Abstract
The VO2 polymorphs, i.e., VO2(A), VO2(B), VO2(M1) and VO2(R), have a wide spectrum of functionalities useful for many potential applications in information and energy technologies. However, synthesis of phase pure materials, especially in thin film forms, has been a challenging task due to the fact that the VO2 polymorphs are closely related to each other in a thermodynamic framework. Here, we report epitaxial stabilization of the VO2 polymorphs to synthesize high quality single crystalline thin films and study the phase stability of these metastable materials. We selectively deposit all the phases on various perovskite substrates with different crystallographic orientations. By investigating the phase instability, phonon modes and transport behaviours, not only do we find distinctively contrasting physical properties of the VO2 polymorphs, but that the polymorphs can be on the verge of phase transitions when heated as low as ~400 °C. Our successful epitaxy of both VO2(A) and VO2(B) phases, which are rarely studied due to the lack of phase pure materials, will open the door to the fundamental studies of VO2 polymorphs for potential applications in advanced electronic and energy devices.
Similar content being viewed by others
Introduction
Vanadium dioxides (VO2) are strongly correlated d1 electron systems and are known to have several polymorphs, which include VO2(A), VO2(B), VO2(M1) and VO2(R). While the chemical formula is the same, their crystalline and electronic structures are completely different and highly complex, exhibiting many interesting electrical, optical and chemical properties owing to the strong electron correlation1,2,3. Among the aforementioned VO2 polymorphs, the rutile VO2(R) and the monoclinic VO2(M1) have been the most widely studied phases due primarily to their metal-to-insulator transition (MIT) temperature close to room temperature (68 °C)1,2,3. Since this phase transition is accompanied by a huge change in resistivity by three orders of magnitude, VO2(R) and VO2(M1) have attracted tremendous attention for the electronic and optical applications, such as smart windows4, frequency-agile metamaterials5,6 and electrical switches7−9.
The monoclinic VO2(B) phase has also been explored. However, the focus has been on utilization of the open framework, which originates from the edge-sharing VO6 octahedra10−12. Such open framework makes VO2(B) a promising energy material, which can be used as electrodes in Li-ion batteries13. Chen et al. first reported the growth of textured VO2(B) films on (001)-oriented SrTiO3 substrates14, but pure phase could be stabilized only at thin (<25 nm) films. It is known that the growth of single crystalline VO2(B) is very challenging due to the complex crystal structure14,15. Similarly, the study of the tetragonal VO2(A) has so far been very limited15−17 as compared to other VO2 polymorphs, due to the difficulty in synthesizing phase pure crystals. Thus, their physical properties and potential for technical applications have not been much explored.
One of the main reasons for the difficulty in preparing phase pure VO2 polymorphs is the narrow range of phase diagram3 and, more importantly, the VO2 polymorphs are closely related to each other in a thermodynamic framework10,12,16. For example, it has been shown that the VO2(A) and VO2(B) phases are metastable in bulk and undergo an irreversible phase change into VO2(R) upon heating10,12,16, resulting in a mixture of VO2 polymorphs. The formation of such mixed phases hinders the accurate understanding of the physical properties of the VO2 polymorphs. Hence, preparation of phase pure and high quality crystalline materials has been one of the major challenges in VO2 research.
Epitaxial stabilization of crystalline materials by formation of low energy interface is a well-known approach to creating pure phase materials18,19,20,21,22. Because the stability of these non-equilibrium materials is affected by both thermodynamic and kinetic factors, the highly non-equilibrium film growth conditions offered by pulsed laser epitaxy (PLE) provide a unique opportunity to discover a wide range of materials with unprecedented functionalities.
Here, we report comparatively the physical properties of four VO2 polymorphs (i.e., R, M1, A and B phases) epitaxially stabilized by PLE on various perovskite substrates with different crystallographic orientations, i.e., ABO3(001), ABO3(011) and ABO3(111). Distinctively contrasting phase stability, lattice motions and transport properties reported here will provide useful information to develop VO2-based electronic devices and energy materials.
