Abstract
Lepidopteran midgut aminopeptidases N (APNs) are phylogenetically divided into eight clusters, designated as APN1–8. Although APN1 has been implicated as one of the receptors for Cry1Ac in several species, its potential role in the mode of action of Cry2Ab has not been functionally determined so far. To test whether APN1 also acts as one of the receptors for Cry1Ac in Helicoverpa zea and even for Cry2Ab in this species, we conducted a gain of function analysis by heterologously expressing H. zea APN1 (HzAPN1) in the midgut and fat body cell lines of H. zea and the ovarian cell line of Spodoptera frugiperda (Sf9) and a loss of function analysis by RNAi (RNA interference) silencing of the endogenous APN1 in the three cell lines using the HzAPN1 double strand RNA (dsRNA). Heterologous expression of HzAPN1 significantly increased the susceptibility of the three cell lines to Cry1Ac, but had no effects on their susceptibility to Cry2Ab. Knocking down of the endogenous APN1 made the three cell lines resistant to Cry1Ac, but didn’t change cell lines susceptibility to Cry2Ab. The findings from this study demonstrate that HzAPN1 is a functional receptor of Cry1Ac, but not Cry2Ab.
Similar content being viewed by others
Introduction
Aminopeptidases N (APNs) are a class of broad-specificity metalloaminopeptidases that sequentially and preferentially remove neutral amino acids from the N-terminus of a range of peptides in microorganisms, plants and animals1. They share two conserved signature motifs: a catalytic zinc binding/gluzincin motif HEXXH-(X18)-E and an N-terminal amino acid substrate-binding exopeptidase motif GXMEN2,3. In the catalytic HEXXH-(X18)-E motif, the two histidine residues and the distant gluatamic acid residue constitute zinc ligands, whereas the gluatamic acid residue between the two histidine residues and zinc ion catalyze the sequential release of N-terminal neutral amino acids3. By virtue of the two conserved motifs, APNs efficiently retrieve amino acids from dietary proteins and endogenous proteins degraded during protein turnover and thus play important roles in nutrition, protein maturation, peptide hormone level regulation, stress response, cell signaling, cell cycle control and cellular processes involved in health and diseases1,4.
In recent years, lepidopteran midgut APNs are widely studied because of their potential role as one of the receptors for the Bacillus thuringiensis (Bt) crystal insecticidal toxins (Cry toxins)5,6, which are used extensively in sprays and transgenic crops to control lepidopteran insect pests7,8. Lepidopteran midgut APNs are 100 to 180 kDa and mainly bound to microvillar membranes of midgut cells via a C-terminal glycosyl phosphatidyl inositol (GPI) anchor. Over 140 APN cDNAs have been cloned from more than 20 lepidopteran species (http://www.ncbi.nlm.nih.gov/). Phylogenetic analysis has resolved them into 8 clusters, designated as APN1–8 respectively, which are derived from multiple gene duplications9,10. Each lepidopteran species usually has no more than one APN for each cluster and their amino acid sequence identity ranges from 23 to 40% between different clusters of the same species and from 50 to 95% between members of the same cluster in different species9.
Although Cry1Ac and Cry2Ab are two of the most widely used Bt Cry toxins in transgenic crops8,11, studies on defining the role of insect APNs as a receptor for Cry toxins have been mostly focused on Cry1Ac. Among the 8 clusters of lepidopteran APNs, there is no evidence for involvement of APN4, APN7 and APN8 as a receptor for Cry1Ac, Cry2Ab, or other Cry toxins12,13,14. Conflicting evidence exists for APN2, because 1) it can bind to Cry1Ac in Helicoverpa armigera15, but not in Lymantria dispar16; and 2) it is not linked to Cry1Ac resistance in Plutella xylostella17. APN3 and APN5 can bind to Cry1Ac in H. armigera18 and P. xylostella17 respectively, but their involvement in Cry1Ac resistance has not been documented. APN6 seems not to be a receptor for Cry1Ac and Cry2Ab because it can not bind to Cry1Ac in H. armigera19 and is not involved in Cry1Ac and Cry2Ab resistance in Trichoplusia ni14,20. In stark contrast, various studies with APN1 from Manduca sexta21, L. dispar22,23, P. xylostella24, H. armigera15,25 and T. ni20 consistently demonstrate that it is one of the midgut receptors for Cry1Ac.
So far, the only study examined APN’s role in the mode of action of Cry2Ab shows that APN5 can bind to Cry2Ab in P. xylostella26. Whether APN1 also serves as one of the receptors for Cry2Ab still remains unclear. Presence of cross-resistance between Cry1Ac and Cry2Ab27,28,29 and detection of a Cry1Ac- and Cry2Ab-binding protein with a similar size (120 ~ 130 kDa) of APN1 in the midgut and fat body cell lines of H. zea by ligand blot suggest a possibility of APN1 as a shared receptor for Cry2Ab and Cry1Ac (Wei & Li, unpublished manuscript). To test this hypothesis, we conducted a gain of function analysis by heterologously expressing H. zea APN1 (HzAPN1) in the midgut and fat body cell lines of H. zea and the ovarian cell line of Spodoptera frugiperda (Sf9) and a loss of function analysis by knocking down the endogenous APN1 in the three cell lines using the HzAPN1 double strand RNA (dsRNA). Heterologous expression of HzAPN1 significantly enhanced the susceptibility of the three cell lines to Cry1Ac, but not to Cry2Ab. Knocking down of the endogenous APN1 made the three cell lines more tolerant to Cry1Ac, but didn’t change their susceptibility to Cry2Ab. The data demonstrate that APN1 is a receptor for Cry1Ac, but not for Cry2Ab.
