Abstract
Human females exhibit greater social interest and skills relative to males, appearing in infancy, suggesting biological roots; however, male and female infants may be treated differently, potentially causing or amplifying sex differences. Here, we tested whether sex differences in social motivation emerge in infant monkeys (n = 48) reared in a controlled postnatal environment. Compared to males, females at 2–3 weeks looked more at conspecifics’ faces (d = 0.65), especially the eyes (d = 1.09) and at 4–5 weeks exhibited more affiliative behaviors (d = 0.64), including gesturing, looking and proximity to familiar and unfamiliar human caretakers. In sum, converging evidence from humans and monkeys suggests that female infants are more social than males in the first weeks of life and that such differences may arise independent of postnatal experience. Individual differences in social interest have wide-ranging developmental consequences, impacting infants’ social interaction quality and opportunities for learning. Understanding the evolution of sex differences and their developmental emergence is necessary to best support infants with varying levels of sociality.
Similar content being viewed by others
Introduction
In humans, sex differences appear at the level of the brain, cognition and behavior1,2, across numerous domains, including physical and mental health3,4, personality5 and sexuality6. Females, compared to males, exhibit greater social sensitivity7 and stronger verbal ability8, while males outperform females on mental rotation9 and the analysis or construction of systems10. Sex differences in social behavior are already evident in infancy11. Female neonates, compared to males, make more eye contact12, are more likely to orient to faces13 and voices14, are rated as more cuddly15 and exhibit stronger emotion contagion (e.g., contagious crying16) and imitation17. Despite converging evidence of sex differences in social sensitivity early in ontogenetic development, the causes of these differences and contributions of early experience, remain unresolved.
According to one view, sex differences may, at least in part, be a consequence of evolutionary pressures, reflecting a history during which males and females faced different challenges for survival and reproduction18. According to this perspective, selective pressures may partially explain some of these sex differences19,20. For example, across most mammals, females are the primary caretakers, a role that may have increased females’, but not males’, social interest and skill interpreting nonverbal expressions, as such interests and skills, in theory, might increase offspring survival, preparing caregivers to recognize and respond to infants’ needs21,22,23. While such evolutionary proposals remain to be fully tested, they are consistent with the evidence to date.
Complicating matters, however, are reports that male and female infants are treated differently from the day of birth and throughout infancy24,25,26; therefore, differential early caregiver or other environmental stimulation may cause or contribute to sex differences. For example, in the first months of life, males are touched more and handled more roughly27, but females are verbally stimulated more28 and mothers spend more time in synchronous coordination with sons29,30, but interact more overall with daughters31. Even if such differential treatment is small32, it may nonetheless contribute to different socialization33. The extent to which sexually dimorphic behaviors reflect inborn or natural biological differences, independent of parental influence, remains untested.
Nonhuman primate (NHP) studies can begin to address this challenge34,35, as there is greater control over NHPs’ early experiences, potentially eliminating postnatal environmental causes of sex differences. In addition, macaques, like humans, are highly social and engage in complex face-to-face infant-adult interactions36. Newborn macaques possess good visual acuity and we can assess their visual attention with remote eye tracking37,38. Infant and juvenile macaques exhibit sex differences in a variety of behaviors—rough-and-tumble play, peer preferences, social grooming and infant interest—that parallel sex differences in humans39,40. As in humans, macaque mothers treat male and female infants differently, for example, grooming female infants more than males and responding more to males’ separation vocalizations41,42,43. In fact, macaques are one of the only species, besides humans, in which the early social environment has been shown to influence sex differences in behavior44. However, no study, to date, has controlled human or NHP infants’ environment from birth; therefore, the extent to which sex differences are experience-independent, or due to infants’ early experiences, have yet to be explored.
In this study, we assessed sex differences in nursery-reared macaque infants, raised in homogenous, controlled environments (see Suppl. Info.). Remote eye tracking revealed that females, compared to males, looked more at videos of expressive conspecific faces and especially the eye region (Fig. 1) at 2 to 3 weeks of age. Furthermore, in a human interaction test, females displayed more affiliative behaviors (e.g., facial gestures, close proximity) to familiar and unfamiliar social partners, compared to males when 4 to 5 weeks old. In sum, in the absence of different postnatal environments, across two tasks, females appeared more social than males. While the long-term consequences of these individual differences are currently unknown in macaques, in humans, diminished social motivation in infancy may signify individuals at risk for poor developmental outcomes45. Our results offer compelling evidence that, through this novel approach, we can begin to disentangle biological (postnatal experience-independent) and experiential influences on sexually dimorphic behaviors, such as social interest.