Results and Discussion
In order to selectively grow VO2 polymorphs, commercially-available perovskite-oxide substrates, including TbScO3 (TSO), SrTiO3 (STO), (LaAlO3)0.3(SrAl0.5Ta0.5O3)0.7 (LSAT), LaAlO3 (LAO) and YAlO3 (YAO), were used. As summarized in Table 1, we were able to epitaxially grow (1) the tetragonal VO2(A) phase on (011)-oriented STO and LAO substrates; (2) the monoclinic VO2(B) phase on a wide selection of (001)-oriented substrates, including pseudo-cubic TSO, STO, LSAT, LAO and pseudo-cubic YAO; and (3) the monoclinic VO2(M1) phase on (111)-oriented STO, LSAT and LAO substrates, which commonly have a 3m surface symmetry.
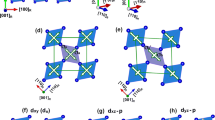
The selective growth occurs due to preferential in-plane lattice matching of perovskite-oxide substrates with the VO2 polymorphs. VO2(B) has a low-symmetry monoclinic structure (space group of C2/m (12)) with lattice constants of a = 12.03 Å, b = 3.69 Å, c = 6.42 Å and β = 106.6o, as summarized in Table 1 and as schematically shown in Fig. 1a. Various X-ray diffraction (XRD) scans, including θ−2θ scans shown in Fig. 2a and ϕ scans shown in Fig. 2d, for VO2(B) films on (001)STO (aSTO = 3.905 Å) confirmed the following epitaxy relationship: (001)VO2(B) || (001)STO and [100]VO2(B) || [100]STO (see Fig. 2g). The lattice mismatch  was −2.6% for [010]VO2(B) || [010]STO and +5.8% for [100]VO2(B) || [100]STO, where the negative and positive signs indicate compressive and tensile strain, respectively.
was −2.6% for [010]VO2(B) || [010]STO and +5.8% for [100]VO2(B) || [100]STO, where the negative and positive signs indicate compressive and tensile strain, respectively.
XRD θ−2θ scans of (a) VO2(B), (b) VO2(A) and (c) VO2(M1) thin films on STO (001), (011) and (111) substrates, respectively. The inset in (c) shows XRD scans of the VO2(R) phase (red line) obtained at 100 °C by heating the VO2(M1) film (blue line), which is above the Tc = 68 °C. From φ scans shown in (d) VO2(B), (e) VO2(A) and (f) VO2(M1) and VO2(R) thin films, in-plane lattice matching is schematically illustrated as shown in (g–i).
VO2(A) has a tetragonal structure (space group of P42/ncm (138)) with lattice constants of a = b = 8.43 Å and c = 7.68 Å, as schematically shown in Fig. 1b. We found that the single crystalline VO2(A) phase could be grown best on (011)STO with the following epitaxy relationship: (100)VO2(A) || (011)STO and [010]VO2(A) || [011]STO (see Fig. 2h), as confirmed by XRD θ−2θ scans (see Fig. 2b) and ϕ scans (see Fig. 2e). The mismatches along the two orthogonal directions, i.e., [010]VO2(A) || [011]STO and [001]VO2(A) || [100]STO are −1.7 and +1.7%, respectively.
The VO2(M1) phase has a low-symmetry monoclinic structure (space group of P21/c (14)) with lattice constant of a = 5.38 Å, b = 4.52 Å, c = 5.74 Å and β = 122.6o, as schematically shown in Fig. 1c. There have been several reports on the successful growth of VO2(M1) films on substrates with a 3m surface symmetry23 such as (0001)Al2O3, (111)MgAl2O4, (111)MgO and (0001)ZnO. In our study, we mainly attempted to grow epitaxial films on (111)STO substrates to unify the substrates for VO2 polymorph films. Since the XRD peak positions of VO2(M1) are very close to those of the STO substrate, the film peaks are hardly observed in Fig. 2c. Nevertheless, as confirmed by XRD ϕ scans (see Fig. 2f), VO2(M1) could be grown on (111)STO with the following epitaxy relationship: (010)VO2(M1) || (111)STO and [001]VO2(M1) || [ 11]STO, as illustrated in Fig. 2i (see the left portion). The lattice mismatch is −3.8% along the [001]VO2(M1) || [
11]STO, as illustrated in Fig. 2i (see the left portion). The lattice mismatch is −3.8% along the [001]VO2(M1) || [ 11]STO and +2.6% along the [100]VO2(M1) || [
11]STO and +2.6% along the [100]VO2(M1) || [ ]STO.
