Abstract
Titanium dioxide (TiO2) is an attractive anode material for energy storage devices due to its low-volume-change and high safety. However, TiO2 anodes usually suffer from poor electrical and ionic conductivity, thus causing dramatic degradation of electrochemical performance at rapid charge/discharge rates, which has hindered its use in energy storage devices. Here, we present a novel strategy to address this main obstacle via using nanoarchitectured TiO2 anode consisting of mesoporous TiO2 wrapped in carbon on a tunnel-like etched aluminum substrate prepared by a simple one-step approach. As a result of this nanoarchitecture arrangement, the anode exhibits excellent rate performance and superior cyclability. A rate up to 100 C is achieved with a high specific capacity of about 95 mA h g−1 and without apparent decay after 8,000 cycles.
Similar content being viewed by others
Introduction
Lithium-ion batteries (LIBs) have been widely applied in portable electronic devices due to their superior properties such as high energy density, light weight and low toxicity1,2,3,4,5,6. Titanium dioxide (TiO2), as a promising high-performance anode for LIBs, has attracted increasing attention in recent years because of its low volume expansion, low cost as well as non-toxicity7,8,9,10,11. Moreover, the relatively high lithium insertion/extraction voltage (higher than 1.5 V vs. Li/Li+) can efficiently avoid the formation of SEI layers and dendritic lithium, improving the batteries safety compared to its carbon counterparts1,8,9,12,13. However, low electrical conductivity (ca. 10−12–10−7 S cm−1) and Li+ diffusivity (ca. 10−15–10−9 cm2 s−1) hinder its practical application in high-power LIBs12,14,15,16,17,18. To address these issues, shortening the characteristic dimensions of TiO2 or/and coating TiO2 with conductive layer have been widely considered as efficient approaches for enhancing ion and electron transport kinetics in batteries. And various strategies have been developed so far, such as exploiting TiO2 with a variety of nanostructures (e.g., nanosphere19, nanowire20, nanotube21, nanoribbon22, etc.), preparing hybrid with carbon-based materials (e.g., TiO2/C23, TiO2/CNTs24, TiO2/graphene12, TiO2/fullerene25, etc.). Unfortunately, the application of these nanoscaled TiO2 to specific areas always suffers from low volumetric energy density, poor inter-particle contacts and self-aggregation upon deep cycling. In this regard26, mesoporous TiO2 is proposed to offer a clever solution to achieve high volumetric energy densities for LIBs. Nevertheless, only modest improvements in rate performance have been observed, because the mesoporous structures primarily address ion but not electron transport27. More efforts, therefore, are urgently needed to develop integrated electrode including active materials and architecture substrate.
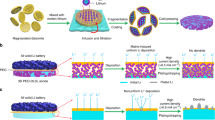
A perfect electrode with excellent rate performance and high capacity requires the simultaneous minimization of five resistances present during charging/discharging (Fig. 1c): (1) ion transport in the electrolyte, (2) ion transport in the electrode, (3) electrochemical reactions in the electrode, (4) electron transport in the electrode and (5) electron conduction in the current collector27. Comparing with conventional electrodes (Fig. 1a), nanoarchitectured electrode with a three-dimensional interpenetrating network of hybrid nanoscaled active materials and nano- or micro- structured current collectors seems to be a promising avenue to meet these requirements, as stated below27,28,29,30. First, hybrid nanoscaled active materials can evidently shorten the ion diffusion length and offer tremendous active surface areas, which benefits (2) and (3). Second, nano- or micro- structured current collectors are capable of providing efficient pathways for ion and electron transport through the entire electrode architecture, which is conductive to (1), (4) and (5). In addition, nano- or micro- structured current collectors have much larger surface areas and less mass than the planar bulky ones do, which dramatically increases the loading of active materials and decreases the total mass of the electrode, thus improving the volumetric and gravimetric energy density. Since the pioneering work by Taberna in 200631, the fabrication of these structures, such as coaxial nanoarrays32, metal foam33, porous metals34, conducting polymer scaffolds35 and inverse opal matrix27, has drawn worldwide interest. In most of the previous reports, however, the fabrication usually required somewhat complex chemical and physical processes and cost a lot, which is not in favor of the large-scale manufacture.