Results
Binding profiles of Cry1Ac and Cry2Ab in H. zea larval midgut BBMV
As in H. zea midgut and fat body cell lines (Wei & Li, unpublished manuscript), ligand blot detected two binding bands of 210 and 130 kDa for Cry1Ac (Fig. 1A, left panel) and four binding proteins of 130 and 87, 57 and 42 kDa for Cry2Ab (Fig. 1A, right panel) in the brush border membrane vesicles (BBMV) isolated from H. zea larval midguts. Western blot analysis of H. zea larval midgut BBMV (Fig. 1B) with the antibody directed against APN1 from H. armigera30, a sister species of H. zea, revealed that the common binding band of 130 kDa by Cry1Ac and Cry2Ab was co-localized with H. zea APN1.
Impact of heterologous expression of HzAPN1 on cytotoxicities of Cry1Ac and Cry2Ab
Western blot showed that Sf9 cells and H. zea midgut and fat body cells transfected with pAc-HzAPN1 produced significantly more APN1 proteins than the three cell lines transfected with the empty vector pAc (control cells) (Fig. 2; Pmidgut = 0.035, Pfat body = 0.004 and PSf9 = 0.048). More precisely, the protein level of APN1 in the pAc-HzAPN1 transfected midgut, fat body and Sf9 cells was 1.83, 1.55 and 1.97 times more than that in the corresponding pAc-transfected control cells.
Expression level of APN1 in the cells transfected with pAc or pAc-HzAPN1 plasmids.
For pAc (empty vector control) and pAc-HzAPN1 plasmids, three independent transfections were done for each cell line and the protein extracts from each of the three transfections were analyzed by Western blot. The lower panel picture of HzAPN1 and β-actin bands is a representative of the three Western blots. The average expression of HzAPN1 relative to that of β-actin calculated by Image J quantification of the three Western blots is showed in the upper panel. Each error bar represents the standard error of the mean from three transfection replicates. Significant differences in relative expression of HzAPN1 between pAc and pAc-HzAPN1 transfected cells of each cell line are indicated with an asterisk (*) (P < 0.05, Student’s t-test).
When the above pAc- and pAc-HzAPN1-transfected cells were exposed to 15 μg/ml of the activated Cry1Ac, mortality increased from none (−2.64%) for pAc-transfected Sf9 cells to 48.05% for pAc-HzAPN1 transfected Sf9 cells (Table 1). Consistent with the higher APN1 increase in the pAc-HzAPN1 transfected midgut cells than in the pAc-HzAPN1 transfected fat body cells (Fig. 1), the mortality of the former increased by 55.95%, whereas it was 20.90% for the latter. When the concentration of activated Cry1Ac was doubled, mortality was increased significantly (for at least two to three times) from 24.19 (pAc control) to 76.17% (pAc-HzAPN1) for midgut cells, 23.77 to 51.57% for fat body cells and 10.61 to 44.35% for Sf9 cells, respectively (Table 1). When the pAc and pAc-HzAPN1 transfected cells were treated with 2.5 or 3.75 μg/ml of the activated Cry2Ab, no significant mortality enhancement by heterologous expression of HzAPN1 was observed regardless of cell line and Cry2Ab concentration (Table 1).
Effect of knocking down APN1 on cytotoxicities of Cry1Ac and Cry2Ab
Western blot detected 7.87-fold reduction in the protein level of APN1 in H. zea midgut cells transfected with 50 nM HzAPN1 dsRNA, compared with H. zea midgut cells transfected with 50 nM DsRed dsRNA (control cells; Pmidgut < 0.0001) (Fig. 3). Likewise, transfection of 50 nM HzAPN1 dsRNA also resulted in 2.47- (Pfat body = 0.0098) and 3.74-fold (PSf9 < 0.0001) decrease in the protein level of the endogenous APN1 in H. zea fat body cells and Sf9 cells, respectively (Fig. 3).
Expression level of APN1 in the cells transfected with DsRed or HzAPN1 dsRNA.
For DsRed (dsRNA control) and HzAPN1 dsRNA, three independent transfections were conducted for each cell line and the protein extracts from each of the three transfections were analyzed by Western blot. The lower panel picture of APN1 and β-actin bands is a representative of the three Western blots. The average expression of HzAPN1 or SfAPN1 (Sf9 cells) relative to that of β-actin calculated by Image J quantification of the three Western blots is displayed in the upper panel. Each error bar represents the standard error of the mean from three transfection replicates. Significant differences in relative expression of HzAPN1 or SfAPN1 (Sf9 cells) between DsRed and HzAPN1 dsRNA transfected cells of each cell line are indicated with an asterisk (*) (P < 0.05, Student’s t-tests).
