Abstract
Sex is pivotal for reproduction, healthcare and evolution. In the fish medaka, the Y-chromosomal dmy (also dmrt1bY) serves the sex determiner, which activates dmrt1 for male sex maintenance. However, how dmy makes the male decision via initiating testicular differentiation has remained unknown. Here we report that autosomal gsdf serves a male sex initiator. Gene addition and deletion revealed that gsdf was necessary and sufficient for maleness via initiating testicular differentiation. We show that gsdf transcription is activated directly by dmy. These results establish the autosomal gsdf as the first male sex initiator. We propose that dmy determines maleness through activating gsdf and dmrt1 without its own participation in developmental processes of sex initiation and maintenance. gsdf may easily become a sex determiner or other autosomal genes can be recruited as new sex determiners to initiate gsdf expression. Our findings offer new insights into molecular mechanisms underlying sex development and evolution of sex-controlling genes in vertebrates.
Similar content being viewed by others
Introduction
The majority of animal species have both sexes, male and female, for sexual reproduction. Sex development is a multi-step process including sex determination, initiation, differentiation and maintenance, culminating in the production of sperm or eggs for germline transmission. Defects in each of these steps may lead to sex abnormalities including infertility and sex reversal. Animal sex control has an impact in animal husbandry and even human healthcare1. Sex is determined by environmental and genetic factors2. A hallmark of genetic mechanisms underlying sex determination and development is that they show remarkable diversity and do not follow the evolutionary history, because they can arise independently and rapidly, leading to enormous variation between even closely related species. For example, even master sex-determining genes or sex determiners (SDs) so far identified in different animal taxa show considerable differences in sequence and activity of their products3. The primary role of an SD is to determine the initial sex by triggering testicular or ovarian differentiation of a sexually bi-potential gonad. The presence of an SD determines the genetic sex, whereas the onset of gonadal differentiation towards a testis or an ovary delineates primary sex initiation. SDs act at the top of hierarchical cascades to control sex differentiation4. For example, sry in mammals initiates testicular differentiation through activating its direct target sox95,6. Notably, the cascades downstream of SDs also vary enormously from one animal to another4.
The enormous diversity of genetic sex determination mechanisms is a long-standing mystery and also a major challenge for understanding sex development2. Fish have sex-determination mechanisms ranging from environmental to different modes of genetic determination and thus provide a paradigm for studying sex plasticity and development. In particular, medaka (Oryzias latipes) is an excellent vertebrate model for sex development7 and reproductive biotechnology8,9. This fish is the first vertebrate that showed crossing-over between X and Y chromosomes10, induction of sex reversal11 and most importantly, offered the first vertebrate SD besides the mammalian sry, namely dmy12, dmrt1y13 or dmrt1bY14. Most recent studies have revealed female germ stem cell markers capable of making intrinsic sperm-egg fate decision in medaka15. It is known that dmy activates dmrt116, which in turn maintains testicular differentiation, as dmrt1 mutation causes male-to-female sex reversal after the initiation of testicular differentiation17. However, how dmy exerts its primary role in male decision via triggering testicular differentiation has remained unknown. Paradoxically, there are several cases where dmy is dispensable for maleness13,18,19, which points to the presence of autosomal gene(s) essential for male sex initiation in this organism14,18,20.
Recently, the gene gsdf is emerging as a novel sex related factor in several distantly related fish species. This teleost-specific gene21 encodes the gonadal soma derived factor, which belongs to the transforming growth factor-β superfamily22. In medaka, gsdf is located on chromosome 12 and is predominantly expressed in the Sertoli cells and granulosa cells in mature gonads23. Here we show this autosomal gene gsdf acts as a male sex initiator downstream of dmy and renders itself a prime candidate for the searched autosomal gene essential for maleness.
Results
gsdf addition causes masculinization
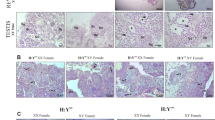
It has been reported that two genes are sufficient to induce medaka masculinization upon transgenic addition, one is dmy24 and the other is gsdf Y, a Y-chromosomal copy of gsdf acting as the SD in O. luzonesis25. We asked whether the medaka autosomal gsdf was similar to gsdf Y in function. To this end, pGsdf2Agfp expressing the Gsdf and GFP from the β-actin promoter was constructed (Fig. 1a) and microinjected into 174 early medaka embryos at the 1-cell stage. Out of 108 adults obtained, eight XY and nine XX fish contained the transgene (Table 1) as determined by genotyping (lanes 1–4; Fig. 1b). While all F0 adults without the transgene detected in the caudal fin developed to either females or males in accordance with their genotype, five of the nine transgenic XX adults were found to be males in phenotype, producing a 56% masculinization rate in F0 animals. Three of them (lanes 2–4, Fig. 1b) were fertile and used to mate with normal XX females. A total of 219 F1 adults were analyzed, all of them were XX as expected from the mating scheme (Table 1). We obtained a total of 68 transgenic adults in F1 generation from the three founders (48, 14 and 6 respectively). Transgene expression was observed ubiquitously - as expected for the promoter used - in developing embryos (Fig. 1c,d) and adult organs such as the heart (Fig. 1e) and testis (Fig. 1f). Notably, GFP-expressing gonads in XX adults were mature testes (Fig. 1f). On the gonadal sections immunostained with an anti-GFP antibody, a normal XY fish displayed a typical architecture of a mature testis containing many sperm but lacked GFP staining (Fig. 1g,g’), whereas a gsdf-transgenic XX fish did show strong GFP staining and the absence of detectable female germ cells but the presence of a testicular structure and many sperm (Fig. 1h,h’). Medaka adults display easily distinguishable secondary sex characteristics26. Specifically, the hindmost rays are separated from other rays in the dorsal fin in male but linked together in female and the anal fin is parallelogram-shaped in male but triangular-shaped in female (Fig. 2a,b). All the 68 transgenic fish invariantly displayed typical male secondary sex characteristics (Fig. 2c), producing a 100% efficiency for masculinization. Taken together, gsdf is fully comparable to SDs, dmy and gsdfY, in the ability to cause masculinization of genetic females at gonadal, gamete and organismal levels.