Here, we present a novel strategy for the fabrication of nanoarchitectured electrode consisting of mesoporous TiO2 wrapped by carbon on a tunnel-like etched aluminum foil (Fig. 1b) via an inexpensive one-step approach. The etched aluminum foil is chosen as the structure support for the electroactive TiO2 because of its large surface area (0.3–1.5 m2 g−1), high conductivity (1–3 × 107 S m−1), excellent mechanical flexibility (tensile strength: 20–30 MPa; bending strength: 40–80 round cm−1), low cost (15–25 $ Kg−1) and commercial availability. The preparation process is schematically illustrated in Fig. 1d. To start with, a TiO2 precursor was prepared via mixing tetrabutyl titanate and isopropyl alcohol. Trace of hydrogen fluoride was also added into the precursor to eliminate the oxide on aluminum foil and improve electrical contact of TiO2 with support. The etched aluminum foils were then dipped into the obtained precursor for anchoring TiO2. After calcined in atmosphere at temperature of 400, 450, 500 °C, respectively, for 10 mins, a nanoarchitectured TiO2 electrode was obtained (denoted as TO/Al-400, TO/Al-450, TO/Al-500 or TO/Al hereafter). Finally these electrodes were directly used as binder-free anodes in LIBs.
Results and Discussion
The field-emission scanning electron microscope (FE-SEM) images of etched Al substrate are shown in Fig. 2a,b and Figure S1 (see the supporting information). It is observed that the etched Al substrate is porous framework made of tunnel-like hole with uniform diameter of about 1 μm and length of tens of micrometers. These holes perpendicular to the surface benefits the transport of electrolyte. After dip-coating and calcination, the Al substrates are covered by a layer of TiO2. FE-SEM examination (Fig. 2d,e,g,h,j,k) shows that TiO2 are uniformly anchored onto the Al substrate to form a dense layer with the thickness of about 100 nm. The thickness changes few with the calcination temperature, which agrees well with the tiny weight loss in Figure S2b. Energy-dispersive X-ray spectroscopy (EDX) measurement indicates the presence of Ti, O, C and Al elements after dip-coating (see Fig. 2f,i,l). There is no F peak observed because of the trace amount of fluorine. However, a very weak peak of F 1s can be observed for TO/Al-400, TO/Al-450 and TO/Al-500 samples via X-ray photoelectron spectroscopy (XPS), which has higher detection sensitivity (as Figure S3(a)).
Grazing incidence X-ray diffraction (GIXRD) analysis in Fig. 3a shows that after annealing, the deposited TiO2 converts into crystal TiO2 identified as anatase (JCPDS card No. 01-071-1167), as characterized by the appearance of peaks from (101), (004), (200), (105), (211), (204), (116), (220) and (215). And these peaks become intensive and sharp with increasing annealing temperature, which indicates the crystallinity of TiO2 is improved. The Raman spectra in the range of 30–800 cm−1 (see Fig. 3b) show the normal modes of anatase at 166, 212, 405, 522 and 643 cm−1, assigned to Eg, Eg, B1g, A1g and Eg modes, respectively36,37. The intensity of Eg(1) increases with increasing annealing temperature, indicating the crystallinity becomes better, which corresponds to the result of GIXRD. The Raman spectra in the range of 1000–1800 cm−1 are also collected to detect the residual carbon in TiO2 film and presented in Fig. 3c. There exist two bands at ~1380 cm−1 and ~1590 cm−1, named D band and G band, respectively. The D band corresponds to disordered carbon or defective graphitic structure, while the G band, identified as the tangential vibration of carbon atoms, is a characteristic feature of graphitic layers38,39. It is shown, from Fig. 3c, that these samples contain the carbon with partial graphitization, which is highly desirable for the application as electrode material due to its high conductivity. The amount of this kind of carbon varies with the annealing temperature. Before 500 °C, the intensity of D and G band is high, meaning a lot of carbon remained in samples. However, the intensity of these two bands decreases dramatically when annealed at 500 °C, indicating the amount of residuary carbon drops sharply. This is caused by combustion of carbon as shown in Figure S2c. The optical images of these samples (Fig. 3d) display that the color of TO/Al-400 and TO/Al-450 is darker than that of TO/Al-500, which suggests that more carbon exists in TO/Al-400 and TO/Al-450 comparing to TO/Al-500. The content of carbon in these samples is calculated via Equation S1-S4. The results reveal that the percentage of carbon in TO/Al-400, TO/Al-450 and TO/Al-500 is 50–53%, 44–49% and 21–28%, respectively. Here it is important to note that in all samples Ti element exists as the form of TiO2 because there is only + 4 Ti in TO/Al samples demonstrated by XPS measurement, as shown in Figure S3(b).