When the above HzAPN1 dsRNA-transfected H. zea midgut cells were exposed to 150 μg/ml of the activated Cry1Ac, a 33.79% decrease in mortality was exhibited when compared with that of the DsRed dsRNA-transfected H. zea midgut cells (Table 2). Due to the lower knocking down activities of the endogenous APN1 in the other two cell lines (Fig. 3), HzAPN1 dsRNA only produced a 19.29% and a 13.12% reduction in mortality for H. zea fat body cells and Sf9 cells, respectively (Table 2). In contrast, no significant mortality reduction by knocking down of the endogenous APN1 was seen in the three insect cell lines when they were treated with 30 μg/ml of the activated Cry2Ab (Table 2).
Discussion
Both Cry1Ac and Cry2Ab are expected to have two or more receptors in the larval midguts of Lepidopteran insects (Wei & Li, unpublished manuscript). Documentation of cross-resistance between the two toxins implies that they may share one common receptor27,28,29, although cross-resistance can occur through receptors-independent mechanisms. Recognition of one protein band with the same size of APN1 (120 ~ 130 kDa) by both Cry1Ac and Cry2Ab antibodies in the midgut and fat body cell lines of H. zea (Wei & Li, unpublished manuscript) and by Cry2Aa antibody in Spodoptera littoralis BBMV31 suggest that the potential common receptor could be APN1. This present study was conducted to test whether this hypothesis is true or not.
By ligand blot and Western blot, we confirmed that H. zea larval midgut BBMV, like H. zea midgut and fat body cell lines (Wei & Li, unpublished manuscript), also had a 130 kDa protein band that was recognized by Cry1Ac, Cry2Ab and APN1 antibodies (Fig. 1). Heterlogous expression of HzAPN1 significantly increased the susceptibilities of Sf9, H. zea midgut and fat body cell lines to activated Cry1Ac (Fig. 2 and Table 1), whereas RNAi knocking down of the endogenous APN1 made the three cell lines significantly less susceptible to activated Cry1Ac (Fig. 3 and Table 2). In contrast, neither heterologous expression of HzAPN1 nor RNAi knocking down of the endogenous APN1 affected the susceptibilities of the three cell lines to activated Cry2Ab (Tables 1 and 2). The data suggest that the 130 kDa band recognized by Cry1Ac antibody is HzAPN1, but the 130 kDa band recognized by Cry2Ab is an unknown protein. Moreover, the data directly demonstrate that HzAPN1 is one of the receptors for Cry1Ac, but not for Cry2Ab in H. zea.
While APN1 has been implicated as one of the receptors for Cry1Ac in a number of species15,21,23, this is the first report that functionally characterized APN1 as a receptor of Cry1Ac in H. zea. This is consistent with the result of Sivakumar et al., who confirmed that APN1 from H. armigera (HaAPN1)32, the closest orthologue of HzAPN1, acted as a receptor of Cry1Ac in Sf21 cells. To our knowledge, the current study is also the first report that experimentally excluded APN1 as the basis of cross-resistance between the two toxins—the common receptor of Cry1Ac and Cry2Ab. However, this study cannot be extrapolated to rule out the possibility of other APNs as one of the receptors for Cry2Ab. First, the size of the protein band (120 ~ 130 kDa) recognized by Cry2Ab antibody not only matches with the size of APN1, but also falls into the size range of other APNs. In addition, APN5 from P. xylostella (PxAPN5) was reported to bind to Cry2Ab26. Further functional analyses of HzAPN5 and other APNs from H. zea are needed to resolve this issue.
The fact that RNAi knocking down of the endogenous APN1 protected not only midgut cells but also fat body and ovarian (Sf9) cells from the toxic effects of Cry1Ac (Fig. 3 and Table 2) suggests that APN1 may be expressed in both midgut and non-gut hemocoelic tissues at least in some species. Consistent with this notion, APN1 has been shown to be expressed in midgut, malpighian tubule, salivary gland and fat body in T. ni33, fat body, malpighian tubule and salivary gland in Achaea janata34, midgut, fat body, malpighian tubule, epidermis and hemolymph in S. exigua35, midgut, fat body, Malpighian tubule and carcass in Epiphyas postvittana36 and midgut and Malpighian tubule in B. mori37. The toxic effects of Cry1Ac and Cry2Ab on in vitro cultured fat body and ovarian (Sf9) cells observed in this study and Wei et al. (Wei & Li, unpublished manuscript) as well as the effects of intrahemocoelic injections of several Cry1 toxins on the growth and survival of Lymantria dispar and Neobellieria bullata larvae38 and of A. janata larvae34 further strengthen the notion of APNs expression in the above non-gut hemocoelic tissues. Apparently, these receptors-expressing non-gut hemocoelic tissues can serve as additional in vivo target tissues of Cry toxins, as long as these toxins can cross the insect midgut epithelium and reach the haemolymph when fed orally. Detection of small amounts of orally ingested Cry toxins in the haemolymph of Lygus Hesperus39 proves that Cry toxins can naturally cross the insect gut into the haemolymph at least in some species, but probably at a very low speed. Novel protein conjugation/fusion technology capable of speeding up the crossing of the haemocoelic-active Cry toxins across the insect gut into haemolymph is needed to further enhance the efficacy of Cry toxins by virtue of targeting both midgut and non-gut hemocoelic tissues.