Masculinization by ectopic gsdf expression.
(a) Map of pGsdf2Agfp. The 2.5 kb β-actin promoter, gsdf, gfp and self-cleavage sequence 2A as well as flanking restriction sites are indicated. (b) Genotype and phenotype of F0 adults. (c–f) Transgenic GFP expression in F1 embryos at 1 dpf (c) or 4 dpf (d) and adult heart (e) and testis (f). (g,h) Cross sections of adult gonads immunostained with an anti-GFP antibody, showing testicular structure and presence of many sperm of a wildtype XY gonad (g) and a XX gonad transgenic for pGsdf2Agfp. (g’,h’) Larger magnification of areas framed in (g) and (h), highlighting the absence in (g’) and presence in (h’) of GFP (arrows). sm, sperm. Scale bar, 50 μm.
Sexual phenotype of medaka adults.
(a) wtXX female, showing a triangular-shaped anal fin and connected dorsal fin rays. (b) wtXY male, showing a parallelogram-shaped anal fin and the separation (arrow) of hindmost dorsal fin rays. (c) Male phenotype of gsdf2Agfp-transgenic XX fish. (d) Male phenotype of gsdf+/− XY fish. (e) Female phenotype of mtXX fish, showing dramatically enlarged abdomen. (f) Female phenotype of mtXY fish, showing dramatically enlarged abdomen. mt, homozygous gsdf−/−mutant; wt, wildtype. Scale bar, 0.5 cm.
gsdf deletion causes feminization
In order to determine the exact role of gsdf in maleness, we then analyzed the gsdf deletion phenotype. Several fish bearing subtle mutations in the gsdf locus were obtained by using zinc finger nucleases (ZFN) (Supplementary Fig. 1a,b)27. A male founder carrying a null gsdf allele was chosen to produce a mutant family. This allele contained a 4-bp insertion and predicted truncated translation (Supplementary Fig. 1c). Upon crossing with wildtype females, this fish produced 80 F1 adults, five of which had the null gsdf allele and developed into XX females (n = 3) and XY males (n = 2). Intercrossing between F1 heterozygotes (gsdf+/−) produced 120 F2 adults and a typical Mendelian segregation of wildtype (n = 35; gsdf+/+), gsdf+/− (n = 62) and homozygous animals (n = 23; gsdf−/−) (Table 2). gsdf disruption led to multiple phenotypes in adulthood and the most prominent was feminization, which was the focus of this study. All the heterozygotes developed as female (n = 25) or male (n = 37) strictly according to their genetic sex (Fig. 2d). Interestingly, all the 23 gsdf−/− adults including 13 fish of the XY chromosomal constitution were found to be female (Fig. 2e,f). The gsdf+/− F2 fish were selected to produce the progeny and the Mendelian segregation also occurred in the resultant F3 generation, where all gsdf+/− fish (n = 129) again developed according to genetic sex, whereas gsdf−/− adults (n = 56; 19 XX and 37 XY) were invariantly female in phenotype (Table 2). The Mendelian segregation in F2 and F3 generations and the fertility of phenotypically female gsdf−/− XY adults demonstrated that gsdf is dispensable for survival and its disruption has caused 100% feminization at the gamete and organismal levels.
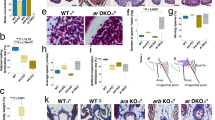
We examined the histology of adult gonads and spatial gene expression patterns by fluorescence in situ hybridization (FISH) and immunofluorescence (IF). We first focused on dmrt1 and foxl2. The former is expressed in Sertoli cells and essential for the maintenance of testicular differentiation17. The latter is predominantly expressed in the granulosa cells of the medaka ovary28. The dmrt1 riboprobe hybridized to the dmy transcripts due to sequence similarity29. On the gonadal sections, the dmrt1 or dmy transcripts were evident in the Sertoli cells surrounding spermatogonia, spermatocytes and sperm of the wildtype XY (wtXY) testis (Fig. 3a) but hardly detectable in the wildtype XX (wtXX) ovary (Fig. 3b). In the gsdf-mutant XY (mtXY) gonad, the dmrt1 riboprobe produced a barely detectable signal (Fig. 3c), possibly due to its cross hybridization to the dmy transcripts. On the other hand, the foxl2 mRNA was absent in wtXY testis (Fig. 3a) but present in follicular cells of the wtXX ovary and more importantly, the mtXY gonad (Fig. 3b,c), suggesting that the mtXY gonad is comparable to the wtXX ovary in architecture and gene expression. We then analyzed the expression of vasa and gsdf. vasa was expressed predominantly in spermatogonia of adult testes (Fig. 3d) and pre-meiotic oocytes of adult ovaries from wtXX fish and mtXY fish (Fig. 3e,f), in accordance with its reported expression pattern30,31. The gsdf signal peaked in putative Sertoli cells surrounding spermatogonia and sperm, remained easily detectable in putative Sertoli cells surrounding spermatocytes of the wtXY testis (Fig. 3d) and was moderately detectable in the somatic cells surrounding developing oocytes and those residing in the interstitium of the wtXX ovary (Fig. 3e), which is consistent with its reported expression pattern23. Notably, the gsdf signal was hardly detectable in the mtXY gonad (Fig. 3f). IF by using an anti-Gsdf antibody (αGsdf) revealed the localization of Gsdf protein also in somatic cells surrounding developing oocytes of the wtXX ovary (Fig. 3g). Gsdf protein was, however, completely absent in the mtXY gonad (Fig. 3h), demonstrating that the mutation by 4-bp insertion leads to gsdf disruption at RNA, protein and thus functional levels. Therefore, gsdf disruption causes full feminization of an XY gonad into a functional ovary.
Gonadal histology and gene expression.