(a) GIXRD patterns of TO/Al-400, TO/Al-450, TO/Al-500 along with reference patterns of TiO2 anatase phase (JCPDS 01-071-1167) and Al (JCPDS 01-089-4037); (b,c) Raman spectra of blank Al substrate, TO/Al-400, TO/Al-450 and TO/Al-500 in the range of 0-800 cm−1 and 1000-1800 cm−1, respectively; (d) optical images of blank Al substrate, TO/Al-400, TO/Al-450 and TO/Al-500.
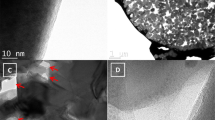
Closer observations by FE-SEM and transmission electron microscope (TEM) on morphology and structure of the anchored TiO2 film are given in Fig. 4 and Figure S4. A high-magnification SEM image (Fig. 4a) exhibits the TiO2 film is porous, which is further manifested by TEM measurements as shown in Fig. 4b. High angle annular dark field-scanning transmission electron microscope (HAADF-STEM) image (Fig. 4c and Figure S4) discovers typical mesoporous characteristics with a narrow distribution in range of 1–10 nm in the TiO2 film, which is consistent with the result of Brunner-Emmet-Teller (BET) measurement (Figure S5). EDX mapping (Fig. 4d–g) detects a large amount of carbon distributed within the TiO2 film. High-resolution TEM (HRTEM) (Fig. 4h–j) analysis reveals TiO2 film possesses a nanonetwork in which TiO2 nanocrystals are wrapped by carbon. With the increase in annealing temperature, the amount of carbon decreases and more anatase TiO2 appears. The lattice fringes with a distance of 0.237 nm are observed in Fig. 4h–j, corresponding to the (004) interplane spacing of anatase.
To evaluate the LIBs applications of these unique TO/Al binder-free electrodes, the electrochemical performance was investigated. Figure 5a shows the cyclic voltammograms (CV) of the TO/Al electrodes annealed at different temperature at a slow scan rate of 0.1 mV s−1. A pair of cathodic/anodic peaks centered at about 1.72/2.02 V, corresponding to the lithium insertion/extraction in anatase lattice, can be observed. The gap between anode and cathode peaks is 0.30, 0.30 and 0.39 V for TO/Al-400, TO/Al-450 and TO/Al-500, respectively, indicating weaker polarization in TO/Al-400 and TO/Al-450 than that in TO/Al-500. Electrochemical impedance spectra (EIS) show the charge transfer resistance increases in order of TO/Al-450, TO/Al-400 and TO/Al-500 (see Figure S6 and Table S1). This attributes to the different amount of carbon and various crystallinity of TiO2. The CV curves at different scan rate indicates that the peak current density perfectly scales with the square root of scan rate (Figure S7), evidencing a semi-infinite diffusion process in the TiO2 film at high current rate. According to Randles-Sevick equation13,40, the Li+ apparent chemical diffusion coefficient increases in order of TO/Al-400 (1.47 × 10−13 cm2 s−1) <TO/Al-500 (2.61 × 10−13 cm2 s−1) <TO/Al-450 (4.45 × 10−13 cm2 s−1). Figure 5b presents the initial discharge-charge voltage profiles at a current density of 0.3 C (where 1C rate represents one-hour completely charge or discharge the practical capacity determined experimentally) within a cut-off voltage window of 1.0-3.0 V. The initial discharge/charge capacities are found to be 730/328, 632/335 and 473/270 mA h g−1 (based on the mass of TiO2) for TO/Al-400, TO/Al-450 and TO/Al-500, respectively. The irreversible capacity in the first cycle is mainly due to the