Methods
Insects and insect cell lines
Three insect cell lines and one susceptible strain of H. zea were used in this study. The H. zea susceptible strain was established with a few thousand eggs purchased from Benzon Research Inc. (Carlisle, PA) in October 2014 and maintained on wheat germ-containing diets at 27 ± 1 °C, 60 ± 10% RH and a photoperiod of 14 L:10 D40. The H. zea midgut cell line RP-HzGUT-AW1(MG) and H. zea fat body cell line BCIRL-HzFB33(FB), generously provided by Dr. Cynthia L.Goodman (BCIRL, USDA, ARS)41, were routinely maintained with Excell 420 insect serum-free medium (SAFC Bioscience, Lenexa, KS) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone-QB perbio, Logan, UT), 50 U/ml penicillin, 50 mg/ml streptomycin and 12 mg/ml gentamycin (Invitrogen, CA) in an incubator at 28 °C. Sf9 cell line derived from pupal ovarian tissue of Spodoptera frugiperda was cultured in Sf-900 II SFM medium (GIBCO/BRL/Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum, 50 U/ml penicillin, 50 mg/ml streptomycin and 12 mg/ml gentamycin in the same incubator.
Toxins and antibody
Cry1Ac and Cry2Ab protoxin were supplied by Biotechnology Research Laboratory, Institute of Plant Protection, Chinese Academy of Agricultural Sciences. Activated Cry1Ac and Cry2Ab were prepared from the corresponding protoxin as described by Wei et al. (Wei & Li, unpublished manuscript). Anti-Cry1Ac antibody and anti-HaAPN1 antibody were obtained from Dr. Chenxi Liu of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences30. Preparation of anti-Cry2Ab antibody was described in Wei et al. (Wei & Li, unpublished manuscript).
Ligand blot detection of CryAc and Cry2Ab binding proteins in H. zea larval midgut BBMV
Brush border membrane vesicles (BBMV) for ligand blot and Western blot (see below) were prepared from the 5th instar larvae midguts of the susceptible strain of H. zea using a differential centrifugation method42,43. When dissected out, midguts were open to remove peritrophic membranes and gut contents, cleaned in ice-cold 0.7% NaCl solution, placed on filter paper for a few seconds to remove excessive water, weighed and stored at −80 °C. The frozen midguts taken out from the freezer were homogenized in nine-fold volume (w/v) of ice-cold buffer A (300 mM mannitol, 5 mM EGTA, 17 mM Tris-HCl, 1 mM PMSF), incubated with one volume of 24 mM MgCl2 on ice for 15 min and centrifuged at 2500 × g and 4 °C for 15 min. The supernatant was centrifuged at 30,000 × g and 4 °C for 30 min. The resulting pellet was suspended in buffer B (150 mM Mannitol, 2.5 mM EGTA, 8.5 mM Tris-HCl, 1 mM PMSF), left on ice up to 4 h and then centrifuged at 30,000 g and 4 °C for 15 min. The final pellet was suspended in buffer C (150 mM NaCl, 5 mM EGTA, 1 mM PMSF, 20 mM Tris-HCl, 1% CHAPS) and used as the BBMV preparation. The protein concentrations of the BBMV preparations were determined by the Bradford protein assay, using bovine serum albumin (BSA) as a standard (Bradford, 1976). When conducted ligand blot, 8 μg protein of the larval midgut BBMV preparation was separated on a 10% SDS-PAGE gel and electroblotted to a polyvinylidene difluoride (PVDF) membrane (Thermo scientific) in the transfer buffer (25 mM Tris, 192 mM glycine, 10% methanol, pH 8.3). The follow-up procedures for detection of Cry1Ac or Cry2Ab binding proteins using the anti-Cry1Ac antibody or anti-Cry2Ab antibody were the same as described in Wei et al. (Wei & Li, unpublished manuscript).
Western blot detection of APN1 in larval midgut BBMV and cell line protein extracts
The larval midgut BBMV was prepared as above and the protein extracts of the three insect cell lines transfected with the control plasmid pAc, pAc-HzAPN1, DsRed double strand RNA (dsRNA), or HzAPN1 dsRNA (see preparation and transfection of pAc-HzAPN1 plasmid and dsRNA below) were prepared as described by Wei et al. (Wei & Li, unpublished manuscript). Six μg protein of the larval midgut BBMV and/or the protein extract of each cell line transfected with pAc, pAc-HzAPN1, DsRed dsRNA, or HzAPN1 dsRNA were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel and electroblotted to a polyvinylidene difluoride (PVDF) membrane (Thermo scientific) in the transfer buffer (25 mM Tris, 192 mM glycine, 10% methanol, pH 8.3). The membrane was blocked with phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) containing 0.1% Tween 20 (Bio-Rad) (PBST buffer) and 5% milk (Amresco) at 4 °C overnight or room temperature for at least 2 h. The membrane was then hybridized at 4 °C with the primary antibody anti-HaAPN1 antiserum (adding to the blocking buffer at 1:15,000 dilution) for about 14 h, washed three times of 15 min each with PBST buffer, probed at 4 °C with the horseradish peroxidase (HRP)-conjugated secondary antibody (ZSGB-BIO, China) (1:15,000) for 2 h, washed three times of 15 min each with PBST buffer and visualized with immobilon western chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA, USA)30.