Results
Eye Tracking Test
We first carried out a 3 × 3 × 2 mixed design ANOVA on look durations to the face, with the within-subjects factors of Expression (Fear, Lipsmacking [LPS], Threat) and Phase (Expression, Still, Turn) and the between-subjects factor of Sex (Female, Male). There was a main effect of Phase, F(2,60) = 12.40, p < 0.001, ηp2 = 0.246, in which infants looked more during the period of Expression (M = 2.34, SD = 0.65) and Turn (M = 2.29, SD = 0.76), compared to Still (M = 1.98, SD = 0.64), t(47) > 4.19, ps < 0.001, ds > 0.61. There was a main effect of Sex, F(1,38) = 6.58, p = 0.014, η2 = 0.148, in which females looked more (M = 2.41 sec, SD = 0.55) than males (M = 2.03 sec, SD = 0.61), Fig. 2a. There were no other effects, ps > 0.05.
Eye tracking task results.
Across both measures, there were main effects of sex: females (gray) > males (red), *ps < 0.05. Error bars reflect standard error of the mean. (a) Look durations to faces. (b) The eye-mouth index (EMI) reflects the relative amount of time looking to the eye and mouth regions of the face, with values of .50 indicating equal looking to eyes and mouth and values above .50 indicating more looking to the eyes.
We next carried out a 3 × 3 × 2 mixed design ANOVA on the Eye-Mouth-Index (EMI), with the within-subjects factors of Expression and Phase and the between-subjects factor of Sex. This analysis revealed a main effect of Phase, F(2,60) = 10.10, p < 0.001, ηp2 = 0.252, in which there was a lower EMI (more looking to the mouth) for the periods of Expressions (M = 0.69, SD = 0.17) compared to either Still (M = 0.81, SD = 0.12) or Turn (M = 0.79, SD = 0.19), t(47) > 3.75, ps ≤ 0.001, ds > 0.54. There was a main effect of Sex, F(1,30) = 7.07, p = 0.012, η2 = 0.191, in which females had higher EMI (M = 0.83, SD = 0.06) compared to males (M = 0.70, SD = 0.14) (Fig. 2b; Fig. S1). There were no other effects, ps > 0.05.
Human Interaction Test
We carried out three 2 × 2 mixed-design ANOVAs, one on each composite measure—Affiliative Social, General Arousal and Stress/Anxiety—with the between subjects factor Sex and the within subjects factor of Person Type (Stranger, Familiar), Fig. 3. The ANOVA on Affiliative Social revealed a main effect of Sex, F(1, 46) = 5.04, p = 0.030, η2 = 0.099, in which female infants were more social (M = 0.23, SD = 0.62) compared to males (M = −0.18, SD = 0.68). There were no main effects of Sex for either General Arousal nor for Stress/Anxiety, F(1, 46) = 0.022, p = 0.882 and F(1, 46) = 0.016, p = 0.899, respectively. There were no main effects or interactions for the factor Person Type for any of the composite measures (Social/Affiliation: F(1,46) = 0.031, p = 0.861, F(1,46) = 1.987, p = 0.165; General Arousal: F(1, 46) = 0.002, p = 0.964, F(1,46) = 0.129, p = 0.721; Stress/Anxiety: F(1, 46) = 0.001, p = 0.976, F(1, 46) = 0.173, p = 0.680, respectively).
Discussion
There is considerable variability in infants’ social interest37. One factor that seems to predict infants’ sociality is sex7,11,12,13,14,15,16,17; however, the causes of these sex differences and the role of the early environment, in particular, have yet to be uncovered. Here, we tested whether infants reared in controlled homogenous environments from birth would still exhibit sex differences in social interest. We found consistent sex differences in infants’ social interest: females, compared to males, exhibited greater social interest and affiliative behavior. These results are striking because infants were reared in carefully controlled environments, making environmental (e.g., caregiving) causes, theorized to account for sex differences in humans24,25,26,27,28,29,30,31,32,33, unlikely. An internal quality assessment of caregiver training protocols confirmed that caregivers were not more sensitive to female than male infants (see Suppl. Info.). Note that we do not want to make any claims as to the generalizability of this observation; rather, we believe it shows that the specific training protocols at this facility were effective in preventing caregiver bias. Thus, it is unlikely that our findings of greater social interest among females can be attributed to differences in caregivers’ behavior. The present study is the first (in any primate species, including humans) to provide evidence of experience-independent sex differences in sociality present or emerging soon after birth.
