink significantly hence reducing the overall seafood supplies.
Methods
Collection and acclimation of bivalves
Adult T. granosa (9.5 ± 1.4 g), M. edulis (28.0 ± 5.2 g) and M. meretrix (50.0 ± 8.1 g) were collected from Yueqing, Wenzhou, China in August 2014. After cleaning off the epizoa, bivalve individuals were acclimatized in a 1000 L plastic tank at an ambient water temperature of 26 ± 3 °C and pH 8.07 ± 0.05 with flowing sand filtered seawater. The sample were fed with microalgae (Tetraselmis chui) at the satiation feed rate daily for 7 days prior to experiment.
Seawater acidification
The sand-filtered seawater used in the experiment was obtained from Qingjiang Bay, Zhejiang Province (28°28′N and 121°11′E) with pH at 8.07 ± 0.05, salinity at 20 ± 0.5‰ and the average background Cd concentration of 9.8 ± 0.2 μg/L. During the experiment, the bivalves were maintained under manipulated pCO2 conditions, with one ambient group at pH 8.1 (current concentration of pCO2) as the control and two experimental groups at pH 7.8 and 7.4 representing the pH values predicted by the Intergovernmental Panel on Climate Change (IPCC) to occur at 2100 and 2300, respectively3. The desired pH values were achieved by continuous aeration with ambient air or air-CO2 mixture into the filtered seawater in 60-L plastic tanks. The air-CO2 mixture was obtained by mixing dry CO2-free air and pure CO2 gas at known flow rates using flow controllers. The pH of each experimental trial was verified daily with a portable pH metre (Sartorius PB-10) to ensure there was no substantial pH change during the course of experiment.
Cadmium accumulation assay
The experiments were performed using analytical grade salts of Cd (NO3)2·4H2O. Stock solutions were prepared in deionized water at 1 M, a concentration high enough to prevent weighing errors and salinity fluctuation. The experimental Cd concentration (0.05 mg/L) was chosen on the basis of the reported safe concentrations of these bivalve species45,46,47.
After one week of acclimation, the bivalves (40 T. granosa, 15 M. edulis and 20 M. meretrix) were randomly assigned to plastic tanks with a total seawater volume of 20 L containing approximately 0.05 mg/L Cd and maintained in the three desired pCO2 conditions. Bivalves were fed with T. chui and the seawater was replaced daily with pH pre-adjusted seawater to maintain the desired pCO2 level. After seawater replacement, Cd was added to achieve the designed experimental Cd concentration in the water column. The Cd accumulation assay was conducted for a 30day duration.
Metal concentration analysis
Seawater samples were collected from each experimental trial every ten days to determine the effect of the CO2-driven acidification on the concentration of Cd2+ and Ca2+ in the water column. These water samples were stored in properly labelled preparation bottles at 4 °C and were used for subsequent Cd2+ and Ca2+ concentration analyses. The water samples (200 mL) were digested with 5 mL of a di-acid mixture (HNO3:HClO4 = 9:4) on a hot plate and filtered with a glass microfibre filter paper (Advantec Toyo) for the analysis of Cd2+ and Ca2+ using a flame atomic spectrophotometer (WFX-130A, Beijing Rayleigh Analytical Instruments Co., Ltd, China), according to the National Standard of China (GB 17378.4-2007, the section “seawater analysis” in “The speciation for marine monitoring”)48 at detection limits of 0.01 and 0.001 μg/L for Cd and Ca, respectively.