]STO.
While the three VO2 phases listed above are accessible at room temperature from as grown films, we also tried to access to the VO2(R) phase via a structural phase transition by heating a VO2(M1) film above the Tc (68 °C). The VO2(R) has a tetragonal structure (space group of P42/mnm (136)) with lattice constants of a = b = 4.55 Å and c = 2.86 Å, as schematically shown in Fig. 1d. As shown in the inset of Fig. 2c, we were able to confirm the phase transition into the VO2(R) phase by performing an XRD θ−2θ scans at 100 °C, which is higher than the Tc. Both VO2(R) and VO2(M1) phases on (111)STO are (010)-oriented. The epitaxial relationship for VO2(R) on (111)STO is illustrated in Fig. 2i (see the right portion) as follows: (010)VO2(R) || (111)STO and [100]VO2(R) || [ 11]STO with the lattice mismatch of +4.9% along the [100]VO2(R) || [
11]STO with the lattice mismatch of +4.9% along the [100]VO2(R) || [ 11]STO direction and −3.6% along the [001]VO2(R) || [01
11]STO direction and −3.6% along the [001]VO2(R) || [01 ]STO direction.
]STO direction.
Among the growth parameters, we found that a proper choice of the substrate temperature, Ts, is critical, in particular for VO2(A) and VO2(B) phases on perovskite substrates. As shown in Table 1, we could reproducibly grow VO2(A) and VO2(B) phases when Ts was lower than 430 °C. On the other hand, the growth of VO2(M1) phase was quite insensitive to Ts as we confirmed the growth of high quality films in a wide temperature window (400 ≤ Ts ≤ 600 °C).
To evaluate the thermal stability of VO2 polymorphs, epitaxial films of VO2(A), VO2(B) and VO2(M1) phases were heated up to 600 °C. We kept the samples in vacuum (~0.37 Torr) to avoid spontaneous oxidation into the V2O5 phase24. Fig. 3a,b show the phase evolution of VO2(B)/STO(001) and VO2(A)/STO(011), respectively, characterized by XRD θ−2θ scans as a function of temperature. In case of VO2(B) on STO(001), upon heating, XRD peaks corresponding to (00l) VO2(B) disappeared above 430 °C and then the (330) VO2(A) peak subsequently appeared above 440 °C, indicating the formation of polycrystalline VO2(A). When we further increased Ts, the VO2(A) phase disappeared above 470 °C and the polycrystalline VO2(R) phase appeared above 520 °C. This transformation, i.e., VO2(B) → VO2(A) → VO2(R), indicates that the structural frameworks are similar among the phases. The first transition to A-phase is known to associate with the realignment of VO6 octahedra from edge shared to face shared10 and, the second transition to the R-phase is attributed to the reorientation of the half of the VO6 octahedra10.
Real time XRD θ−2θ scans of (a) VO2(B)/STO(001) and (b) VO2(A)/STO(011) samples as a function of temperature in 0.37 Torr of air. A clear phase change was observed from both samples, indicating that the phases are in close proximity with each other. The phase changes were, however, irreversible upon cooling. It is also worth noting that there are temperature gaps where the XRD peaks are hardly seen before showing up the polycrystalline phases. They are 470–520 °C in (a) and 430–470 °C in (b). We attribute this to the films in metastable state undergoing polycrystallization during the phase transition, even though it is hard to clearly identify due to the suppressed XRD peaks.
As shown in Fig. 3b, the VO2(A)/STO(011) also revealed similar thermal stability. The peaks corresponding to (l00) VO2(A) disappeared above 430 °C and polycrystalline VO2(R) was subsequently formed above 470 °C. The phase transitions of both VO2(B) and VO2(A) were irreversible upon cooling. The irreversible phase transformation of VO2 polymorphs is similar to what was observed in other binary oxide polymorphs. For example, TiO2 is known to undergo a transition from the anatase to the rutile phase via brookite25. As the crossover instability in TiO2 polymorphs was understood by closely balanced enthalpy among these phases26, further thermodynamic studies will be useful to understand the phase instability in VO2. The thermal instability of VO2(A) and VO2(B) explains the formation of mixed phase VO2 polymorphs with VO2(R) as an impurity phase often observed from films grown above 430 °C. The observation of MIT at 68 °C in VO2(A) and VO2(B) films grown above 430 °C clearly indicates inclusion of VO2(R) as an impurity phase14. We note that, on the other hand, the VO2(M1) phase was converted into VO2(R) at ~68 °C upon heating and was stable up to 600 °C (data not shown). Upon cooling, VO2(R) was converted back to VO2(M1), indicating a reversible phase evolution with good thermal stability.