Cryosections of adult gonads were subjected for FISH with antisense riboprobes of dmrt1 plus foxl2, gsdf plus vasa or IF with antibodies against Vasa plus Gsdf and analyzed by fluorescence microscopy. (a–c) FISH analysis, showing the expression of dmrt1/dmy (red) and foxl2 (green) transcripts. (d–f) FISH analysis. The gsdf RNA signal is evident in somatic cells surrounding spermatogonia (sg), spermatocytes (sc) and sperm (sm) of the wtXY testis (d), somatic cells surrounding (arrows) oocytes of various stages (I-IV) and residing in the interstitium (asterisk) of the wtXX ovary (e), but barely detectable in the mtXY gonad (f). (g,h) IF analysis, showing the expression of Vasa (red) and Gsdf protein (green). The Gsdf protein is seen in somatic cells surrounding (arrows) oocytes of the wtXX ovary (g) but absent in the mtXY gonad (h). mt, homozygous gsdf−/−mutant; wt, wildtype. Scale bar, 50 μm.
gsdf disruption alters global gene expression
We analyzed global gene expression profiles to gain insights into the molecular mechanism underlying feminization upon gsdf disruption. Next-generation sequencing of adult gonads of from wtXY, wtXX and mtXY fish produced a total of 35073 unigenes, 25588 of which were widely expressed in gonads of the three genotypes. The wtXX ovary and wtXY testis had 27872 and 32212 unigenes, respectively. The mtXY gonad generated 32153 unigenes, indicating that it is not different from the wtXY testis in total number of expressed unigenes (Fig. 4a). However, a comparison of globally differential gene expression profile in log fold change revealed the mtXY gonad is more similar to the wildtype ovary than to the wildtype testis (Fig. 4b). This similarity became more evident when a comparison was focused on 400 most differentially expressed genes, where 314 unigenes (78.5%) were expressed at either low (n = 242) or high levels (n = 72) in wtXX and mtXY gonads compared to the wtXY testis (Fig. 4c). Interestingly, 17 and 69 unigenes were highly expressed only in the wtXX and mtXY gonads, respectively (Fig. 4c). Thus, gsdf disruption alters global gene expression in favor of ovarian development.
Gene expression profile in adult gonads.
(a) Transcriptome analysis. Venn diagram, showing numbers of unigenes expressed in wtXY, wtXX and mtXY gonads. (b) MA-plots, showing the comparisons of global gene expression profiles. Each gene is represented as a dot. Red dots, representing significantly differentially expressed genes (P < 0.05); y-axis, the log2 fold change; x-axis, the average of counts (mean expression). (c) Heat map of 400 top differentially expressed genes. (d) RT-PCR analysis, showing expression of ovarian (blue) and testicular markers (red). vasa and β-actin served a germ cell marker and a loading control. Numbers of PCR cycles are indicated in the parenthesis. (e,f) ddPCR analyses, showing the relative mRNA levels of ovarian markers (e) and testicular markers (f). Values are relative copy numbers per μl of ddPCR templates normalized to β-actin and presented as means ± s.e.m from three independent experiments. Significance levels of difference (*P < 0.05; **P < 0.01) were obtained by comparisons to wtXY as the control.
We then examined the gonadal expression profile of genes as markers or essential players for female and male development. Genes chosen were 2 ovarian markers foxl2 and cyp19a1 (aromatase)28,32 and 5 testicular markers gsdf, dmy12,13, sdgc, dmrt1 and sox9b. sdgc is linked closely to dmy and expressed predominantly in spermatogonia but weakly in early oocytes33. dmrt1 and sox9b are autosomal male markers, as dmrt1 is expressed in the Sertoli cell lineage and essential for the maintenance of testis differentiation17; while sox9b is expressed in both sexes during early gonadal sex differentiation34 but maintained mainly in the XY gonads later during testicular tubules development35. vasa was used as a germ cell marker30,36. An RT-PCR analysis revealed that in the mtXY gonad, both ovarian markers foxl2 and cyp19a1 were dramatically increased to a level comparable to that in the wtXX ovary, whereas testicular markers dmrt1 and sox9b were concurrently decreased obviously (Fig. 4d). Notably, the mutant gsdf RNA became hardly detectable in both XX and XY gonads (Fig. 4d), in accordance with reported decay of nonsense transcripts37. Interestingly, dmy was significantly up-regulated in the mtXY gonad, perhaps due to a decreased level of dmrt1 capable of suppressing dmy expression16. More importantly, the sdgc RNA was barely detectable in the mtXY gonad, indicating the absence of spermatogonia (Fig. 4d). A quantitative droplet digital PCR (ddPCR) analysis validated the RT-PCR results. Specifically, the expression of foxl2 and cyp19a1 in the mtXY gonad was approximately 3 times lower than in the wtXX ovary, but significantly higher than in the wtXY testis (Fig. 4e). The expression of dmrt1 was dramatically downregulated in the mtXY gonad than in the wtXY testis (Fig. 4f), indicating that gsdf may directly or indirectly activate or maintain its expression. Interestingly, dmy was significantly upregulated in the mtXY gonad than in the wtXY testis, demonstrating that gsdf is not necessary for dmy expression. Thus, gsdf disruption causes gonadal feminization at the molecular level and gsdf may play an important role also in regulating gene expression favoring testicular versus ovarian development and function.
Feminization via ovarian differentiation
In order to distinguish whether feminization upon gsdf disruption comes from either male-to-female sex reversal after the initiation of testicular differentiation or ovarian differentiation without the initiation of testicular differentiation in genetically male embryos, we determined the initial primary sex of differentiating gonads. In medaka, sex dimorphism is easily detectable in embryos shortly before and after hatching, when primordial germ cells (PGCs) in a male gonad are entering into mitotic arrest and thus dramatically fewer than in its female counterpart, where germ cells continue propagation and enter into meiosis29,38. To visualize PGCs in live embryos, a gsdf+/− male was crossed to a Vg female homozygous for transgene Olvasa-gfp that expresses GFP specifically in germ cells and embryos with GFP-labeled PGCs from the F3 generation were used for PGC observation (Supplementary Fig. 3). As expected, these F3 embryos had various genotypes as determined by genomic PCR genotyping. These embryos at stage 39 (1 day before hatching) displayed two major types in terms of PGC number, namely the female type of more PGCs and male type of fewer PGCs. XX gonads had a similarly larger PGC number (~100) in wildtype (n = 8), gsdf+/− (n = 9) and gsdf−/− (n = 8) embryos (Fig. 5a–c), which was characteristic of ovarian differentiation and in accordance with a female phenotype in adulthood (Fig. 2a,e). In contrast, XY gonads had a similarly smaller PGC number (~30) in wildtype (n = 6) and gsdf+/− (n = 6) embryos (Fig. 5d,e), which was in accordance with testicular differentiation and a male phenotype in adulthood (Fig. 2b,d). Strikingly, the number of PGCs in mtXY gonads examined (n = 10) increased to ~90 (Fig. 5f) and was in accordance with ovarian differentiation as in XX gonads. Taken together, feminization upon gsdf disruption is not a consequence of male-to-female sex-reversal but rather results from ovarian differentiation in the absence of testicular differentiation, demonstrating that gsdf is a male sex initiator, because it is necessary for the initiation of testicular differentiation, the first step of male development.