After exposure to Cd-contaminated seawater for 30 days at different pCO2 levels, five live individuals of each species were taken out for the Cd accumulation analysis. The individuals were dissected on ice and the gills, mantle and adductor muscles were peeled off and weighed separately. To obtain the dry mass, the different tissues were dehydrated in the oven to a constant weight at 75 °C. Dried tissues were first homogenized with a standard Teflon tissue homogenizer, followed by nitric acid digestion (1 g of each sample). Once the samples cooled down to room temperature, the sample digestions were filtered with a glass microfibre filter paper (Advantec Toyo) and diluted to 50 ml in volumetric flasks with deionized water. The concentrations of cadmium were then determined using a flame atomic spectrophotometer (WFX-130A), according to the National Standard of China (GB05009-15-2003)49 with a detection limit of 5 μg/kg. Three replicates were examined for each pCO2 level to obtain the average concentration. The Cd concentrations in the various tissues were then calculated and expressed in mg kg−1 dry weight. Similarly, after 30 days exposure, the entire soft body of M. meretrix was peeled off to determine Cd concentration (C) for THQ analysis. Cd concentrations in the whole soft body were then calculated and expressed in mg kg−1 wet weight.
Appropriate quality assurance procedures and precautions were carried out to ensure results reliability. Samples were carefully handled to avoid contamination, all the plastics and glasswares were cleaned by soaking in dilute HNO3 and then rinsed with distilled water prior to use and reagents of analytical reagent grade were used. A standard reference materials (GBW08571) obtained from the National Research Center for Standard Reference Materials (Beijing China) was used in the analysis to ensure measurement accuracy. A recovery experiment was carried out by spiking the already analyzed sample and recoveries were found to be within ±5% of certified values.
Expression analysis of pgp-5 gene
Total RNA of T. granosa was extracted from the gills, which were considered the main entry site for toxic metals in bivalves, with the RNAprep Pure Tissue Kit (Tiangen, DP431) according to the protocols provided by the manufacturer. RNA integrity was checked by gel electrophoresis and quantified spectrophotometrically with NanoDrop 1000 UV/visible spectrophotometer (Thermo Scientific). First strand cDNA was synthesized from high-quality total RNA using the M-MLV First Strand Kit (Invitrogen, C28025-032) following manufacturer’s instructions. Real-time quantitative PCR were conducted on the CFX96TM Real-Time System (Bio-Rad) in triplicates, in a total volume of 10 μL consisting of 5 μL of 2× Super Mix (Bio-Rad, 172-5201AP), 0.5 μL of each primer (10 μM), 1 μL of cDNA template and 3 μL of double-distilled water. The following amplification protocol was used: 95 °C for 5 min, followed by 40 cycles (94 °C for 20 sec, 61 °C for 20 sec and 72 °C for 20 sec). A melting curve analysis (MCA) was used to confirm the specificity and reliability of the PCR products. The 18S rRNA was employed as a reference for the calculation of the relative expression levels. The primers used are listed in Table 3 and all primers were synthesized by Sangon Biotech (Shanghai, China).
Health risk assessment
The EDI of Cd was determined by the equation: EDI = (EFr × ED × MS × C)/(WAB × TA), where EFr is the exposure frequency50 (350 day/year); ED is the exposure duration (70 years), which is equivalent to the average lifetime of adults51; MS is the average food meal size (21.43 g/person/day according to the dietary intake survey52); C is the obtained Cd concentration in the soft body of M. meretrix; WAB represents the average body weight53, adults (70 kg); and TA is the average exposure time for noncarcinogens (70 years × 365 day/year according to Wang et al.54). The THQ values of Cd through consumption of M. meretrix raised in different pCO2 levels were then estimated by the equation: THQ = EDI/RfD with the data obtained in the present study. Accoding to JECFA55, 0.83 × 10−3 μg/g/day was used as the oral intake reference dose (RfD).
Statistics analysis
One-way analysis of variances (one-way ANOVAs) followed by post-hoc Tukey tests were performed to compare the Cd levels within various tissues, the Ca2+ and Cd2+ concentrations of the seawater, EDI and THQ values at different pCO2 levels. The analyses were performed using the “R” statistical software packages (R Development Core Team, 2012), T-tests were conducted to detect whether there was a significant alteration in the gene expression compared to that of the control. All of the data are presented as mean ± SD and a p-value at p < 0.05 was taken as statistically.
Additional Information
How to cite this article: Shi, W. et al. Ocean acidification increases cadmium accumulation in marine bivalves: a potential threat to seafood safety. Sci. Rep. 6, 20197; doi: 10.1038/srep20197 (2016).