A duplicate SDS-PAGE gel of the larval midgut BBMV and/or cell line protein extracts was electrophoresed simultaneously to detect the reference protein β-actin. The procedure for detection of β-actin was identical to that for APN1 except that the membrane blot was hybridized with anti-β-actin antibody (sc-69879, Santa Cruz Biotechnology, USA) (1:3000) for 2 h at room temperature with constant shaking and then probed with a goat anti-mouse IgM-HRP (Santa Cruz Biotechnology, USA) (1:3000) for 1 h at room temperature with constant shaking.
Construction of pAC-HzAPN1 construct
The open reading frame (ORF) of HzAPN1 was PCR-amplified from the plasmid HzAPN1-pGEMT (Zhang & Li, unpublished manuscript) with the gene-specific primers APN-5′ASCI (5′-GGCGCGCCGACCAGCGTGGAGTCACAA-3′) and APN-3′NOTI (5′-GCGGCCGCTTAAGCCATATTAACAACGAGAGTCA-3′). PCR reaction (20 μL) contained 1 μL of HzAPN1-pGEMT (Zhang & Li, unpublished manuscript), 1 μL of LongAmp® Taq DNA polymerase (New England Biolabs, Ipswich, MA), 1.6 μL of dNTP (5 mM), 2 μL of primer mix (25 mM each), 4 μL of 5× longAmp Taq reaction buffer and 10.4 μL ddH2O. The PCR reaction was initiated at 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 60 °C for 30 s and 65 °C for 1 min and a final extension of 5 min at 65 °C. The PCR products were TA-cloned into pGEM®-T vector (Promega, Madison, WI), released by double digestion with ASCI and NOTI (New England Biolabs, Ipswich, MA) and subcloned into the expression vector pAc/V5-His A (Invitrogen) via the ASCI and NOTI enzyme sites.
Production of HzAPN1 and DsRed dsRNA
A 658 bp cDNA sequence of HzAPN1 was PCR-amplified from HzAPN1-pGEMT (Zhang & Li, unpublished manuscript) using the primers HZAPN1 dsRNA-F (5′-GGATCCTGT CTGACTCCCTTGACTCTGCTC-3′) and HzAPN1 dsRNA-R (5′-CGGTTGTATTC GTGGATTGATAGCCT-3′). The PCR reaction (20 μL) was composed of 1 μL of HzAPN1-PGEMT, 1 μL of LongAmp® Taq DNA polymerase (New England Biolabs, Ipswich, MA), 1.6 μL of dNTP (5 mM), 2 μL of primer mix (25 mM each), 4 μL of 5× longAmp Taq reaction buffer and 10.4 μL ddH2O. The PCR conditions were 94 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 56 °C for 30 s and 65 °C for 1 min and a final extension of 5 min at 65 °C. The resultant PCR product was TA-cloned into the pGEM-T Easy-vector to generate the template plasmid HzAPN1-pGEMT for production of HzAPN1 dsRNA. Likewise, a 480-bp cDNA sequence of DsRed was PCR-amplified from pBac [3 × P3-DesRedaf] with the primers DsRED-F (TGCAGGTGACCAAGGGC) and DsRED-R (CGTTGTGGGAGGTGATGT) and TA-cloned into the pGEM-T Easy-vector to generate the template plasmid DsRed-pGEMT for production of the DsRed dsRNA (i.e. control dsRNA).
We used two pairs of universal primers complementary to the flanking sequence of the multiple cloning sites of the pGEM-T Easy-vector to PCR-amplify two in vitro transcription templates (one for sense RNA, one for antisense RNA) from the template plasmids DsRed-pGEMT and HzAPN1-pGEMT, respectively. The primer pair T7 pGEMTeasy Fwd (5′-GGTGTAATACGACTCACTATAGGG-3′) and pGEMTeasy Rev (5′-CAAGCTATGCATCCAACGCGTTGGGAG-3′) were used to PCR-amplify the sense template that had a T7 RNA polymerase promoter sequence at the 5′ end of its sense strand. Another primer pair T7 pGEMTeasy Rev (5′-GGTGTAATACGACTCACTATAGGGCAAGCTATGCATCCAACGCGTTGGGAG-3′) and pGEMTeasy Fwd (5′-CGAATTGGGCCCGGACGTCGCA-3′) were used to PCR-amplify the antisense template that had a T7 RNA polymerase promoter sequence at the 5′ end of its antisense strand. The resulting sense and antisense template PCR products were cleaned, quantified and combined at 1:1 ratio. Then the combined sense and antisense templates were used to make dsRNA by simultaneous in vitro transcription of both the sense and antisense RNA using the RiboMax Large Scale RNA Production System (Promega) according to the product manual. Following in vitro transcription, the DsRed and HzAPN1 dsRNA products were cleaned with MEGAclear Kit (Ambion Inc.), quantified and stored at −80 °C for subsequent transfection.