PGC abundance at early sexual differentiation.
Hatching F3 embryos (9 dpf) from crosses between gsdf+/− fish and Vg fish bearing GFP-labeled germ cells were analyzed by confocal microscopy and then genotyped. GFP-positive PGCs (green) are clearly visible in the gonad. (a–c), XX gonads, showing similar abundance of PGCs in normal (a), gsdf+/− (b) and gsdf−/− (c) embryos. (d–f), XY gonads, highlighting fewer PGCs in normal (d) and gsdf+/− embryos (e) than XX embryos (a–c), whereas the PGC number in gsdf−/− embryos (f) is comparable to that in XX embryos (a–c). Numbers of PGCs are presented as means ± SD with number of embryos examined are given in parenthesis. Scale bar, 50 μm.
gsdf acts directly downstream of dmy
The experiments described so far demonstrate that gsdf is essential for early testicular differentiation, a role similar to dmy12,13. This similarity provoked us to investigate whether dmy and gsdf had direct or indirect relationship in action. Two putative binding sites of Dmy are present within the 4-kb gsdf upstream regulatory region (Fig. 6a) and can be specifically amplified for quantification (Fig. 6b). In the testis of medaka transgenic for dmy:GFP (also dmrt1bY:GFP)16, both sites showed approximately 2-fold enrichment of binding by dmy:GFP as determined by Chromatin immunoprecipitation (ChIP) using a GFP antibody (Fig. 6c). An in vitro reporter assay was performed to see whether dmy controls gsdf transcription in medaka embryonic stem cell line MES139 and spermatogonial cell line SG38. Both cell lines were transfected with pDmy:cherry and cells expressing the Dmy:Cherry fusion protein were not different from nontransgenic control cells in phenotype (Fig. 6d–e’ and Supplementary Fig. 4a,b). Transgenic cells were isolated by fluorescence-activated cell sorting (Supplementary Fig. 4c) and analyzed for altered dmy and gsdf RNA expression. Both cell lines lacked a detectable level of dmy transcripts (Fig. 6f) but a low level of gsdf transcripts (Fig. 6g). As expected, dmy was expressed at a high level in the sorted transgenic cells (Fig. 6f), where a nearly 2-fold increase in the gsdf RNA level was also observed (Fig. 6g). Taken together, gsdf is a direct target of dmy for transcriptional activation and conforms to its genetic hypostasis to dmy in action.
gsdf is a direct target of dmy.
(a) Dmy target sites. Shown are the positions and sequences of two putative Dmy binding sites within the 4-kb medaka gsdf promoter as well as the transcription start point (+1). Bases in red and blue or grey match the most and second most frequent bases or do not match in respective positions of the reported matrix. (b) Specificity of PCR-based assay. (c) ChIP assay, showing PCR products from immunoprecipitated DNA fragments. Notably, significant binding by Dmy is clearly seen for both binding site 1 and site 2 at P < 0.01. The immunoprecipitated DNA fragments of the control group were obtained from ΔDmy::GFP-transgenic fish (without the DNA binding domain, DM) by the same anti-GFP antibody. Controls show no enrichment while both sites reveal nearly two-fold enrichment of binding by dmy::GFP. (d–e’) Phenotype of medaka stem cell lines MES1 (d,d’) and SG3 (e,e’) at 72 h post transfection with pDmy:cherry. Scale bar, 20 μm. (f–g) ddPCR analysis of dmy (f) and gsdf (g) expression. −, control cells; +, sorted cells positive for transgene-expressed Cherry and thus Dmy. A nearly 2-fold increase in gsdf RNA is clearly seen in both MES1 and SG3 cell lines by transgenic dmy expression (g). dmy expression was presented as relative copy numbers per μl of ddPCR templates normalized to β-actin and presented as means ± s.e.m from three independent experiments. gsdf expression was presented as relative gene expression levels to the controls and presented as means ± s.e.m from three independent experiments. *P < 0.05; **P < 0.01 from comparisons between transgenic and non-transgenic control cells.
Discussion
Previously we and others have established dmy as the male SD in medaka, as it is the only functional gene within the 258-kb region unique to the Y chromosome13,40 and shows necessity12 and sufficiency24 for maleness. However, dmy is not a sufficient factor to ensure the maleness and may also be paradoxically dispensable for maleness in several cases. For example, medaka XY embryos can develop into functional females after estrogen treatment in the presence of dmy expression13 and XX embryos can develop into functional males after treatment with either androgen41,42 or high temperature43,44 in the absence of dmy. Notably, dmy expression is independent of sex phenotype and remains unaltered by feminizing13 and masculinizing29 factors45. In addition, medaka shows a strain difference in frequency of spontaneous XX males18. The fact that dmy is not always necessary and sufficient for maleness is obviously in consistence with an important notion: dmy acts upstream in the genetic hierarchy13 and serves as the male SD only, whereas autosomal genes are responsible for male sex differentiation and maintenance13,14,18,20. Indeed, autosomal dmrt1 behaves as a male sex maintainer, because its mutation leads to male-to-female sex reversal after the initiation of testicular differentiation17. However, its addition did not masculinize genetic females24 apparently due to its inability to induce testicular initiation or differentiation, as its expression is not detectable until 20 days post hatching after testicular differentiation13,29 via transcriptional activation by dmy16 and can be elevated by masculinizing factors like high temperature46.