Since the VO2 polymorphs have distinct structures, one can expect highly contrasting vibrational characteristics of lattice. Thus, identifying the phonon mode is a good measure of phase purity. In order to comparatively understand the phonon modes, Raman spectroscopy was carried out for the VO2 polymorphs by growing films on LAO substrates. The latter were used because dominant Raman spectral features of LAO are isolated at very low wavelength (32 and 123 cm−1)27. As shown in Fig. 4, the VO2 polymorphs revealed contrasting Raman spectra compared to each another. As compared to Raman data available from nanostructured materials28,29,30,31, we were able to confirm the phase purity of our epitaxial films.
In addition to the phase confirmation, the Raman spectra from VO2 provide more detailed information about the local structure. There are three sets of V−O modes29 within wavenumber of 100−1100 cm−1. At low wavenumber (<400 cm−1), the bands are assigned to V−O−V bending modes; at intermediate wavenumber (400−800 cm−1), the bands are attributed to V−O−V stretching modes; and at high wavenumber (>800 cm−1), the bands are assigned to V=O stretching modes of distorted octahedra and distorted square-pyramids. As shown in Fig. 4a, the phonon modes in epitaxial films of VO2(B) were mainly observed at low and intermediate wavenumbers (152, 263 and 480 cm−1), indicating that bending and stretching modes of V−O−V are dominant in VO2(B). On the other hand, as shown in Fig. 4b, the phonon modes in VO2(A) were mainly observed at high and intermediate wavenumbers (152, 485 and 887 cm−1), which imply that the stretching modes of V−O−V and V=O are dominant lattice motions in VO2(A). The phonon modes in VO2(M1) are very complex and composed of stretching and bending of V−O−V and zigzag chains of V−V. The phonon modes in VO2(R) dominantly include stretching modes of V−O−V, which indicate that the crystal structure of VO2(R) is more symmetric than VO2(M1)30,32,33.
While the transport properties of VO2(M1) and VO2(R) have been extensively studied1,2,3,8,9,24,32,33,34, the physical properties of VO2(B) and VO2(A) phases have not been much explored due to difficulty in preparing phase pure thin films. Figure 5 shows the transport characteristics of VO2(B), VO2(A) and VO2(M1) films grown on STO substrates. VO2(A) showed a monotonic decrease of resistivity as increasing the temperature, typical for insulators. While still insulating over the temperature range we measured, VO2(B) revealed more or less semiconducting behaviours with much smaller resistivity compared to that of VO2(A), i.e.,  and
and  . The resistivity in our VO2(A)/STO(011) is higher than that reported in VO2(A)/STO(001)15 by one order of magnitude. The reason is unclear, but one can consider that the film on (001)STO is under a different strain state or that the growth on a (001)STO substrate may include a small amount of VO2(B) since their thermal phase boundary is relatively low10,12,16, as shown in Fig. 3. In the case of VO2(M1) phase, we also observed the MIT at 340 K from VO2(M1) to VO2(R) phase change upon heating, similarly observed from many previous studies1,2,3,8,9,24,32,33,34. The MIT accompanied a sudden decrease of the resistivity by 3−4 orders of magnitude, which is comparable to high quality epitaxial films grown on Al2O3(0001)24. This excellent performance could be attributed to the high crystallinity of our epitaxial films (Δω < 0.1o). The transition temperature is consistent with structural phase transition from VO2(M1) to VO2(R), as shown in XRD θ−2θ scan in the inset of Fig. 2c. We note that the transport properties of the films grown on LAO substrates were almost identical except for slightly decreased resistivity for films on LAO (data not shown).