Cell transfection and toxicity bioassay
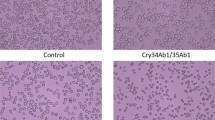
H. zea midgut, fat body and Sf9 cells were seeded onto a 12-well plate (9 × 105 cells/well) and incubated at 28 °C, respectively. For heterologous expression of HzAPN1 in the three cell lines, cells were transfected with 2 μg/well of pAc (control, empty vector) or pAC-HzAPN1 plasmids using Cellfectin (Invitrogen; 8 μl per well) for 5 h. For RNA interference (RNAi) silencing of endogenous APN1 in the three cell lines, cells were transfected with 50 nM of DsRed (control) or HzAPN1 dsRNA for 5 h. We then replaced the transfection mixture of each well with 1.5 ml of supplemented Excell 420 (midgut and fat body cells) or Sf-900 II SFM medium (Sf9 cells) and incubated the cells at 28 °C for 64 hours. The cells in each independent transfection replicate were reseeded onto three wells in a 96-well micro-plate at 10000 cells in 100 μL per well. After 2 h of attachment, the cells were treated with activated Cry1Ac or activated Cxry2Ab. The concentrations for bioassays of the cells transfected with pAc (control) or pAc-HzAPN1 plasmids were 15 and 30 μg/ml for activated Cry1Ac and 2.5 and 3.75 and μg/ml for activated Cry2Ab), respectively. The concentrations for bioassays of the cells transfected with DsRed dsRNA (control) and HzAPN1 dsRNA were 150 μg/ml for activated Cry1Ac and 30 μg/ml for activated Cry2Ab, respectively. Cell mortality was determined by replacing 35 μL medium from each well with 35 μL of 0.4% trypan blue (AMRESCO®, BioExpress, Ohio, USA) and counting the numbers of stained (dead cells) and unstained (live cells) in two 400x fields of view from the central parts of each well under an inverted microscope (VistaVision, VWR) after 4.5 h for Cry1Ac or 5.5 hours for Cry2Ab(Wei & Li, unpublished manuscript). Each plasmid or dsRNA was independently transfected into each cell three times and each independent transfection was bioassayed three times with control or a given concentration of Cry1Ac or Cry2Ab (3 × 3 = 9 replicates).
Statistical Analysis
Cell mortality was calculated by dividing the number of dead cells (the number of cells before toxin treatment – the number of unstained cells after treatment) by the number of cells before treatment and corrected with the Abbott formula44. Significant differences in mortality between the control plasmid pAc (or the DsRed control dsRNA) and pAc-HzAPN1 (or HzAPN1 dsRNA) were compared using Student t-test with α = 0.05 (JMP 8; SAS Institue, Inc.). Mortality values of different treatments were arcsine transformed before analysis.
The band intensities of the target protein APN1 and the reference protein β-actin on all the Western blot pictures of the protein extracts from the three cell lines transfected with pAc, pAc-HzAPN1, DsRed dsRNA, or HzAPN1 dsRNA were quantified by densitometry using Image J software (NIH, v1.46). The relative expression level of APN1 in each control or treatment cells was calculated by dividing the band intensity of APN1 by the band intensity of β-actin. Significant differences in the relative expression of APN1 between the control plasmid pAc (or the DsRed control dsRNA) and pAc-HzAPN1 (or HzAPN1 dsRNA) were evaluated by Student’s t-test (JMP 8; SAS Institue, Inc.).
Additional Information
How to cite this article: Wei, J. et al. APN1 is a functional receptor of Cry1Ac but not Cry2Ab in Helicoverpa zea. Sci. Rep. 6, 19179; doi: 10.1038/srep19179 (2016).
References
Sanz, Y. Aminopeptidases. In J. Polaina & A. P. MacCabe (eds.), Industrial Enzymes, Springer. 243–260 (2007).
Albiston, A. L., Ye, S. & Chai, S. Y. Membrane bound members of the M1 family: more than aminopeptidases. Protein Pept Lett, 10, 491–500 (2004).
Terra, W. R. & Ferreira, C. Biochemistry and molecular biology of digestion. In Gilbert, L. I. (ed.), Insect Molecular Biology and Biochemistry (ed.). Elsevier B.V., 365–416 (2012).
Luan, Y. & Xu, W. The structure and main functions of aminopeptidase N. Curr Med Chem. 9, 639–647 (2007).
Pardo-López, L., Soberón, M. & Bravo, A. M. Bacillus thuringiensis insecticidal toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev. 37, 3–22 (2013).