While this manuscript was under revision, another group also reported that gsdf mutation has induced XY gonads to undergo ovarian differentiation, suggesting its potential role as an endogenous inducer of testicular development47. Here our study has provided sufficient evidence and established gsdf as a male sex initiator acting downstream of dmy, thus serving as a prime candidate for the searched autosomal gene essential for maleness. First, gsdf addition is sufficient for masculinization in the absence of dmy and gsdf disruption causes feminization without compromising dmy expression, pointing to its hypostasis to dmy in action. Second, feminization upon gsdf disruption results from the initiation of ovarian differentiation despite the presence of dmy, demonstrating that the earliest and perhaps the primary role of gsdf is to initiate testicular differentiation, which cannot be replaced by dmy13. Finally, the fact that Dmy protein binds to the gsdf promoter and activates gsdf transcription convincingly reveals that gsdf acts downstream of dmy. Consistent with this notion is a recent report that the gsdf mRNA reduces dramatically by up to 28 folds in the dmy knockout XY gonad48. Reportedly, gsdf expression temporospatially correlates with the initiation of testicular differentiation and is suppressed by feminizing factors such as female hormone estrogen23 but activated by masculinizing factors such as male hormone androgen and high temperature49. This expression pattern is what exactly expected for a male sex initiator. Interestingly, unlike the recent report showing that some genetically male adults did not demonstrate female phenotype after gsdf disruption47, here we have revealed a 100% feminization rate of XY adults, indicating that the adult sexual phenotype might be affected by multiple factors. Moreover, gsdf disruption causes the alteration of global gene expression and dmrt1 down-regulation in adult gonads, suggesting the potential involvement of gsdf in subsequent processes such as male sex maintenance besides acting as a male sex initiator.
In model organisms such as Drosophila and C. elegans4, a single genetic cascade responsible for sex determination and development comprises multiple genes. This study together with previous reports reveals two unusual features for the genetic male sex determination system in medaka, where dmy, gsdf and dmrt1 constitute the core sex determination system and form two cascades. One comprises dmy and gsdf for male sex initiation and differentiation, the other comprises dmy and dmrt1 for male sex maintenance (Fig. 7a). As mentioned above, gsdf and dmrt1 are involved in male sex initiation and maintenance, whereas dmy itself is not necessary for and apparently not involved in, these two early developmental processes. We propose that dmy determines maleness through activating the male sex initiator gsdf and the male sex maintainer dmrt1 without its own participation in developmental processes of sex initiation and maintenance.
Sex determination and its evolution in Oryzias.
(a) The core cascade controlling sex development in medaka. The black horizontal line depicts developmental day(s) post fertilization (dpf) or hatching (dph). Shown are key developmental events including testis initiation (TI), testis differentiation (TD) and testis formation (TF). Horizontal boxes depict developmental RNA expression stages of dmy, gsdf and dmrt1. Arrows depict transcriptional activation of gsdf and dmrt1 by dmy. Indicated are the primary roles for gsdf in testis initiation and differentiation and for dmrt1 in testis maintenance. (b) Hypothetical evolution of sex determiners in the genus Oryzias. gsdf may easily become a sex determiner (namely gsdfY in O. luzonesis) via acquiring proper temporospatial expression (bent arrow) or preferentially recruit gsdf-regulating genes as new sex determiners (namely dmy in O. latipes and sox3Y in O. dancena). Shown are potential or putative binding sites (black) for Dmrt1, Sox3 and an unknown transcription factor in the prototype gsdf promoter. These binding sites may be used (orange) by transcription factors such as Dmrt1 and Sox3 for activation (horizontal arrows) of proper temporospatial gsdf expression to initiate testicular differentiation.
SDs demonstrate enormous diversity across animal phyla in general as well as conservation within certain taxa such as sry in many mammals and transformer (tra) in several insects2. There is an example that certain genes such as dmrt1 or its paralog can be repeatedly recruited as the SD in a wide variety of organisms including medaka12, smooth tongue sole (Cynoglossus semilaevis)50, chicken51 and frog52. These facts raise a question as to what genes can preferentially become SDs. Our identification of autosomal gsdf as a male sex initiator has a direct and important implication on the evolution of SDs as well as sex chromosomes. It is generally accepted that autosomes bearing genes capable of exerting a male determining function may evolve into Y chromosomes that will compete and possibly replace the present Y chromosome14, ultimately leading to the tremendous variety of SDs in lower vertebrates due to the plasticity of homomorphic sex chromosomes53. Here we propose that autosomal genes performing key male sex functions like gsdf may serve as a core in understanding the evolution of SDs and cascades (Fig. 7b). On one hand, gsdf may easily evolve into a male SD capable of competing and even replacing the existing SD dmy, which is exactly what has occurred in O. luzonesis (Fig. 7b), where gsdf has undergone allelic divergence into gsdfY and gsdfX, with gsdfY serving as the SD via acquiring early XY-specific high expression for testicular differentiation by itself25. On the other hand, other autosomal genes can be recruited as new SDs to initiate gsdf expression, which is exactly what has happened in medaka and its close relative O. dancena, where the sex determiner sox3Y functions early in XY embryos to induce gsdf expression54. However, it remains to be determined whether gsdf is the male sex initiator and activated by sox3Y directly or indirectly in this species. Therefore, an autosomal sex initiator like gsdf may play a critical role in the evolution of SDs and sex chromosomes.
Methods
Fish and chemicals
Fish work was performed in strict compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Advisory Committee for Laboratory Animal Research in Singapore and approved by this committee (Permit Number: 27/09). The medaka strain Hd-rR and Vg were maintained under an artificial photoperiod of 14-h light to 10-h darkness at 26~28 °C as described55. Medaka embryos were maintained at 27 ± 2 °C and staged as described56. Chemicals were purchased from Sigma, enzymes were from New England Biolabs and PCR reagents were from TaKaRa unless otherwise indicated.