interfacial reaction between TiO2 and the electrolyte, which is common in most lithium intercalation hosts7,41. As annealing temperature rises, the coulombic efficiency is improved, which maybe owns to the loss of H2O and O-H groups on the surface of TiO2 caused by the heat (see Figure S8 and Table S2). After couple cycles, nonetheless, the coulombic efficiency can be raised to nearly 100%, as shown in Figure S9a. From the second cycle onwards, the TiO2 electrodes show good reversibility. And at the end of 200 discharge-charge cycles, a reversible capacity of 317 (TO/Al-400), 316 (TO/Al-450) and 233 (TO/Al-500) mA h g−1 can be retained. In present work, the electrochemical performance of blank Al substrate and pyrolytic carbon on Al substrate were also measured and the results are shown in Fig. 5a, Figure S10 and S11. Within operating voltage window of 1.0–3.0 V, the capacity contributed by Al (<1 mA h g−1) or pyrolytic carbon (<12 mA h g−1) is negligible because the lithium intercalation within Al or carbon mainly occurs at potential far below 1.0 V42,43.
Electrochemical performance of TO/Al-400, TO/Al-450 and TO/Al-500 in the voltage window of 1–3 V.
(a) CV curves of blank Al substrate, TO/Al-400, TO/Al-450 and TO/Al-500 at scan rate of 0.1 mV s−1; (b) initial charge/discharge curves at a current rate of 0.3 C; (c), rate capability tests from 0.3 to 100 C; (d) cycle performance at a current rate of 100 C after 0.3 C for the first 3 cycles.
Benefiting from the unique structure, the TiO2 electrode exhibits excellent rate performance, as shown in Fig. 5c. The results reveal that TO/Al-400 and TO/Al-450 present better reversibility and higher capacity than that of TO/Al-500 at different current rates. This mainly profits from the high amount of carbon. Compared with TO/Al-400, TO/Al-450 shows more dominant performance especially at high discharge/charge rates. For example, TO/Al-450 electrode delivers charge capacity of 153 mA g−1 at 20 C, 117 mA g−1 at 50 C and 95 mA g−1 at 100 C, whereas the values for TO/Al-400 are 141 mA g−1, 99 mA g−1 and 78 mA g−1, respectively. This is possibly caused by the better crystallinity of TO/Al-450. Importantly, after more than forty cycles tested under various current rates even up to 100 C, the capacities of TO/Al electrodes can recover to the initial value at different current rates, indicating its high reversibility of lithium ion insertion/extraction in the electrode. The specific energy density and specific power density calculated from discharge profiles are also displayed as the Ragone plot as shown in Figure S12. Apparently, the maximum specific energy density of 141 Wh kg−1 is achieved for our TO/Al-450 sample at highest specific power density of 14.1 kW kg−1. Further, long cycling performance at high current rates of 20, 50 and 100 C are also investigated and shown in Fig. 5d and Figure S9b,c. The TO/Al electrodes exhibits excellent stability and superior cyclability and it, even after 8000 cycles at 100 C, still affords a remarkable capacity of 97 mA h g−1 for TO/Al-450, which is better than TO/Al-500 (60 mA h g−1) and TO/Al-400 (81 mA h g−1). This can be explained that TO/Al-450 has high amount of carbon as well as good crystallinity. These exciting performances are better than most of the reports on nano-TiO2.