Adang, M. J., Crickmore, N. & Jurat-Fuentes, J. L. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. Adv Insect Physiol. 47, 39–87 (2014).
Sanahuja, G., Banakar, R., Twyman. R., Capell, T. & Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol J. 9, 283–300 (2011).
James, C. Global Status of Commercialized Biotech/GM Crops: 2014. ISAAA Brief No. 49. ISAAA: Ithaca, NY (2014).
Crava, C. M. et al. Study of the aminopeptidase N gene family in the lepidopterans Ostrinia nubilalis (Hübner) and Bombyx mori (L.): sequences, mapping and expression. Insect Biochem. Mol. Biol. 40, 506–515 (2010).
Hughes, A. L. Evolutionary diversification of aminopeptidase N in Lepidoptera by conserved clade-specific amino acid residues. Mol Phylogenet Evol. 76, 127–133 (2014).
Tabashnik, B. E., Bre′vault, T. & Carrie’re, Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 31, 510–521 (2013).
Stephens, E. et al. The N-linked oligosaccharides of aminopeptidase N from Manduca sexta. Eur J Biochem. 271, 4241–4258 (2004).
Yang, Z. et al. Expression of cadherin, aminopeptidase N and alkaline phosphatase genes in Cry1Ac-susceptible and Cry1Ac-resistant strains of Plutella xylostella (L.). J Appl Entomol. 7, 539–548 (2012).
Song, X., Kain, W., Cassidy, D. & Wang, P. Resistance to Bacillus thuringiensis toxin Cry2Ab in Trichoplusia ni is conferred by a novel genetic mechanism. Appl. Environ. Microbiol. 10.1128/AEM.00593-15 (2015).
Rajagopal, R. et al. Recombinantly expressed isozymic aminopeptidases from Helicoverpa armigera midgut display differential interaction with closely related Cry proteins. Biochem. J. 370, 971–978 (2003).
Valaitis, A. P., Mazza, A., Brousseau, R. & Masson, L. Interaction analyses of Bacillus thuringiensis CryIA toxins with two aminopeptidases from gypsy moth midgut brush border membranes. Insect Biochem Mol Biol. 27, 529–539 (1997).
Chang, X. et al. Determining the involvement of two aminopeptidase Ns in the resistance of Plutella xylostella to the Bt toxin Cry1Ac: Cloning and study of in vitro function. J Biochem Mol Toxic. 2, 60–70 (2012).
Wang, G., Liang, G., Wu, K. & Guo, Y. Gene cloning and sequencing of aminopeptidase N3, a putative receptor for Bacillus thuringiensis insecticidal Cry1Ac toxin in Helicoverpa armigera (Lepidoptera: Noctuidae). Eur. J. Entomol. 102, 13–19 (2005).
Angelucci, C. et al. Diversity of aminopeptidases, derived from four lepidopteran gene duplications and polycalins expressed in the midgut of Helicoverpa armigera: identification of proteins binding the delta-endotoxin, Cry1Ac of Bacillus thuringiensis. Insect Biochem Mol Biol. 38, 685–696 (2008).
Tiewsiri, K. & Wang, P. Differential alteration of two aminopeptidases N associated with resistance to Bacillus thuringiensis toxin Cry1Ac in cabbage looper. Proc. Natl. Acad. Sci. USA 34, 14037–14042 (2011).
Flores-Escobar, B., Rodríguez-Magadan, H., Bravo, A., Soberón, M. & Gómez, I. Differential role of Manduca sexta aminopeptidase-N and alkaline phosphatase in the mode of action of Cry1Aa, Cry1Ab and Cry1Ac toxins from Bacillus thuringiensis. Appl. Environ. Microbiol. 15, 4543–4550 (2013).
Garner, K. J., Hiremath, S., Lehtoma, K. & Valaitis, A. P. Cloning and complete sequence characterization of two gypsy moth aminopeptidase-N cDNAs, including the receptor for Bacillus thuringiensisCry1Ac toxin. Insect Biochem. Mol. Biol. 29, 527–535 (1999).
Valaitis, A. P. Localization of Bacillus thuringiensis Cry1A toxin-binding molecules in gypsy moth larval gut sections using fluorescence microscopy. J Invertebr Pathol. 2, 69–75 (2011).
Chang, W. X. Z., Gahan, L. J., Tabashnik, B. E. & Heckel, D. G. A new aminopeptidase from diamondback moth provides evidence for a gene duplication event in Lepidoptera. Insect Mol. Biol. 8, 171–177 (1999).
Zhang, S. et al. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol. 7, 421–429 (2009).
Xu, L. et al. Cloning, prokaryotic expression and homology modeling analysis of midgut aminopeptidase gene PxAPN5 in Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomologica Sinica 57, 1272–1280 (2014).
Tabashnik, B. E. et al. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc. Natl. Acad. Sci. USA 29, 11889–11894 (2009).
Brévault, T. et al. Potential shortfall of pyramided transgenic cotton for insect resistance management. Proc. Natl. Acad. Sci. USA. 110, 5806–5811 (2013).