Plasmids
Plasmid pGsdf2Agfp expressing a chimeric mRNA between medaka gsdf and gfp was constructed, which predicts a nascent fusion protein that is self-cleaved by 2A sequence into Gsdf and GFP. Vectors pZN1gsdf and pZN2gsdf expressing ZFNs for targeting the medaka gsdf gene were described previously27. Plasmid pDmy:cherry expressing a fusion between Dmy and Cherry was constructed by inserting dmy coding sequence with BamHI and EcoRI into the pCS2cherryHis backbone. Plasmids for synthesizing riboprobes were constructed by TA-cloning of PCR products into pGEM-T Easy vectors (Promega). Recombinant plasmids were sequenced on the 3130XL Sequencer (Applied Biosystems). Sequence analyses were run on Vector NTI and DNAman software.
Microinjection
Microinjection of DNA and RNA into 1-cell stage embryos was done as previously described36. Microinjection of synthetic mRNAs for ZFNs into medaka embryos was described27. The operated embryos were reared to adulthood.
Fish sexing
Phenotypic sex was judged based on the secondary sex characteristics, especially the shapes of the dorsal and anal fins in medaka adults26. Genotypic sex (XX or XY) was determined by PCR genotyping with the primer set, PG17.5 and PG17.6 amplifying dmy and dmrt1 transcripts as described previously12.
PCR analyses
Isolation of genomic DNA and total RNA were done as described9,27. Primers used for PCR are listed in Supplementary Table 1. Genotyping PCR was run in a 25-μl volume containing 50 ng of genomic DNA and genotyping primers for 35 cycles (94 °C for 15 s, 60 °C for 15 s and 72 °C for 30 s). RT-PCR was done as described9. PCR products were then separated on 1% agarose gels or 8% polyacrylamide gels and documented on a bio-imaging system (VilberLourmat).
RNA quantification was performed on the QX200 automated Droplet Digital PCR system (Bio-rad, Hercules, CA). Briefly, cDNA templates were first mixed with 2× QX200 ddPCR EvaGreen Supermix to 20-μl volume. The mixture was transferred to a 96-well plate (Eppendorf) and subjected to oil droplets generation on the automated generator (Bio-Rad, Hercules, CA). PCR in oil droplets was run for 40 cycles of 94 oC for 30 s and 60 °C for 1 min. Droplets were read by QX200 Droplet Reader (Bio-Rad, Hercules, CA) and analyzed with the QuantaSoft software (Bio-Rad, Hercules, CA) that determines concentration of target cDNA as copies per microliter (copies/μl) from the fraction of positive droplets using Poisson statistics57. The copy number of each mRNA was further normalized with β-actin.
Fluorescence in situ hybridization
Riboprobe synthesis, cryosectioning and fluorescence in situ hybridization were performed as described previously58. The vasa, foxl2 probes were labeled with fluorescein isothiocyanate (FITC) and gsdf, dmrt1 probes with digoxigenin (DIG) (Roche, Germany). Horseradish peroxidase-conjugated anti-FITC and anti-DIG antibodies were used for signal detection. The TSA Plus Fluorescein/TMR system was used (PerkinElmer Inc., Waltham, MA) to amplify the fluorescence signals.
Immunofluorescence
Immunostaining and nuclear staining with DAPI on gonadal cryosections were done as described31. A monoclonal mouse anti-Gsdf antibody (αGsdf; generated by using peptide EEPAASPAST) and a polyclonal rabbit anti-Vasa primary antibody (αVasa)59 were used as primary antibodies at a 1:200 dilution. Goat anti-mouse antibody (Alexa Fluor 488, abcam) and anti-rabbit secondary antibody (Alexa Fluor 543, abcam) were used as secondary antibodies at a 1:200 dilution. Slides were mounted with Gold Antifade Reagent (Invitrogen) for microscopy.
In vivo chromatin precipitation
Binding sites for transcription factors were predicted using the matrix60 and the Regulatory Sequence Analysis Tools portal (http://rsat.ulb.ac.be/rsat/, last accessed March 15, 2015). Testis samples (20 mg) from dmrt1bY:GFP transgenic fish and GFP antibody (Upstate) were used for in vivo chromatin immunoprecipitation (ChIP) by using the EpiQuik Tissue Chromatin Immunoprecipitation kit (Epigentek)16,61. After tissue disaggregation and cell re-suspension, DNA was sheared by sonication (9 pulses of 10s with an amplitude of 10%)16. ChIP procedure and analysis of DNA enrichment by real-time PCR were as described16,62.
Cell transfection
Medaka embryonic stem cell line MES1 and spermatogonial cell line SG3 were maintained as described8,39. Cell transfection was performed by using the TransIT-X2 Dynamic Delivery System (Mirus). Cells were transfected at a density of ~80% confluence and propagated or analyzed at 24–72 h post transfection.
Microscopy
Adult fish were observed with stereomicroscope (M205FA, Leica) and photographed with the Evolution VF digital camera (MediaCybernetics) and digital camera SELP1650 (SONY). Slides were observed and photographed with the LSM 510 Meta confocal microscope (ZEISS). Cultured cells were observed with Axiovert upright microscope (Zeiss) and photographed with the AxioCam digital camera (Zeiss).
RNA sequencing and bioinformatics
RNA samples from adult gonads were sequenced as 2 × 100-bp paired-end reads on the HiSeq 2000 platform (Illumina) by the custom service provider AITbiotech PTE LTD (Singapore). Clean reads were mapped to the medaka genome (http://www.ensembl.org/index.html) by using the reads mapper Tophat2 (version 2.0.13, http://ccb.jhu.edu/software/tophat/). Aligned sequences were assembled into transcripts by using Cufflinks (version 2.2.0, http://cole-trapnell-lab.github.io/cufflinks/cufflinks/). Cuffmerge (http://cole-trapnell-lab.github.io/cufflinks/cuffmerge/) was used to merge assemblies into a master transcriptome for analyzing differentially expressed genes (DEGs). Htseq-count63 was used to count the transcripts in the final transcriptome assembly as measurement of relative expression levels. Venn diagram was plotted against the reads of unigenes across samples. Count-table was passed to DESeq264 to perform DEG analysis. A cutoff of FDR < 0.1 (Benjamini Hochberg method) was for MA-plots comparisons. Red dots represent statistically significant DEGs. A heat map was plotted for 400 top DEGs by sample clustering and visualization.