References
McInerney, F. A. & Wing, S. L. The Paleocene-Eocene thermal maximum: a perturbation of carbon cycle, climate and biosphere with implications for the future. Annu. Rev. Earth Planet. Sci. 39, 489–516 (2011).
Kurihara, H. & Shirayama, Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 274 (2004).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Gattuso, J. P., Frankignoulle, M., Bourge, I., Romaine, S. & Buddemeier, R. W. Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change 18, 37–46 (1998).
Miles, H., Widdicombe, S., Spicer, J. I. & Hall-Spencer, J. Effects of anthropogenic seawater acidification on acid-base balance in the sea urchin Psammechinus miliaris. Mar. Pollut. Bull. 54, 89–96 (2007).
Riebesell, U. et al. Reduced calcification of marine plankton in response to increased atmospheric CO2 . Nature 407, 364–367 (2000).
Havenhand, J. N., Buttler, F. R., Thorndyke, M. C. & Williamson, J. E. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 18, R651–R652 (2008).
Lannig, G., Eilers, S., Pörtner, H. O., Sokolova, I. M. & Bock, C. Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas—changes in metabolic pathways and thermal response. Mar. Drugs 8, 2318–2339 (2010).
Bibby, R., Widdicombe, S., Parry, H., Spicer, J. & Pipe, R. Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis. Aquat. Biol. 2, 67–74 (2008).
Talmage, S. C. & Gobler, C. J. Effects of past, present and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc. Natl. Acad. Sci. USA. 107, 17246–17251 (2010).
Pascal, P. Y., Fleeger, J. W., Galvez, F. & Carman, K. R. The toxicological interaction between ocean acidity and metals in coastal meiobenthic copepods. Mar. Pollut. Bull. 60, 2201–2208 (2010).
Kim, E., Jee, J., Steiner, H., Cormet-Boyaka, E. & Boyaka, P. Chronic exposure to cadmium alters gut immune homeostasis and innate immunity (MUC8P. 810). J. Immunol 192, 198.111–198.111 (2014).
Reichelt-Brushett, A. & Harrison, P. The effect of copper, zinc and cadmium on fertilization success of gametes from scleractinian reef corals. Mar. Pollut. Bull. 38, 182–187 (1999).
Inglot, P. et al. Cadmium-induced changes in genomic DNA-methylation status increase aneuploidy events in a pig Robertsonian translocation model. Mutat. Res.-Gen. Tox. En. 747, 182–189 (2012).
FAO. Yearbook: Fishery and Aquaculture Statistics 2012 (FAO, 2014).
Chen, C., Qian, Y. Z., Chen, Q. & Li, C. Y. Assessment of Daily Intake of Toxic Elements Due to Consumption of Vegetables, Fruits, Meat and Seafood by Inhabitants of Xiamen, China. J. Food Sci. 76, T181–T188 (2011).
Fang, Z., Cheung, R. Y. H. & Wong, M. H. Heavy metal concentrations in edible bivalves and gastropods available in major markets of the Pearl River Delta. J. Environ. Sci. 13, 210–217 (2001).
Millero, F. J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 22, 72 (2009).
Kikkawa, T., Kita, J. & Ishimatsu, A. Comparison of the lethal effect of CO2 and acidification on red sea bream (Pagrus major) during the early developmental stages. Mar. Pollut. Bull. 48, 108–110 (2004).
Haines, T. A. & Brumbaugh, W. G. Metal concentration in the gill, gastrointestinal tract and carcass of white suckers (Catostomus commersoni) in relation to lake acidity. Water Air Soil Poll. 73, 265–274 (1994).
Wiener, J. G., Martini, R. E., Sheffy, T. B. & Glass, G. E. Factors influencing mercury concentrations in walleyes in northern Wisconsin lakes. T. Am. Fish. Soc. 119, 862–870 (1990).
Lacoue-Labarthe, T. et al. Effects of ocean acidification on trace element accumulation in the early-life stages of squid Loligo vulgaris. Aquat. Toxicol. 105, 166–176 (2011).