Wei, J. et al. Cross-resistance and interactions between Bt toxins Cry1Ac and Cry2Ab against the cotton bollworm. Sci Rep 5, 10.1038/srep07714 (2015).
Liu, C. et al. Antisera-mediated in vivo reduction of Cry1Ac toxicity in Helicoverpa armigera. J. insect physiol. 7, 718–724 (2010).
Mandal, C. C. et al. Prediction-based protein engineering of domain I of Cry2A entomocidal toxin of Bacillus thuringiensis for the enhancement of toxicity against lepidopteran insects. Protein Eng Des Sel. 20, 599–606 (2007).
Sivakumar, S., Rajagopal, R., Venkatesh, G. R., Srivastava, A. & Bhatnagar, R. K. Knockdown of aminopeptidase-N from Helicoverpa armigera larvae and in transfected Sf21 cells by RNA interference reveals its functional interaction with Bacillus thuringiensis insecticidal protein Cry1Ac. J. Biol. Chem. 282, 7312–7319 (2007).
Nan, X. Gene cloning localization and expression of midgut aminopeptidase N isozymes from the Cabbage Looper, Trichoplusia Ni. Northwest University of Science and Technology, PhD thesis (2012).
Ningshen, T. J., Aparoy, P., Ventaku, V. R. & Dutta-Gupta, A. Functional interpretation of a non-gut hemocoelic tissue aminopeptidase N (APN) in a Lepidopteran insect pest Achaea janata. PLoS ONE 11, e79468 (2013).
Ren, X., Ma. Y., Cui, J. & Li, G. RNA interference-mediated knockdown of three putative aminopeptidases N affects susceptibility of Spodoptera exigua larvae to Bacillus thuringiensis Cry1Ca. J. Insect Physiol. 67, 28–36 (2014).
Simpson, R. M., Poulton, J. & Markwick, N. P. Expression levels of aminopeptidase N genes in the light brown apple moth, Epiphyas postvittana. Insect Sci. 15, 505–512 (2008).
Xia, Q. et al. Microarray-based gene expression profiles in multiple tissues of the domesticated silkworm, Bombyx mori. Genome Biol. 8, R162 (2007).
Cerstians, A. et al. Effect of Bacillus thuringiensis Cry1 toxins in insect hemolymph and theirneuro toxicity in brain cells of Lymantria dispar. Appl Environ Microbiol 9, 3923–3927 (2001).
Brandt, S. L. et al. Interaction of two Bacillus thuringiensis δ-endotoxins with the digestive system of Lygus hesperus. Curr. Microbiol. 48, 1–9 (2004).
Waldbauer, G. P. & Friedman, S. Self-selection of optimal diets by insects. Annu. Rev. Entomol. 36, 43–63 (1991).
Goodman, C. L. et al. Development and partial characterization of heliothine cell lines from embryonic and differentiated tissues. In Vitro Cell Dev. Biol. Anim. 40, 8994 (2004).
Wolfersberger, M. G. et al. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86A, 301–308 (1987).
Chen, L. et al. Proteomic analysis of novel Cry1Ac binding proteins in Helicoverpa armigera (Hübner). Arch Insect Biochem. 2, 61–73 (2010).
Abbott, W. S. A Method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267 (1925).
Acknowledgements
This research was supported by the U.S. Department of Agriculture Biotechnology Risk Assessment Grant 2011-33522-30729, National Natural Science Foundation of China (Grant 31228019, 31210103921 and 31321004), State Key Laboratory for Biology and of Plant Diseases and Insects (grants SKLOF201402 and SKLOF201504) and the China Scholarship Council for a scholarship to Jizhen Wei.
Author information
Authors and Affiliations
Contributions
X.L. and J.W. conceived and designed the experiments. J.W. and M.Z. performed the experiments. X.L. and J.W. analyzed the data. X.L., J.W., G.L., K.W., Y.G. and X.N. wrote the manuscript. J.W. and X.L. shared in the scoping and writing responsibilities. All authors have read and approved the manuscript for publication.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Wei, J., Zhang, M., Liang, G. et al. APN1 is a functional receptor of Cry1Ac but not Cry2Ab in Helicoverpa zea. Sci Rep 6, 19179 (2016). https://doi.org/10.1038/srep19179
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19179
This article is cited by
-
The evaluation of resistance risk to Cry2Ab and cross-resistance to other insecticides in Helicoverpa armigera
Journal of Pest Science (2024)
-
Performance insights into spray-dryer microencapsulated Bacillus thuringiensis cry pesticidal proteins with gum arabic and maltodextrin for effective pest control
Applied Microbiology and Biotechnology (2024)
-
Differences in midgut transcriptomes between resistant and susceptible strains of Chilo suppressalis to Cry1C toxin
BMC Genomics (2020)
-
Functional roles of cadherin, aminopeptidase-N and alkaline phosphatase from Helicoverpa armigera (Hübner) in the action mechanism of Bacillus thuringiensis Cry2Aa
Scientific Reports (2017)
-
Aminopeptidase N1 is involved in Bacillus thuringiensis Cry1Ac toxicity in the beet armyworm, Spodoptera exigua
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.