Caregiver-infant interactions are complex and multimodal, varying across cultures and contexts: some occurring primarily through tactile stimulation (e.g., holding, patting, stroking), while others rely more on visual (e.g., mutual gaze, facial gestures) or verbal interactions36,46,47. Thus, infant sociality can be expressed in different ways across various cultural contexts. Despite this variability, certain key features appear universal in human and nonhuman primate infants, including an early attraction to faces38. Here, we found sex differences in sociality across two tasks. First, in an eye tracking task, in which 2- to 3-week-old monkey infants viewed affiliative, fearful and threatening monkey facial expressions, females, compared to males, spent more time looking at faces and spent a greater proportion of time looking to the eyes. Similarly, in a human-interaction task, in which 4- to 5-week-old monkey infants were presented with unexpressive human models attempting eye contact, females, compared to males, engaged in more affiliative behaviors towards both familiar and unfamiliar humans. Together, these results suggest that macaque infants in controlled postnatal environments exhibit sex differences in social interest and affiliation, with females appearing more interested in social interactions than males. Our data suggest that such differences are unlikely to be exclusively due to different postnatal environments, as the postnatal environment was controlled in the present study. Rather, there appear to be experience-independent sex differences in social behavior in early infancy and the present results begin to reveal the nature of these sex differences that are not due to social experiences. While the present study does not rule out the possibility that experiences may also contribute to sex differences—and we agree with others24,25,26 that they likely do—it suggests that differential experiences are unnecessary for the initial expression of sex differences in social behaviors in infant monkeys. These data provide additional support for the hypothesis that sex differences in social behavior can arise independent of social mechanisms48.
Our data are consistent with reports in human infants that females are drawn more to biological motion and faces compared to males13,17,49,50. The present study did not include nonsocial control stimuli, which may be more engaging for males; future assessments that include social and nonsocial stimuli presented in direct competition51 could help clarify sex differences in infants’ relative visual interest. Nonetheless, the present paradigm revealed female infants, compared to males, looked longer at facial expressions, suggesting females may find faces intrinsically more rewarding.
Our results are also consistent with findings in human infants that females, compared to males, spend more time in eye contact12,25, a difference that persists through childhood and into adulthood52. In the present study, this sex difference may reflect the fact that eye contact is one of the first ways in which infants can engage in social exchanges, which, in humans, is speculated to be foundational for later social skills53. Indeed, newborn monkeys who look more at the eye region of faces are also better at imitating facial gestures37 and imitation predicts later social skills, such as gaze following (i.e., the ability to look where another individual is looking)35,54.
In addition, differential parental behavior towards infants as a function of infant sex may, at least in part, stem from and amplify initial biological differences. Adult macaques—much like adult humans—differentially treat infants depending on their sex41,42,43,46; however, it is unclear how this differential treatment may impact infants’ early social interest. Future studies in infant NHP may be fruitful in this regard, as they allow us to explore the extent to which natural maternal interactions or other specific aspects of infants’ early social experiences may drive or dampen early sex differences. Further work is needed on these potential feedback loops and interactions; conclusions about causality are therefore premature at present.
While studies in human infants have found that females are better at discriminating facial expressions than males55, we did not find any differential looking across our facial expression types. This may be because 2- to 3-week-old infant monkeys do not understand these expressions until around 2 to 3 months of age56. While these infants had previously seen human models lipsmacking, in unrelated studies (see Methods), they had no exposure to adult monkeys producing these expressions, nor did they have any previous exposure to open-mouth threat expressions or fear grimace expressions, as were shown here. Here we did not explicitly test facial expression discrimination, nor did we record infants’ other behavioral reactions beyond their viewing patterns (e.g., their emotional reactions or facial expressions). Many questions, therefore, remain regarding newborn emotion processing. For example, in human neonates, contagious crying—hypothesized to reflect an early form of empathy in infants—appears stronger in females than males16, but such assessments have yet to be carried out in infant NHP.