Clearly, the exceptional electrochemical performance of TO/Al electrode is originated from the unique structure merits. First, mesoporous TiO2 offers infinite open channels to facilitate the Li+ ion transport and huge surface area (The surface areas of TiO2 and carbon deposited on Al substrate after calcined at 400, 450 and 500 °C is about 85, 81 and 67 m2 g−1, respectively. see Figure S5) to favor the Li+ ion insertion/extraction. Second, the ultrathin mesoporous structure greatly shortens the ionic diffusion length and improves the architecture stability. Third, the etched Al substrate has numerous tunnel-like holes which provides efficient pathways for electrolyte transport through the whole electrode and evidently shortens the electronic transport distance. Fourth, the 3D network of TiO2 wrapped by carbon not only enhances the electronic conductivity of the electrode, but also restrains the aggregation of titanium clusters and maintains the nanostructure during cycles. The last, the introduction of trace HF maybe give rise to a better electrical contact and lower internal resistances via cleaning the Al surface, resulting in an excellent rate performance (Figure S13).
Conclusions
In summary, we have presented a simple strategy to fabricate high-rate rechargeable anodes. As-prepared 3D nanoarchitectured electrode provides numerous pathways for ions and electrons transport as well as extremely shortened ionic/electronic diffusion length. Moreover, the processes do not involve any complex equipment and are entirely compatible with many battery chemistries. The approach may even enable the use of those materials with low ion and electron conductivities because of the short diffusion length provided by nanoarchitectured electrode. However, further research is needed to increase the loading mass of active material and to reduce the content of carbon in active material, promoting the application of this approach to practical batteries. A viable route to this end could be to adjust the diameter and length of pore in Al substrate and to use Ti precursor with less carbon content. We believe that the nanoarchitecture concept we have described may hold great promise for the development of LIBs with high power and energy densities and can be extended as a general approach to other systems such as supercapacitors.
Methods
Material
All chemicals were of analytical grade and used as received without further purification. Tetrabutyl titanate (TBT), isopropanol, sodium hydroxide, hydrochloric acid and hydrofluoric acid were purchased from Sinopharm Chemical Reagent Co., Ltd., China. Deionized water was prepared by Milli-Q-Reference water system (Millipore Co., USA). High purity (99.99%) commercially etched aluminum foils with tunnel-like pores (110 μm, 630 V/0.61 μF cm−2, Zhaoqing Huafeng Co. Ltd, China) were used as substrates.
Electrode Preparation
The binder-free electrodes were prepared by dip-coating using TiO2 precursor, followed by heat-treatment in air, as illustrated in Fig. 1d. Typically, TBT was firstly dissolved in isopropanol solution containing 2.5 mmol L−1 hydrogen fluoride to form a homogenous precursor with a molar ratio of 500 mmol L−1. Then, tunnel-like Al substrates were conducted to dip into the above solution for deposition of TiO2. After which, the resultant samples were rapidly pushed into tube furnace at desired temperature (400, 450 and 500 °C, respectively). After 10 min, they were pulled out immediately and put in atmosphere, a nanoarchitectured TiO2 electrodes were obtained. These electrodes were directly used as binder-free anodes in LIBs.