. The resistivity in our VO2(A)/STO(011) is higher than that reported in VO2(A)/STO(001)15 by one order of magnitude. The reason is unclear, but one can consider that the film on (001)STO is under a different strain state or that the growth on a (001)STO substrate may include a small amount of VO2(B) since their thermal phase boundary is relatively low10,12,16, as shown in Fig. 3. In the case of VO2(M1) phase, we also observed the MIT at 340 K from VO2(M1) to VO2(R) phase change upon heating, similarly observed from many previous studies1,2,3,8,9,24,32,33,34. The MIT accompanied a sudden decrease of the resistivity by 3−4 orders of magnitude, which is comparable to high quality epitaxial films grown on Al2O3(0001)24. This excellent performance could be attributed to the high crystallinity of our epitaxial films (Δω < 0.1o). The transition temperature is consistent with structural phase transition from VO2(M1) to VO2(R), as shown in XRD θ−2θ scan in the inset of Fig. 2c. We note that the transport properties of the films grown on LAO substrates were almost identical except for slightly decreased resistivity for films on LAO (data not shown).
Overall, as explained above, the VO2 polymorphs revealed a wide range of electronic ground states, i.e., metal [VO2(R)], semiconductor [VO2(B)] and insulator [VO2(A) and VO2(M1)], depending on their crystal structure. This wide range of electronic ground states makes VO2 highly attractive over other transition metal dioxides, since most other binary oxides are either metal (CrO2: α-phase and β-phase) or insulator (TiO2: rutile, brookite and anatase). While it is not the main focus of this paper, it is worth mentioning that Goodenough32,34 obtained a semiempirical expression for the room temperature critical V−V separation Rc ≈ 2.92−2.94 Å for localized and itinerant 3d electrons in vanadium oxides.

This semiempirical criterion indicates that VO2 polymorphs can be either metal or insulator depending on the V−V separation in the distinguishable crystal structures. The VO2(R) phase has a uniform V−V separation of R = 2.88 Å (ref. 32), resulting in a metallic ground state. As shown in Fig. 1c, the VO2(M1) phase has zigzag V−V chains of R = 2.65 Å and 3.12 Å (ref. 32). The VO2(A) phase also has zigzag V−V chains of R = 3.25 Å, 3.11 Å and 2.77 Å (ref. 17) as shown in Fig. 1b. The insulating behaviours that we have observed for those M1 and A-phases are attributed to the localized electrons in shorter V−V chains with R = 2.65 Å [VO2(M1)] and 2.77 Å [VO2(A)]. Thus, overall transport behaviours of our epitaxial thin films can be well explained by Goodenough’s criterion32,34. Since VO2 polymorphs have a wide range of physical properties and, in particular, VO2(B) phase is on the verge of becoming a metal, our report on epitaxial synthesis of high quality thin films can open the door to the discovery of novel phenomena and physical properties by deliberate control of the order parameters by various means, including strain, dimensionality, confinement, etc., which can be accessible via epitaxial heterostructuring.
In conclusion, we grew epitaxial films of VO2 polymorphs. For the growth of phase pure VO2 polymorphs, a careful selection of the growth conditions was necessary especially for the temperature and oxygen pressure. Depending on the crystal orientation of substrates, we found that different phases of VO2 could be selectively grown, i.e., VO2(B)/ABO3(001), VO2(A)/ABO3(011), VO2(M1)/ABO3(111) and VO2(R)/ABO3(111). Such phases revealed unique phonon modes due to the distinctly different crystal structures and physical properties in spite of the same chemical composition. Since the VO2 polymorphs have a wide range of electronic ground states from metal [VO2(R)] and semiconductor [VO2(B)] to insulator [VO2(A) and VO2(M1)], our epitaxial thin films, which are known to be challenging to grow, will expedite our understanding of underlying physics and developing VO2 polymorphs-based electronic devices utilizing the wide selection of the electronic properties from a single composition.
Methods
Epitaxial film growth
We deposited epitaxial films (100 nm in thickness) of VO2 polymorphs on perovskite oxide substrates by pulsed laser epitaxy. We ablated a sintered VO2 target, which contains mainly the M1 phase, by a KrF excimer laser (248 nm in wavelength) at a laser fluence of 1 Jcm−2 and at a laser repetition rate of 10 Hz. By growing thin films under a wide range of P(O2) and Ts (2 mTorr < P(O2) < 25 mTorr and 350 °C < Ts < 600 °C), we found the optimal condition for VO2(A), VO2(B) and VO2(M1), as described in Table 1. It should be noted that V2O3 was formed at P(O2) < 5 mTorr and V2O5 was formed for P(O2) > 25 mTorr, due to the multivalent nature of vanadium24.