Lacoue-Labarthe, T. et al. Effects of increased pCO2 and temperature on trace element (Ag, Cd and Zn) bioaccumulation in the eggs of the common cuttlefish, Sepia officinalis. Biogeosciences 6, 2561–2573 (2009).
Lacoue-Labarthe, T. et al. Temperature and pCO2 effect on the bioaccumulation of radionuclides and trace elements in the eggs of the common cuttlefish, Sepia officinalis. J. Exp. Mar. Bio. Ecol. 413, 45–49 (2012).
Gottesman, M. M. & Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62, 385–427 (1993).
Kurz, C. L., Shapira, M., Chen, K., Baillie, D. L. & Tan, M.-W. Caenorhabditis elegans pgp-5 is involved in resistance to bacterial infection and heavy metal and its regulation requires TIR-1 and a p38 map kinase cascade. Biochem. Biophys. Res. Commun. 363, 438–443 (2007).
Çoğun, H. Y. & Kargın, F. Effects of pH on the mortality and accumulation of copper in tissues of Oreochromis niloticus. Chemosphere 55, 277–282 (2004).
Wangersky, P. J. Biological control of trace metal residence time and speciation: a review and synthesis. Mar. Chem. 18, 269–297 (1986).
Hinkle, P. M., Kinsella, P. A. & Osterhoudt, K. C. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J. Biol. Chem. 262 (1987).
Boughammoura, S., Kessabi, K., Chouchene, L. & Messaoudi, I. Effects of cadmium and high temperature on some parameters of calcium metabolism in the killifish (Aphanius fasciatus). Biol. Trace Elem. Res. 154, 73–80 (2013).
Zhang, B., Shi, Z. R., Wang, X. L., Deng, S. G. & Lin, H. M. Depuration of cadmium from blue mussel (Mytilus edulis) by hydrolysis peptides and chelating metal elements. Food Res. Int. (2015).
Ortega, P., Custódio, M. & Zanotto, F. Characterization of cadmium plasma membrane transport in gills of a mangrove crab Ucides cordatus. Aquat. Toxicol. 157, 21–29 (2014).
Li, Z. H., Li, P. & Randak, T. Protective roles of calcium channel blocker against cadmium-induced physiological stress in freshwater teleost Oncorhynchus mykiss. Water Air Soil Poll. 220, 293–299 (2011).
Roesijadi, G. & Unger, M. Cadmium uptake in gills of the mollusc Crassostrea virginica and inhibition by calcium channel blockers. Aquat. toxicol. 24, 195–205 (1993).
Wang, W. X. & Fisher, N. S. Effects of calcium and metabolic inhibitors on trace element uptake in two marine bivalves. J. Exp. Mar. Biol. Ecol. 236, 149–164 (1999).
Hollis, L., McGeer, J. C., McDonald, D. G. & Wood, C. M. Protective effects of calcium against chronic waterborne cadmium exposure to juvenile rainbow trout. Environ. Toxicol. Chem. 19, 2725–2734 (2000).
Bjerregaard, P. & Depledge, M. H. Cadmium Accumulation in Littorina littorea, Mytilus edulis and Carcinus maenas: the Influence of Salinity and Calcium Ion Concentrations. Mar. Biol. 119, 385–395 (1994).
Lucu, Č. & Obersnel, V. Cadmium influx across isolated Carcinus gill epithelium interaction of lanthanum and calcium with cadmium influxes. J. Comp. Physiol. B 166, 184–189 (1996).
Jezierska, B. & Witeska, M. In Soil and Water Pollution Monitoring, Protection and Remediation 107–114 (Springer, 2006).
Winston, G. W., Moore, M. N., Kirchin, M. A. & Soverchia, C. Production of reactive oxygen species by hemocytes from the marine mussel, Mytilus edulis: lysosomal localization and effect of xenobiotics. Comp. Biochem. Phys. C 113, 221–229 (1996).
Roberts, D. A. et al. Ocean acidification increases the toxicity of contaminated sediments. Global Change Biol. 19, 340–351 (2013).