Our finding that female macaque infants, compared to males, exhibited more social and affiliative behaviors towards both familiar and novel human models suggest that female infant monkeys are more interested in social interactions compared to males. We found no differences in their general arousal (e.g., sleepiness) or behaviors indicative of stranger-anxiety (e.g., self-directed behaviors), which could have been alternative explanations for the observed effects. Nonetheless, these data seem consistent with reports in human infants. In humans, female infants, compared to males, are more responsive to their mother’s voice, initiate more maternal social interactions and spend more time in close proximity to their mothers57,58. In addition, human 3-month-old females smile more than males while interacting with strangers in face-to-face interactions59.
In the human interaction task, the human produced a neutral face, attempting to maintain eye contact with the infant. Although speculative, it is possible that male infants may have been more likely to interact had the human initiated the interaction with a communicative gesture or, at the least, if the human had appeared more responsive to the infant’s interaction attempts. One interpretation of our results is that it may take a more engaging adult partner to attract male infants’ interest relative to females. For example, when mothers were instructed to direct fearful expressions at their infants in a social referencing task (i.e., infants had to use their mother’s expression to respond to an ambiguous situation), mothers’ expressions were less intense when directed at female infants compared to male infants, perhaps reflecting the mothers’ awareness of their infants’ sensitivity to such expressions60. Thus, this human interaction task seems to assess some combination of infants’ ability, interest and persistence in initiating a social interaction, even one that appears failing. A similar task in human infants is the still-face paradigm, in which a parent interacts with the infant normally and then produces an unresponsive still-face61. We are unaware of any reports that female infants try harder to re-engage parents in social interactions during this still-face test, as they appeared to do in the present study; however, in one report female infants did appear more distressed than males61.
In humans, mother-stranger discrimination has been reported to occur earlier in female infants, compared to males, possibly reflecting faster social development in female infants62. We also expected differences in infants’ reactions to familiar compared with novel human models. However, infant monkeys do not generally exhibit fear of strangers or novelty until 2.5- to 3-months-old; here, infants may have been too young to exhibit noticeably different responses to familiar and unfamiliar social partners, at least in this context.
In summary, infant monkeys appear to exhibit experience-independent sex differences in the first month of life, with female infants, compared to males, displaying more visual attention and affiliative behaviors towards social stimuli, including increased gaze to faces and especially the eyes, facial gestures, proximity and touch. The present study is not without limitations, however. We were unable to completely rule-out other potential causes of sex differences, such as more subtle differential treatment by caregivers or research staff, especially beyond 3 weeks of age. However, we think these are unlikely to account for the present findings for two reasons. First, we found infant sex differences within the first 3 weeks of life, so even if infants are treated differently after 3 weeks that cannot account for the present findings. Second, infants participated in only one test with research staff prior to this study (see Suppl Info.), making it unlikely that differential treatment during this standardized interaction is responsible for our findings. Further observations assessing subtler differential treatment of infants, however, are a worthy future direction. Another challenge that needs to be addressed in future work is how to disentangle social skill from social motivation45, because without the later, the former cannot be assessed. Making tasks equally engaging for male and female infants may be difficult, but is nonetheless critical for fairly assessing possible differences in social skills. Finally, the extent to which these findings are generalizable to other cultural contexts, or predictive of social outcomes at later ages, is yet to be determined.
Newborns’ early capacities to engage with social partners—including their interest in faces, eye-contact and other affiliative expressions (e.g., facial gestures)—provide an early window which may ultimately be useful for understanding individual differences and predicting developmental trajectories47. Visual attention to social stimuli seems particularly promising in this regard63. Early sex differences may be related to later behaviors, including sex differences in developmental disorders and disabilities24,64. Finally, our findings are consistent with evolutionary hypotheses about the origin of sex differences in social behavior20, possibly reflecting an evolved mechanism enabling the care of nonverbal infants, ultimately increasing infant survival (i.e., primary caretaker hypothesis21). Determining specific causes of sex differences necessitates further study at multiple levels, including proximate and ultimate causes and their interactions. Nonetheless, studying sex differences across development in humans and NHP in controlled environments may provide important insights into the evolution of sex differences.