Characterization of physical properties
To investigate the dc transport properties, a physical property measurement system (Quantum Design Inc.) was used with Pt contacts in four-probe geometry. X-ray diffraction (XRD) measurements were carried out with a four-circle high-resolution X-ray diffractometer (X’Pert Pro, Panalytical) using the Cu-Kα1 radiation equipped with a hot stage (DHS 900, Anton Paar). High-temperature environmental XRD measurements were conducted under vacuum with base pressure of 0.37 Torr air. Raman spectra were recorded at various temperatures using a temperature control stage (Lincam Scientific Instruments). A Renishaw 1000 confocal Raman microscope was used to measure Raman spectra in back scattering configuration. Each spectrum is a sum average of seven individual spectra taken at different place on the sample through 20× objective. The wavelength of the Raman laser used in these measurements was 532 nm.
Additional Information
How to cite this article: Lee, S. et al. Epitaxial stabilization and phase instability of VO2 polymorphs. Sci. Rep. 6, 19621; doi: 10.1038/srep19621 (2016).
References
Imada, M., Fujimori, A. & Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 70, 1039 (1998).
Basov, D. N., Averitt, R. D., Van der Marel, D., Dressel, M. & Haule, K. Electrodynamics of correlated electron materials. Rev. Mod. Phys. 83, 471 (2011).
Yang, Z., Ko, C. & Ramanathan, S. Oxide electronics utilizing ultrafast metal-insulator transitions. Annu. Rev. Mater. Res. 41, 337 (2011).
Zhou, J. et al. VO2 thermochromic smart window for energy savings and generation. Sci. Rep. 3, 3029 (2013).
Driscoll, T. et al. Mott transition in VO2 revealed by infrared spectroscopy and nano-imaging. Science 325, 1518 (2009).
Liu, M. et al. Terahertz-field-induced insulator-to-metal transition in vanadium dioxide metamaterial. Nature 487, 345 (2012).
Chang, S. H. et al. Oxide double-layer nanocrossbar for ultrahigh-density bipolar resistive memory. Adv. Mater. 23, 4063 (2011).
Nakano, M. et al. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature, 487, 459 (2012).
Jeong, J. et al. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science, 339, 1402 (2013).
Leroux, Ch., Nihoul, G. & Van Tendeloo, G. From VO2(B) to VO2(R): Theoretical structures of VO2 polymorphs and in situ electron microscopy. Phys. Rev. B 57, 5111 (1998).
Chernova, N. A., Roppolo, M., Dillon, A. C. & Whittingham, M. S. Layered vanadium and molybdenum oxides: batteries and electrochromics. J. Mater. Chem. 19, 2526 (2009).
Zhang, S. et al. From VO2(B) to VO2(A) nanobelts: first hydrothermal transformation, spectroscopic study and first principles calculation. Phys. Chem. Chem. Phys. 13, 15873 (2011).
Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115 (1994).
Chen, A. et al. Textured metastable VO2(B) thin films on SrTiO3 substrates with significantly enhanced conductivity. Appl. Phys. Lett. 104, 071909 (2014).
Srivastava, A. et al. Selective growth of single phase VO2(A, B and M) polymorph thin films. APL Mater. 3, 026101 (2015).
Oka, Y., Sato, S., Yao, T. & Yamamoto, N. Crystal structures and transition mechanism of VO2(A). J. Solid State Chem. 141, 594 (1998).
Popuri, S. R. et al. VO2(A): Reinvestigation of crystal structure, phase transition and crystal growth mechanisms. J. Solid State Chem. 213, 79 (2014).
Lotnyk, A., Senz, S. & Hesse, D. Epitaxial growth of TiO2 thin films on SrTiO3, LaAlO3 and yttria-stabilized zirconia substrates by electron beam evaporation. Thin Solid Films 515, 3439 (2007).
Schlom, D. G., Chen, L.-Q., Pan, X., Schmeh, A. & Zurbuchen, M. A. Strain tuning of ferroelectric thin films. J. Am. Ceram. Soc. 91, 2429 (2008).