Beesley, A., Lowe, D. M., Pascoe, C. K. & Widdicombe, S. Effects of CO2-induced seawater acidification on the health of Mytilus edulis. Clim. Res. 37, 215–225 (2008).
Evans, T. G. & Watson-Wynn, P. Effects of Seawater Acidification on Gene Expression: Resolving Broader-Scale Trends in Sea Urchins. Biol. Bull. 226, 237–254 (2014).
Schmidhuber, J. & Tubiello, F. N. Global food security under climate change. Proc. Natl. Acad. Sci. USA. 104, 19703–19708 (2007).
Liu, G. X., Chai, X. L., Shao, Y. Q. & Wu, H. X. Specific death symptoms and organic lesions of blood clam Tegillarca granosa in acute copper, zinc, lead and cadmium exposures. Adv. Mater. Res. 518–523, 490–493 (2012).
Coles, J. A., Farley, S. R. & Pipe, R. K. Alteration of the immune response of the common marine mussel Mytilus edulis resulting from exposure to cadmium. Dis. Aquat. Organ. 22, 59–65 (1995).
Wang, Q., Liu, B., Yang, H., Wang, X. & Lin, Z. Toxicity of lead, cadmium and mercury on embryogenesis, survival, growth and metamorphosis of Meretrix meretrix larvae. Ecotoxicology 18, 829–837 (2009).
State Oceanic Administration (SOA) of China. The specification for marine monitoring, GB 17378.4-2007 (Standards Press of China, 2007).
Standardization Administration of China (SAC). China National Standards Compilation, GB05009-15 (Standards Press of China, 2003).
Hu, J. et al. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere 91, 455–461 (2013).
Bennett, D., Kastenberg, W. & McKone, T. A multimedia, multiple pathway risk assessment of atrazine: the impact of age differentiated exposure including joint uncertainty and variability. Reliab. Eng. Syst. Safe. 63, 185–198 (1999).
Copat, C. et al. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: consumption advisories. Food. Chem. Toxicol. 53, 33–37 (2013).
Abdallah, M. A. Bioaccumulation of heavy metals in mollusca species and assessment of potential risks to human health. B. Environ. Contam. Tox. 90, 552–557 (2013).
Wang, X., Sato, T., Xing, B. & Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 350, 28–37 (2005).
JECFA. Evaluation of certain food additives and the contaminants: Seventy-third report of the joint FAO/WHO expert committee on food additives (WHO, 2010).
Acknowledgements
This work was funded by National Natural Science Foundation of China (No. 31372503), Open Fund of Key Laboratory of Exploration and Preservation of Costal Bio-resources of Zhejiang (No. J2013003, J2015002) and Open Fund of Key Laboratory for Ecological and Environment in Coastal Areas, State Oceanic Administration (No. 201603) awarded to G Liu.
Author information
Authors and Affiliations
Contributions
W.S., Y.H., X.G.Z. and G.X.L. contributed to all aspects of the paper, including study design, statistical analysis and writing and revisions. Z.M.C. and X.L.C. contributed to the design of the study, to substantive analysis of the results and to revisions of the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shi, W., Zhao, X., Han, Y. et al. Ocean acidification increases cadmium accumulation in marine bivalves: a potential threat to seafood safety. Sci Rep 6, 20197 (2016). https://doi.org/10.1038/srep20197
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20197
This article is cited by
-
Lead in the marine environment: concentrations and effects on invertebrates
Ecotoxicology (2022)
-
Pre-to-post COVID-19 lockdown and their environmental impacts on Ghoghla beach and Somnath beach, India
Environmental Science and Pollution Research (2022)
-
Cadmium removal for marine food application: comparative study of different adsorbents
International Journal of Environmental Science and Technology (2022)
-
Determination of cadmium induced acute and chronic reproductive toxicity with Raman spectroscopy
Lasers in Medical Science (2020)
-
Transcriptional analysis reveals physiological response to acute acidification stress of barramundi Lates calcarifer (Bloch) in coastal areas
Fish Physiology and Biochemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.