Statistics
Statistical analyses were calculated by using GraphPad Prism version 6.01. Data consolidated were presented as mean ± s. e. m. or s. d. and p values were calculated by using non-parametric student’s t-test.
Additional Information
How to cite this article: Zhang, X. et al. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 6, 19738; doi: 10.1038/srep19738 (2016).
References
Hall, A. B. et al. A male-determining factor in the mosquito Aedes aegypti. Science 348, 1268–1270, 10.1126/science.aaa2850 (2015).
Bachtrog, D. et al. Sex Determination: Why So Many Ways of Doing It? PLoS Biol 12, e1001899, 10.1371/journal.pbio.1001899 (2014).
Kikuchi, K. & Hamaguchi, S. Novel sex‐determining genes in fish and sex chromosome evolution. Dev Dyn 242, 339–353 (2013).
Wilkins, A. S. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. BioEssays 17, 71–77, 10.1002/bies.950170113 (1995).
DiNapoli, L. & Capel, B. SRY and the standoff in sex determination. Mol Endocrinol 22, 1–9 (2008).
Sekido, R. & Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934 (2008).
Wittbrodt, J., Shima, A. & Schartl, M. Medaka–a model organism from the far East. Nat Rev Genet 3, 53–64, 10.1038/nrg704 (2002).
Hong, Y. et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci USA 101, 8011–8016, 10.1073/pnas.0308668101 (2004).
Yi, M., Hong, N. & Hong, Y. Generation of medaka fish haploid embryonic stem cells. Science 326, 430–433 (2009).
Aida, T. On the inheritance of color in a fresh-water fish, Aplocheilus latipes Temmick and Schlegel, with special reference to sex-linked inheritance. Genetics 6, 554 (1921).
Yamamoto, T.-O. Progeny of artificially induced sex-reversals of male genotype (XY) in the medaka (Oryzias latipes) with special reference to YY-male. Genetics 40, 406 (1955).
Matsuda, M. et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563 (2002).
Nanda, I. et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA 99, 11778–11783, 10.1073/pnas.182314699 (2002).
Schartl, M. A comparative view on sex determination in medaka. Mech Dev 121, 639–645 (2004).
Nishimura, T. et al. foxl3 is a germ cell–intrinsic factor involved in sperm-egg fate decision in medaka. Science 349, 328–331 (2015).
Herpin, A. et al. Transcriptional rewiring of the sex determining dmrt1 gene duplicate by transposable elements. PLoS Genet 6, e1000844 (2010).
Masuyama, H. et al. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res 20, 163–176 (2012).
Nanda, I. et al. Common spontaneous sex-reversed XX males of the medaka Oryzias latipes. Genetics 163, 245–251 (2003).
Kondo, M. et al. Absence of the candidate male sex-determining gene dmrt1b (Y) of medaka from other fish species. Curr. Biol. 13, 416–420 (2003).
Volff, J.-N., Kondo, M. & Schartl, M. Medaka dmY/dmrt1Y is not the universal primary sex-determining gene in fish. TRENDS Genet 19, 196–199 (2003).
Gautier, A., Le Gac, F. & Lareyre, J.-J. The gsdf gene locus harbors evolutionary conserved and clustered genes preferentially expressed in fish previtellogenic oocytes. Gene 472, 7–17 (2011).
Sawatari, E., Shikina, S., Takeuchi, T. & Yoshizaki, G. A novel transforming growth factor-beta superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Dev Biol 301, 266–275, 10.1016/j.ydbio.2006.10.001 (2007).
Shibata, Y. et al. Expression of gonadal soma derived factor (GSDF) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expr Patterns 10, 283–289 (2010).
Matsuda, M. et al. DMY gene induces male development in genetically female (XX) medaka fish. Proc Natl Acad Sci USA 104, 3865–3870 (2007).
Myosho, T. et al. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 191, 163–170, 10.1534/genetics.111.137497 (2012).
Kinoshita, M., Murata, K., Naruse, K. & Tanaka, M. Medaka: Biology, Management and Experimental Protocols. Ch. 5, 117–118 (Wiley, 2009).
Zhang, X., Guan, G., Chen, J., Naruse, K. & Hong, Y. Parameters and efficiency of direct gene disruption by zinc finger nucleases in medaka embryos. Mar Biotechnol (NY) 16, 125–134, 10.1007/s10126-013-9556-6 (2014).
Nakamoto, M., Matsuda, M., Wang, D.-S., Nagahama, Y. & Shibata, N. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem Biophys Res Commun 344, 353–361, http://dx.doi.org/10.1016/j.bbrc.2006.03.137 (2006).
Kobayashi, T. et al. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dyn 231, 518–526, 10.1002/dvdy.20158 (2004).
Shinomiya, A., Tanaka, M., Kobayashi, T., Nagahama, Y. & Hamaguchi, S. The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes. Dev Growth Differ 42, 317–326 (2000).
Yuan, Y., Li, M. & Hong, Y. Light and electron microscopic analyses of Vasa expression in adult germ cells of the fish medaka. Gene 545, 15–22, http://dx.doi.org/10.1016/j.gene.2014.05.017 (2014).
Suzuki, A., Tanaka, M. & Shibata, N. Expression of aromatase mRNA and effects of aromatase inhibitor during ovarian development in the medaka, Oryzias latipes. J. Exp. Zool. 301, 266–273 (2004).
Nishimura, T. et al. Analysis of a novel gene, Sdgc, reveals sex chromosome-dependent differences of medaka germ cells prior to gonad formation. Development 141, 3363–3369, 10.1242/dev.106864 (2014).
Nakamura, S., Kobayashi, K., Nishimura, T., Higashijima, S.-I. & Tanaka, M. Identification of germline stem cells in the ovary of the teleost medaka. Science 328, 1561–1563 (2010).
Nakamoto, M., Suzuki, A., Matsuda, M., Nagahama, Y. & Shibata, N. Testicular type Sox9 is not involved in sex determination but might be in the development of testicular structures in the medaka, Oryzias latipes. Biochem Biophys Res Commun 333, 729–736 (2005).