Method
Subjects
Subjects were 48 healthy, full-term infant rhesus macaques (Macaca mulatta). For the eye tracking task, we tested infants at 2–3 weeks (10–28 days old), including 21 females (M = 18.9 days, SD = 2.2) and 27 males (M = 18.7 days, SD = 2.5). For the human interaction task, we tested infants again at 4–5 weeks (28–37 days old), including 21 females (M = 31.7 days, SD = 2.0) and 27 males (M = 31.4 days, SD = 1.9). Infants were separated from their mothers on the first day of life, after which they were reared in a nursery facility. Infants were tested prior to introduction into social groups with conspecifics. Human caretakers and research staff followed strict protocols ensuring male and female infants were not treated differently. All infants participated in unrelated studies that involved structured social interactions with humans in the first week of life, including neonatal imitation36; because these were structured, they were preformed in the same way for all infants. For details, see the Suppl. Info. The study was approved by the Animal Care and Use Committee, conducted in accordance with the Guide for the Care and Use of Laboratory Animals and complied with the Animal Welfare Act.
Materials and Procedure
Eye movements were recorded via corneal reflection using either a Tobii T60XL (n = 38) or a Tobii TX300 (n = 10) eye tracker, with a remote 61 cm and 58.4 cm monitor, respectively, both with integrated eye tracking technology and a sampling rate of 60 Hertz. We used Tobii Studio software (Tobii Technology, Sweden) to collect and summarize the data.
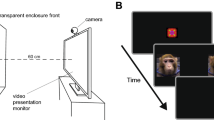
At 2–3 weeks of age, infants viewed three silent video stimuli, depicting an animated adult monkey looking at infants and exhibiting either LPS (an affiliative gesture), fear grimaces, or threats (see Suppl. Info.). The macaque, making eye contact with the viewer, displayed a 5 sec expression (fear grimaces, LPS, or threats), followed by a 5 sec neutral face (eye blinks and small head movements were included to maintain an animated impression). Then the macaque turned away at a 45° angle, breaking eye contact and then turned back to the viewer. This sequence was repeated a second time, for a total duration of 30 sec.
At the beginning of a session, an experimenter held the infant approximately 60 cm from the screen. Each infant was calibrated to Tobii Studio’s five preset locations. Infants were tested with one video per day. Videos were shown in a random order.
At 4–5 weeks of age, infants participated in a human interaction task. A human model was seated in front of the infant’s home cage, 30 cm from the cage front and made eye contact with the infant. During the first 2 minutes of the test, the human model only looked at the infant. During the second 2 minutes of the test the human placed a hand on the infant’s feeder box, located just outside of the infant’s home cage, while continuing to maintain eye contact. Sessions were videotaped (Sony Digital Video HDR-CX560V) with only the infant in view. In total, each session was 4 minutes. We were primarily interested in social behaviors, but also assessed general arousal and anxiety-related behaviors (e.g., self-directed behaviors). In total, we scored 15 behaviors, including affiliative social behaviors: LPS and tongue protrusion facial gesture frequencies, total time looking at model, time touching model’s hand, time in close proximity to model (within arm’s reach of font of cage). Two coders scored behaviors using The Observer XT (Noldus). See Suppl. Info. for details.
Data Analysis
In Tobii Studio we created several Areas of Interest (AOIs) for analysis: Face, Eye and Mouth AOI (see Fig. 1 and Suppl. Info.). We created an Eye-Mouth-Index (EMI) using Eyes / (Eyes + Mouth) in order to compare looking to both areas37. Values closer to 1 indicate more looking to the eyes and values closer to 0 indicate more looking to the mouth.
For the human interaction task, we computed three composite scores by standardizing then averaging individual behavior scores. The Affiliative Social composite included facial gestures and looking, touching, or being in close proximity to model. The General Arousal composite included exploration, locomotion and sleeping. The Stress and Anxiety composite included scratching, fear grimacing, vocalizing, clinging to surrogate, self-clasping, self-sucking and stereotypies. Interobserver reliability was high (see Suppl. Info.).