Material Characterization
The morphology and composition of samples were investigated with field-emission scanning electron microscope (FE-SEM, Quanta 250FEG, FEI, USA) and TEM (JEM-2100, JEOL, Japan). In order to detect the morphology of TiO2 deposited on the surface of Al by TEM, TO/Al electrodes were first eroded with 5 mol·L−1 sodium hydroxide to obtain TiO2 powder as shown in Figure S14. The crystalline structure of TO/Al electrodes was characterized by X-ray diffraction (XRD, X’Pert PRO, PANalytical, Holland) and Raman spectroscopy (Jobin Yvon LabRAM HR800, ENS, Lyon, France). TiO2 evolution on Al substrate during heat-treatment process was investigated with thermogravimetry-differential scanning calorimetry (TG-DSC, TGA/DSC 1, METTLER TOLEDO, Switzerland). The OH group on the surface of TiO2 was detected by Fourier transforms infrared (FTIR, Avatar 360 FTIR ESP, Thermo Nicolet, USA). The elemental chemical state of the samples was examined via X-ray photoelectron spectroscopy (XPS, AXIS ULTRABLD, Kratos, Japan). The surface area and pore-size distribution were measured by Brunner-Emmet-Teller (BET, ASAP 2020, Micromeritics, USA). The content of titanium element in TO/Al samples was detected by an inductively coupled plasma-optical emission spectrometry (ICP-OES, 710, Agilent, USA) after dissolving TO/Al sample in the solution containing 1 mol L−1 hydrochloric acid and 0.1 mol L−1 hydrofluoric acid. The mass of samples was weighed by high-precision analytical balance (XS105DU, METTLER TOLEDO, Switzerland).
Electrochemical Measurements
For the galvanostatic charging-discharging tests, a CR2016 coin-type cell was assembled in argon-filled glove box (Super(1225/750), MIKROUNA, China). In which Al foil loaded with TiO2 was directly used as working electrodes, lithium foil was used as the counter and reference electrodes and a solution of 1.0 M LiPF6 in ethylene carbonate: diethyl carbonate (EC:DEC = 1:1 by weight) was used as the electrolyte. All the tests were conducted on a battery tester (CT2001A, LAND, China; BT2000, Arbin, USA) within 1.0–3.0 V at various current rates. The mass of TiO2 loaded was 0.7–1.0 mg cm−2 (the mass ratio between TiO2 and the Al current collector was 1:30-1:20). The specific capacities are calculated based on the mass of TiO2 in TO/Al samples. The electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) study were carried out on an electrochemical workstation (VMP2, Princeton, USA) in a three-configuration, with TO/Al as the working electrode and Li foil as both reference and counter-electrodes.
Additional Information
How to cite this article: Xianfeng, D. et al. One-step Preparation of Nanoarchitectured TiO2 on Porous Al as Integrated Anode for High-performance Lithium-ion Batteries. Sci. Rep. 6, 20138; doi: 10.1038/srep20138 (2016).
References
Mo, R., Lei, Z., Sun, K. & Rooney, D. Facile Synthesis of Anatase TiO2 Quantum- Dot/GrapheneNanosheet Composites with Enhanced Electrochemical Performance for Lithium-Ion Batteries. Adv. Mater. 26, 2084–2088 (2014).
Wang, Y. & Cao, G. Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv. Mater. 20, 2251–2269 (2008).
Scrosati, B., Hassoun, J. & Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy & Environmental Science 4, 3287–3295 (2011).
Wang, D. et al. Self-assembled TiO2-graphene hybrid nanostructures for enhanced Li-ion insertion. ACS Nano 3, 907–914 (2009).
Liu, C., Li, F., Ma, L.-P. & Cheng, H.-M. Advanced materials for energy storage. Adv. Mater. 22, E28–E62 (2010).
Jeong, G., Kim, Y.-U., Kim, H., Kim, Y.-J. & Sohn, H.-J. Prospective materials and applications for Li secondary batteries. Energy & Environmental Science 4, 1986–2002 (2011).
Liu, S. et al. A Flexible TiO2(B)-based battery electrode with superior power rate and ultralong cycle life. Adv. Mater. 25, 3462–3467 (2013).
Yang, Z. et al. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 192, 588–598 (2009).
Zhu, G.-N., Wang, Y.-G. & Xia, Y.-Y. Ti-based compounds as anode materials for Li-ion batteries. Energy & Environmental Science 5, 6652–6667 (2012).
Ye, J. et al. Nanoporous anatase TiO2 mesocrystals: Additive-free synthesis, remarkable crystalline-phase stability and improved lithium insertion behavior. J. Am. Chem. Soc. 133, 933–940 (2011).