Li, M. et al. Medaka vasa is required for migration but not survival of primordial germ cells. Mech Dev 126, 366–381 (2009).
Frischmeyer, P. A. & Dietz, H. C. Nonsense-mediated mRNA decay in health and disease. Hum Mol Gen 8, 1893–1900 (1999).
Herpin, A. et al. Inhibition of primordial germ cell proliferation by the medaka male determining gene Dmrt I bY. BMC Dev Biol 7, 99, 10.1186/1471-213x-7-99 (2007).
Hong, Y., Winkler, C. & Schartl, M. Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev 60, 33–44 (1996).
Kondo, M. et al. Genomic organization of the sex-determining and adjacent regions of the sex chromosomes of medaka. Genome Res 16, 815–826 (2006).
Yamamoto, T.-O. Effects of 17α-hydroxyprogesterone and androstenedione upon sex differentiation in the medaka, Oryzias latipes. Gen Comp Endocrinol 10, 8–13 (1968).
Yamamoto, T. O. Artificial induction of functional sex‐reversal in genotypic females of the medaka (Oryzias latipes). J Exp Zool 137, 227–263 (1958).
Sato, T., Endo, T., Yamahira, K., Hamaguchi, S. & Sakaizumi, M. Induction of female-to-male sex reversal by high temperature treatment in medaka, Oryzias latipes. Zool Sci 22, 985–988 (2005).
Hayashi, Y. et al. High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol Reprod Dev 77, 679–686 (2010).
Nagahama, Y., Nakamura, M., Kitano, T. & Tokumoto, T. Sexual plasticity in fish: a possible target of endocrine disruptor action. Environ Sci 11, 73–82 (2003).
Hattori, R. et al. Temperature-dependent sex determination in Hd-rR medaka Oryzias latipes: gender sensitivity, thermal threshold, critical period and DMRT1 expression profile. Sex Dev 1, 138–146 (2007).
Imai, T., Saino, K. & Matsuda, M. Mutation of Gonadal soma-derived factor induces medaka XY gonads to undergo ovarian development. Biochem Biophys Res Commun 467, 109–114 (2015).
Luo, D. et al. Direct production of XYDMY− sex reversal female medaka (Oryzias latipes) by embryo microinjection of TALENs. Sci Rep 5, 14057, 10.1038/srep14057 (2015).
Kitano, T., Hayashi, Y., Shiraishi, E. & Kamei, Y. Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Mol Reprod Dev 79, 719–726 (2012).
Chen, S. et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet 46, 253–260 (2014).
Smith, C. A. et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271 (2009).
Yoshimoto, S. et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci USA 105, 2469–2474 (2008).
Otake, H. et al. Heritable artificial sex chromosomes in the medaka, Oryzias latipes. Heredity 105, 247–256 (2010).
Takehana, Y. et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat Commun 5, 10.1038/ncomms5157 (2014).
Hong, N. et al. Accessibility of host cell lineages to medaka stem cells depends on genetic background and irradiation of recipient embryos. Cell Mol Life Sci 67, 1189–1202 (2010).
Iwamatsu, T. Stages of normal development in the medaka Oryzias latipes. Mech Dev 121, 605–618 (2004).
Beliakova-Bethell, N. et al. The effect of cell subset isolation method on gene expression in leukocytes. Cytometry A 85, 94–104, 10.1002/cyto.a.22352 (2014).
Xu, H., Li, Z., Li, M., Wang, L. & Hong, Y. Boule Is Present in Fish and Bisexually Expressed in Adult and Embryonic Germ Cells of Medaka. Plos One 4, e6097, 10.1371/journal.pone.0006097 (2009).
Xu, H., Gui, J. & Hong, Y. Differential expression of vasa RNA and protein during spermatogenesis and oogenesis in the gibel carp (Carassius auratus gibelio), a bisexually and gynogenetically reproducing vertebrate. Dev Dyn 233, 872–882 (2005).
Murphy, M. W., Zarkower, D. & Bardwell, V. J. Vertebrate DM domain proteins bind similar DNA sequences and can heterodimerize on DNA. Bmc Mol Biol 8, 58 (2007).
Hornung, U., Herpin, A. & Schartl, M. Expression of the male determining gene dmrt1bY and its autosomal coorthologue dmrt1a in medaka. Sex Dev 1, 197–206 (2006).
Herpin, A. et al. Divergent expression regulation of gonad development genes in medaka shows incomplete conservation of the downstream regulatory network of vertebrate sex determination. Mol Biol Evol 30, 2328–2346, 10.1093/molbev/mst130 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169, 10.1093/bioinformatics/btu638 (2014).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome biol 11, R106, 10.1186/gb-2010-11-10-r106 (2010).
Acknowledgements
This work was supported by grants to Y.H. from the National Research Foundation Singapore (NRF-CRP7-2010-03) and to M.L. from the National Natural Science Foundation of China (31372520) and the Shanghai Universities First-Class Disciplines Project of Fisheries and by scholarship to X.Z. and F.Z. from the National University of Singapore.
Author information
Authors and Affiliations
Contributions
K.N., Y.N., J.L. and Y.H. designed the study. X.Z., G.G., M.L., F.Z., Q.L. and A.H. did research. X.Z. and Y.H. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, X., Guan, G., Li, M. et al. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci Rep 6, 19738 (2016). https://doi.org/10.1038/srep19738
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19738
This article is cited by
-
Transcriptome characterization of gonadal sex differentiation in Pacific bluefin tuna, Thunnus orientalis (Temminck et Schlegel)
Scientific Reports (2023)
-
dmrt3, nom1, abce1, and pkmyt1 play key roles in gonadal sex determination in Acrossocheilus fasciatus
Aquaculture International (2023)
-
Sex determination mechanisms and sex control approaches in aquaculture animals
Science China Life Sciences (2022)
-
Identification of sex differentiation-related microRNA and long non-coding RNA in Takifugu rubripes gonads
Scientific Reports (2021)
-
Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica
Fisheries Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.