Ethical approval
Research methods were approved by the Animal Care and Use Committee, Eunice Kennedy Shriver National Institute of Child Heath and Human Development, National Institutes of Health (ASP#11-043 and #14-043). The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals and complied with the Animal Welfare Act.
Additional Information
How to cite this article: Simpson, E. A. et al. Experience-independent sex differences in newborn macaques: Females are more social than males. Sci. Rep. 6, 19669; doi: 10.1038/srep19669 (2016).
References
Baron-Cohen, S., Knickmeyer, R. C. & Belmonte, M. K. Sex differences in the brain: implications for explaining autism. Science 310, 819–823. (2005).
Ellis, L. Identifying and explaining apparent universal sex differences in cognition and behavior. Pers. Individ. Dif . 51, 552–561. (2011).
Macintyre, S., Hunt, K. & Sweeting, H. Gender differences in health: are things really as simple as they seem ? Soc. Sci. Med. 42, 617–624. (1996).
Young, L. J. & Pfaff, D. W. Sex differences in neurological and psychiatric disorders. Front. Neuroendocrinol. 35, 253–254. (2014).
Schmitt, D. P., Realo, A., Voracek, M. & Allik, J. Why can’t a man be more like a woman? Sex differences in Big Five personality traits across 55 cultures. J. Pers. Soc. Psychol. 94, 168–182. (2008).
Schmitt, D. P. et al. A reexamination of sex differences in sexuality new studies reveal old truths. Curr. Dir. Psychol. Sci. 21, 135–139. (2012).
Christov-Moore, L., et al. Empathy: gender effects in brain and behavior. Neurosci. Biobehav. Rev. 46, 604–627. (2014).
Stoet, G. & Geary, D. C. Sex differences in mathematics and reading achievement are inversely related: sithin-and across-nation assessment of 10 years of PISA data. PLOS ONE 8, e57988. (2013).
Voyer, D., Voyer, S. & Bryden, M. P. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol. Bull. 117, 250–270. (1995).
Baron-Cohen, S., Richler, J., Bisarya, D., Gurunathan, N. & Wheelwright, S. The systemizing quotient: an investigation of adults with Asperger syndrome or high–functioning autism and normal sex differences. Phil. Trans. R. Soc. B 358, 361–374. (2003).
Alexander, G. M. & Wilcox, T. Sex differences in early infancy. Child Dev. Perspect . 6, 400–406. (2012).
Hittelman, J. H. & Dickes, R. Sex differences in neonatal eye contact time. Merrill-Palmer Q. Behav. Dev . 25, 171–184. (1979).
Connellan, J., Baron-Cohen, S., Wheelwright, S., Batki, A. & Ahluwalia, J. Sex differences in human neonatal social perception. Infant Behav. Dev . 23, 113–118. (2000).
Osofsky, J. D. & O’Connell, E. J. Patterning of newborn behavior in an urban population. Child Dev. 48, 532–536. (1977).
Benenson, J. F., Philippoussis, M. & Leeb, R. Sex differences in neonates’ cuddliness. J. Genet. Psychol. 160, 332–342. (1999).
Sagi, A. & Hoffman, M. L. Empathic distress in the newborn. Dev. Psychol . 12, 175–176. (1976).
Nagy, E., Kompagne, H., Orvos, H. & Pal, A. Gender-related differences in neonatal imitation. Infant Child Dev. 16, 267–276. (2007).
Buss, D. M. Psychological sex differences: origins through sexual selection. Am. Psychol. 50, 164–168. (1995).
Wood, W. & Eagly, A. H. A cross-cultural analysis of the behavior of women and men: implications for the origins of sex differences. Psychol. Bull. 128, 699–727. (2002).
Schmitt, D. P. in The Evolution of Sexuality (eds Shackelford, T. K. & Hansen, R. D. ) pp 221–256 (Springer International Publishing, 2015).
Babchuk, W. A., Hames, R. B. & Thompson, R. A. Sex differences in the recognition of infant facial expressions of emotion: the primary caretaker hypothesis. Ethol. Sociobiol . 6, 89–101. (1985).
Maestripieri, D. & Pelka, S. Sex differences in interest in infants across the lifespan. Human Nature 13, 327–344. (2002).
Taylor, S. E. et al. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 107, 411–429. (2000).