Wang, K., Wei, M., Morris, M. A., Zhou, H. & Holmes, J. D. Mesoporous titania nanotubes: Their preparation and application as electrode materials for rechargeable lithium batteries. Adv. Mater. 19, 3016–3020 (2007).
Yang, S., Feng, X. & Muellen, K. Sandwich-like, graphene-based titania nanosheets with high surface area for fast lithium storage. Adv. Mater. 23, 3575–3579 (2011).
Yan, X. et al. Synthesis and optimizable electrochemical performance of reduced graphene oxide wrapped mesoporous TiO2 microspheres. Nanoscale 6, 4108–4116 (2014).
Zhao, L., Hu, Y.-S., Li, H., Wang, Z. & Chen, L. Porous Li4Ti5O12 coated with N-doped carbon from ionic liquids for Li-ion batteries. Adv. Mater. 23, 1385–1388 (2011).
Guo, Y.-G., Hu, Y.-S., Sigle, W. & Maier, J. Superior electrode performance of nanostructured mesoporous TiO2 (anatase) through efficient hierarchical mixed conducting networks. Adv. Mater. 19, 2087–2091 (2007).
Zhen, M., Su, L., Yuan, Z., Liu, L. & Zhou, Z. Well-distributed TiO2 nanocrystals on reduced graphene oxides as high-performance anode materials for lithium ion batteries. Rsc Advances 3, 13696–13701 (2013).
Song, T. et al. TiO2 nanotube branched tree on a carbon nanofiber nanostructure as an anode for high energy and power lithium ion batteries. Nano Research 7, 491–501 (2014).
Liu, J. et al. Anatase-TiO2/CNTs nanocomposite as a superior high-rate anode material for lithium-ion batteries. J. Alloys Compd. 603, 144–148 (2014).
Wang, H.-E. et al. Facile synthesis and electrochemical characterization of porous and dense TiO2 nanospheres for lithium-ion battery applications. J. Power Sources 196, 6394–6399 (2011).
Armstrong, G., Armstrong, A. R., Bruce, P. G., Reale, P. & Scrosati, B. TiO2(B) nanowires as an improved anode material for lithium-ion batteries containing LiFePO4 or LiNi0.5Mn1.5O4 cathodes and a polymer electrolyte. Adv. Mater. 18, 2597–2600 (2006).
Lan, Y. et al. Titanate nanotubes and nanorods prepared from rutile powder. Adv. Funct. Mater. 15, 1310–1318 (2005).
Kim, S.-W. et al. Fabrication and electrochemical characterization of TiO2 three-dimensional nanonetwork based on peptide assembly. ACS Nano 3, 1085–1090 (2009).
Fu, L. J. et al. Synthesis and electrochemical performance of novel core/shell structured nanocomposites. Electrochem. Commun. 8, 1–4 (2006).
Huang, H., Zhang, W. K., Gan, X. P., Wang, C. & Zhang, L. Electrochemical investigation of TiO2/carbon nanotubes nanocomposite as anode materials for lithium-ion batteries. Mater. Lett. 61, 296–299 (2007).
Kavan, L. Nanomaterials based on carbon and Ti(IV) oxides: some aspects of their electrochemistry. International Journal of Nanotechnology 9, 652–679 (2012).
Saravanan, K., Ananthanarayanan, K. & Balaya, P. Mesoporous TiO2 with high packing density for superior lithium storage. Energy & Environmental Science 3, 939–948 (2010).
Zhang, H., Yu, X. & Braun, P. V. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 6, 277–281 (2011).
Jiang, J. et al. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 24, 5166–5180 (2012).
Long, J. W., Dunn, B., Rolison, D. R. & White, H. S. Three-dimensional battery architectures. Chem. Rev. 104, 4463–4492 (2004).
Rolison, D. R. et al. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 38, 226–252 (2009).
Taberna, P. L., Mitra, S., Poizot, P., Simon, P. & Tarascon, J. M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 5, 567–573 (2006).