Choi, W. S. et al. Atomic layer engineering of perovskite oxides for chemically sharp heterointerfaces. Adv. Mater. 24, 6423 (2012).
Jeen, H. et al. Reversible redox reactions in an epitaxially stabilized SrCoOx oxygen sponge. Nature Mater. 12, 1057 (2013).
Wenbin, W. et al. Room-temperature multiferroic hexagonal LuFeO3 films. Phys. Rev. Lett. 110, 237601 (2013).
Wong, F. J., Zhou, Y. & Ramanathan, S. Epitaxial variants of VO2 thin films on complex oxide single crystal substrates with 3m surface symmetry. J. Cryst. Growth 364, 74 (2013).
Lee, S., Meyer, T. L., Park, S., Egami, T. & Lee, H. N. Growth control of the oxidation state in vanadium oxide thin films. Appl. Phys. Lett. 105, 223515 (2014).
Zhang, H. & Banfield, J. F. Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2 . J. Phys. Chem. B 104, 3481 (2000).
Ranade, M. R. et al. Energetics of nanocrystalline TiO2 . PNAS 99, 6476 (2002).
Gasparov, L., Jegorel, T., Loetgering, L., Middey, S. & Chakhalian, J. Thin film substrates from the Raman spectroscopy point of view. J. Raman Spectrosc. 45, 465 (2014).
Wu, X., Tao, Y., Dong, L., Wang, Z. & Hu, Z. Preparation of VO2 nanowires and their electric characterization. Mater. Res. Bull. 40, 315 (2005).
Hou, J., Zhang, J., Wang, Z., Zhang, Z. & Ding, Z. Structural transition of VO2(A) nanorods studied by vibrational spectroscopies. RSC Adv. 4, 18055 (2014).
Ji, Y. et al. Role of microstructures on the M1-M2 phase transition in epitaxial VO2 thin films. Sci. Rep. 4, 4854 (2014).
Hardcastle, F. D. & Wachs, I. E. Determination of vanadium−oxygen bond distances and bond orders by Raman spectroscopy. J. Phys. Chem. 95, 5031 (1991).
Goodenough, J. B. The two components of the crystallographic transition in VO2 . J. Solid State Chem. 3, 490 (1971).
Zylbersztejn, A. & Mott, N. F. Metal-insulator transition in vanadium dioxide. Phys. Rev. B 11, 4383 (1975).
Goodenough, J. B. Narrow-band electrons in transition-metal oxides. Czech. J. Phys. B 17, 304 (1967).
Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. The Raman and high temperature XRD measurements were conducted as a user project at the Centre for Nanophase Materials Sciences (CNMS), which is sponsored at Oak Ridge National Laboratory by the Scientific User Facilities Division, U.S. Department of Energy.
Author information
Authors and Affiliations
Contributions
S.L. conceived and designed the experiments under supervision of H.N.L. S.L. fabricated the samples and measured electrical transport. S.L. also conducted high temperature XRD measurements with help of J.K.K. I.N.I performed Raman spectroscopic measurements. S.L. and H.N.L. wrote the manuscript and other authors reviewed it.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lee, S., Ivanov, I., Keum, J. et al. Epitaxial stabilization and phase instability of VO2 polymorphs. Sci Rep 6, 19621 (2016). https://doi.org/10.1038/srep19621
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19621
This article is cited by
-
The synthesis of VO2(B) ultrathin nanosheets array and effects of optical radiation on room temperature NO2 gas sensing performance
Journal of Materials Science: Materials in Electronics (2024)
-
Enhancing response characteristics of palladium-doped vanadium pentoxide on a porous silicon substrate as gas sensor synthesized by pulsed laser deposition
Optical and Quantum Electronics (2024)
-
Thermally Induced Switching of Electrical and Optical Characteristics of VO2 Thin Nanostructured Films Fabricated by Radio Frequency Magnetron Sputtering
Arabian Journal for Science and Engineering (2024)
-
Transient dynamics of the phase transition in VO2 revealed by mega-electron-volt ultrafast electron diffraction
Nature Communications (2023)
-
One-dimensional nanostructured vanadium oxides with single-crystalline structure synthesized by cellulose nanocrystal-template-assisted hydrothermal method for Li-ion battery cathodes
Cellulose (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.