Fausto-Sterling, A., Coll, C. G. & Lamarre, M. Sexing the baby: part 1–What do we really know about sex differentiation in the first three years of life? Soc. Sci. Med. 74, 1684–1692. (2012).
Leeb, R. T. & Rejskind, F. G. Here’s looking at you, kid! A longitudinal study of perceived gender differences in mutual gaze behavior in young infants. Sex Roles 50, 1–14. (2004).
Moore, D. S. Sex differences in normal fetuses and infants: a commentary. Child Dev. Perspect . 6, 414–416. (2012).
Lewis, M. State as an infant-environment interaction: an analysis of mother-infant interaction as a function of sex. Merrill-Palmer Q 18, 95–121. (1972).
Stern, M. & Karraker, K. H. Sex stereotyping of infants: a review of gender labeling studies. Sex Roles 20, 501–522. (1989).
Malatesta, C. Z. & Haviland, J. M. Learning display rules: the socialization of emotion expression in infancy. Child Dev. 53, 991–1003. (1982).
Tronick, E. Z. & Cohn, J. F. Infant-mother face-to-face interaction: age and gender differences in coordination and the occurrence of miscoordination. Child Dev. 60, 85–92. (1989).
Clearfield, M. W. & Nelson, N. M. Sex differences in mothers’ speech and play behavior with 6-, 9- and 14-month-old infants. Sex Roles 54, 127–137. (2006).
Lytton, H. & Romney, D. M. Parents’ differential socialization of boys and girls: a meta-analysis. Psychol. Bull. 109, 267–296. (1991).
Rubin, J. Z., Provenzano, F. J. & Luria, Z. The eye of the beholder: parents’ views on sex of newborns. Am. J. Orthopsychiatry 44, 512. (1974).
Meredith, S. L. Comparative perspectives on human gender development and evolution. Am. J. Phys. Anthropol. 156, 72–97. (2015).
Gerson, S., Simpson, E. A. & Paukner, A. Drivers of social cognitive development in human and non-human primate infants. In J. Sommerville, & J. Decety (Eds.), Frontiers in Developmental Science Series: Social Cognition. (Psychology Press, in press).
Ferrari, P. F., Paukner, A., Ionica, C. & Suomi, S. J. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr. Biol. 19, 1768–1772. (2009).
Paukner, A., Simpson, E. A., Ferrari, P. F., Mrozek, T. & Suomi, S. J. Neonatal imitation predicts how infants engage with faces. Dev. Sci. 17, 833–840. (2014).
Simpson, E. A. et al. Face detection and the development of own-species bias in infant macaques. Child Dev. (in press).
Hassett, J. M., Siebert, E. R. & Wallen, K. Sex differences in rhesus monkey toy preferences parallel those of children. Horm. Behav. 54, 359–364. (2008).
Roney, J. R. & Maestripieri, D. in Primate Psychology (ed Maestripieri, D. ) 171–204 (Cambridge: Harvard University Press, 2003).
Jensen, G. D., Bobbitt, R. A. & Gordon, B. N. Sex differences in the development of independence of infant monkeys. Behav . 30, 1–13. (1976).
Mitchell, G. & Brandt, E. M. Behavioral differences related to experience of mother and sex of infant in the rhesus monkey. Dev. Psychol . 3, 149. (1970).
Tomaszycki, M. L., Davis, J. E., Gouzoules, H. & Wallen, K. Sex differences in infant rhesus macaque separation–rejection vocalizations and effects of prenatal androgens. Horm. Behav. 39, 267–276. (2001).
Wallen, K. Nature needs nurture: the interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys. Horm. Behav. 30, 364–378. (1996).
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S. & Schultz, R. T. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. (2012).
Dettmer, A. M. et al. First-time rhesus monkey mothers and mothers of sons, preferentially engage in face-to-face interactions with their infants. Am J Primatol. Advance Online Publication. (in press).
Simpson, E. A., Murray, L., Paukner, A. & Ferrari, P. F. The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Phil Trans Roy Soc B . 369, 1–12 (2014).
Alexander, G. M. & Hines, M. Sex differences in response to children’s toys in nonhuman primates (Cercopithecus aethiops sabaeus). Evol. Hum. Behav. 23, 467–479. (2002).
Alexander, G. M., Wilcox, T. & Woods, R. Sex differences in infants’ visual interest in toys. Arch. Sex. Behav. 38, 427–433. (2008).