Bazin, L. et al. High rate capability pure Sn-based nano-architectured electrode assembly for rechargeable lithium batteries. J. Power Sources 188, 578–582 (2009).
Xiang, J. Y. et al. Electrochemical performances of nanostructured Ni3P-Ni films electrodeposited on nickel foam substrate. J. Power Sources 185, 519–525 (2008).
Cheah, S. K. et al. Self-supported three-dimensional nanoelectrodes for microbattery applications. Nano Lett. 9, 3230–3233 (2009).
Guo, J. & Wang, C. A polymer scaffold binder structure for high capacity silicon anode of lithium-ion battery. Chem. Commun. 46, 1428–1430 (2010).
Giarola, M. et al. Vibrational dynamics of anatase TiO2: Polarized Raman spectroscopy and ab initio calculations. Physical Review B 81, 174305 (2010).
Tian, F., Zhang, Y., Zhang, J. & Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C 116, 7515–7519 (2012).
Dresselhaus, M. S., Jorio, A., Hofmann, M., Dresselhaus, G. & Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 10, 751–758 (2010).
Li, Z. et al. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy & Environmental Science 6, 871–878 (2013).
Wu, M.-S. et al. Electrochemical fabrication of anatase TiO2 nanostructure as an anode material for aqueous lithium-ion batteries. J. Power Sources 185, 1420–1424 (2008).
Wang, Z., Zhou, L. & Lou, X. W. Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 24, 1903–1911 (2012).
Hamon, Y. et al. Aluminum negative electrode in lithium ion batteries. J. Power Sources 97-8, 185–187 (2001).
Kida, Y., Yanagida, K., Funahashi, A., Nohma, T. & Yonezu, I. Electrochemical characteristics of graphite, coke and graphite/coke hybrid carbon as negative electrode materials for lithium secondary batteries. J. Power Sources 94, 74–77 (2001).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Granted No. 50902109), the Natural Science Foundation of Shaanxi Province (Granted No. 2014JM6231) and the Fundamental Research Funds for the Central Universities (Granted No. XJJ2012076). The authors would like to thank Mr. Chuansheng Ma and Mis Juan Feng for their help during TEM and FE-SEM measurements.
Author information
Authors and Affiliations
Contributions
X.D. conceived the concept and designed experiments. Q.W. carried out the synthesis and performed electrochemical measurements. T.F. and X.C. participated in part of the synthesis and electrochemical measurements. L.L. and L.L. conducted FE-SEM characterization. X.M. participated in part of cell assembly. L.X. and X.S. discussed and analyzed the data of measurements. L.L. contributed TEM characterization. X.D. co-wrote the paper. All authors discussed the results and commented on the manuscript. Y.X. supervised the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Du, X., Wang, Q., Feng, T. et al. One-step Preparation of Nanoarchitectured TiO2 on Porous Al as Integrated Anode for High-performance Lithium-ion Batteries. Sci Rep 6, 20138 (2016). https://doi.org/10.1038/srep20138
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20138
This article is cited by
-
Diffusion controlled electrochemical analysis of MoS2 and MOF derived metal oxide–carbon hybrids for high performance supercapacitors
Scientific Reports (2023)
-
Preparation of TiO2/Fe2O3/Al2O3 nanocomposite on rapidly solidified Ti–10Fe–6Al alloy ribbons and their application in supercapacitors
Journal of Materials Science: Materials in Electronics (2018)
-
TiO2-water nanofluid in a porous channel under the effects of an inclined magnetic field and variable thermal conductivity
Applied Mathematics and Mechanics (2018)
-
Improved electrochemical properties of morphology-controlled titania/titanate nanostructures prepared by in-situ hydrothermal surface modification of self-source Ti substrate for high-performance supercapacitors
Scientific Reports (2017)
-
Supercapacitive performance of homogeneous Co3O4/TiO2 nanotube arrays enhanced by carbon layer and oxygen vacancies
Journal of Solid State Electrochemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.