Gluckman, M. & Johnson, S. P. Attentional capture by social stimuli in young infants. Front. Psychol . 4, 527. (2013).
Pierce, K., Conant, D., Hazin, R., Stoner, R. & Desmond, J. Preference for geometric patterns early in life as a risk factor for autism. Arch. Gen. Psychiatry 68, 101–109. (2011).
Levine, M. H. & Sutton-Smith, B. Effects of age, sex and task on visual behavior during dyadic interaction. Dev. Psychol . 9, 400–405. (1973).
Farroni, T., Csibra, G., Simion, F. & Johnson, M. H. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci . 99, 9602–9605. (2002).
Simpson, E. A., Maloney, G., Ferrari, P. F., Suomi, S. J. & Paukner, A. Neonatal imitation and social experience predict gaze following in infant macaques. Sci Rep. (in press).
McClure, E. B. A meta-analytic review of sex differences in facial expression processing and their development in infants, children and adolescents. Psychol. Bull. 126, 424–453. (2000).
Sackett, G. P. Monkeys reared in isolation with pictures as visual input: evidence for an innate releasing mechanism. Science 154, 1468–1473. (1966).
Gunnar, M.R. & Donahue, M. Sex differences in social responsiveness between six months and twelve months. Child Dev. 51, 262–265. (1980).
Wasserman, G.A. & Lewis, M. Infant sex differences: ecological effects. Sex Roles 12, 665–675. (1985).
Farris, M. R. Smiling of male and female infants to mother vs stranger at 2 and 3 months of age. Psychol. Rep. 87, 723–728. (2000).
Rosen, W. D., Adamson, L. B. & Bakeman, R. An experimental investigation of infant social referencing: mothers’ messages and gender differences. Dev. Psychol . 28, 1172–1178. (1992).
Mayes, L. C. & Carter, A. S. Emerging social regulatory capacities as seen in the still-face situation. Child Dev. 61, 754–763. (1990).
Field, T. M., Cohen, D., Garcia, R. & Greenberg, R. Mother-stranger face discrimination by the newborn. Infant Behav. Dev . 7, 19–25. (1984).
Wagner, J. B., Luyster, R. J., Yim, J. Y., Tager-Flusberg, H. & Nelson, C. A. The role of early visual attention in social development. Int. J. Behav. Dev. 37, 118–124. (2013).
Knickmeyer, R. C. & Baron-Cohen, S. Topical review: fetal testosterone and sex differences in typical social development and in autism. J Child Neurol. 21, 825–845. (2006).
Acknowledgements
This work was supported by the Division of Intramural Research, NICHD and NICHD P01HD064653 (to PFF). We thank Timothy Mrozek for stimulus creation, Grace Maloney and Sheila Sutti for collecting eye tracking data, Michelle Miller, Anna Casey, Mandy Riddle, Ryan McNeil, Neal Marquez and Kristen Byers for serving as the models for the human interaction test, Stefano Kaburu for caregiver interaction observations and Kielee Jennings for data entry.
Author information
Authors and Affiliations
Contributions
A.P., P.F.F. and E.A.S. developed the study concept and design. M.S. created the video stimuli. E.A.S. and A.P. collected the data. Y.N. and E.A.S. coded infants’ behaviors. A.P. and E.A.S. analyzed and interpreted the data. E.A.S. wrote the manuscript. E.A.S., A.P., P.F.F. and S.J.S. revised and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Simpson, E., Nicolini, Y., Shetler, M. et al. Experience-independent sex differences in newborn macaques: Females are more social than males. Sci Rep 6, 19669 (2016). https://doi.org/10.1038/srep19669
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19669
This article is cited by
-
Causal evidence for the adaptive benefits of social foraging in the wild
Communications Biology (2021)
-
Developmental shifts in social cognition: socio-emotional biases across the lifespan in rhesus monkeys
Behavioral Ecology and Sociobiology (2018)
-
Preference for novel faces in male infant monkeys predicts cerebrospinal fluid oxytocin concentrations later in life
Scientific Reports (2017)
-
Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques
Psychopharmacology (2017)
-
Neonatal imitation predicts infant rhesus macaque (Macaca mulatta) social and anxiety-related behaviours at one year
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.