Abstract
The relative effects of internal fixation strategies for intertrochanteric fracture after operation remain uncertain. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to address this important issue. We searched PubMed, EMBASE and CENTRAL for RCTs that compared different internal fixation implants in patients with intertrochanteric fracture at 6-month follow-up or longer. We ultimately included 43 trials enrolling 6911 patients; most trials were small in sample sizes and events. Their risk of bias was generally unclear due to insufficient reporting. Because of these, no statistically significant differences were present from most of the comparisons across all the outcomes and no definitive conclusions can be made. However, a number of trials compared two commonly used internal fixation strategies, gamma nail (GN) and sliding hip screw (SHS). There is good evidence suggesting that, compared to SHS, GN may increase the risk of cut out (OR = 1.87, 95% CI, 1.08 to 3.21), re-operation (OR = 1.61, 95% CI, 1.02 to 2.53), intra-operative (OR = 3.14, 95% CI, 1.34 to 7.35) and later fractures (OR = 3.67, 95% CI, 1.37 to 9.83). Future randomized trials or observational studies that are carefully designed and conducted are warranted to establish the effects of alternative internal fixation strategies for intertrochanteric fracture.
Similar content being viewed by others
Introduction
Hip fractures represent a common type of injuries; its number increases rapidly1. By 2050, the number of hip fractures is estimated to surpass 6.3 million2. The 1-year mortality for hip fractures range from 14% to 36%3. Hip fractures include femoral neck and intertrochanteric factures4; 20 to 30 percent of patients died in the first 12 months after an intertrochanteric fracture, especially those elderly with limited activity5,6. Surgical treatment represents the optimal strategy for managing intertrochanteric fractures. It allows early rehabilitation and functional recovery and reduces the risk of postoperative complications7.
Internal fixation is a most common surgical treatment for intertrochanteric fractures3 and intramedually (nails) and extramedually (screws or plates) fixations are two commonly used approaches8. The established benefits of internal fixation treatments are immediate pain relief, rapid mobilization, accelerated rehabilitation and maintenance of independent living.
Several systematic reviews and meta-analysis have tested the effects of different internal fixation implants to provide insight into the options for treating intertrochanteric fractures from 2000 to 20129,10,11,12,13. However, the findings in these studies are inconsistent and the diversity of devices used for intertrochanteric fractures had made it challenging for decision makers to identify the ideal treatment option. Meanwhile, the techniques and implants continue to be modified, which make the previous literature less relevant to current practice.
Therefore, we conducted a systematic review and meta-analysis, aiming to offer a comprehensive assessment of alternative internal fixation treatments for intertrochanteric fractures. The protocol of this study was registered on the PROSPERO database (CRD42014008795).
Materials and Methods
Study selection
We included prospective, randomized controlled trials (RCTs) published in English if they enrolled participants diagnosed with intertrochanteric fractures; compared currently used internal fixation implants; followed up patients for more than 6 months; and reported any of our pre-defined outcome measures of interest.
The currently available internal fixations included gamma nail (GN), ACE nail, holland nail, proximal femoral nail (PFN), proximal femoral nail antirotation (PFNA), intramedullary hip screw (IMHS), sliding hip screws (SHS), dynamic condylar screw (DCS), locking compression plate (LCP), percutaneous compression plate (PCCP), Medoff sliding plate, Targon proximal femoral and less invasive stabilization system (LISS).
The pre-defined outcomes included functional measures (i.e. quality of life scores, function scores), adverse events (i.e. mortality, cut out, non-union, reoperation, intra-operative fracture, later fracture, wound infection and embolism) and procedure measures (i.e. operative time, blood loss and hospital stay).
We excluded studies if patients had subtrochanteric fractures, pathological fractures, or previous femoral fractures.
Data sources and searches
We searched PubMed, EMbase (via OVID) and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception up to May, 2015 (Search strategy in appendix 1). We also searched ClinicalTrial.gov and the reference lists of included studies to identify additional eligible studies. To ensure completeness, we also cross referenced our search results with relevant published Cochrane systematic reviews of extracapsular hip fractures14.
Study procedures
We used standardized pilot-tested data extraction forms for the screening of the abstracts and full texts, assessment of risk of bias and collection of data. Pairs of reviewers (YJJ, LL) independently screened study report for eligibility, assessed risk of bias and collected data from each eligible study. Discrepancies were resolved through discussion.
Risk of bias assessment
We assessed the risk of bias of RCTs using a modified version of the Cochrane Collaboration’s tool15. The items included random sequence generation, allocation concealment, blinding of participants, surgeons or outcome assessors, completeness of outcome data and selective reporting. We included two additional items regarding “standardization of the operative procedures” and “surgeons’ experience clearly defined with operations” because the validity of findings from surgical RCTs depends largely on quality of operation and surgeons’ experience16.
Data extraction
We extracted the following data from each of the eligible studies: study characteristics (publish year, simple size, country, length of follow up), patient characteristics (gender, age, type of fracture); interventions (intramedullary and extramedullary treatments) and outcomes (quality of life scores, function scores, mortality, cut out, non-union, reoperation, operative fracture, later fracture, wound infection, embolism, operative time, blood loss and hospital stay)
Data analyses
In the analysis of quality of life data, we reported the data at the baseline, end of the follow up and the change from the baseline. For functional score data, because of the scanty in the reporting of the baseline data, we compared means at the end of follow up of those outcomes between treatment and control groups, assuming that the randomization has well achieved the balance of the baseline between groups. For each of the comparison, we pooled the quality of life data and the functional scores using weighted mean difference (MD) or standardized mean difference (SMD) if varying measures were used. In the analysis of the operation time, blood loss and hospital stay, we treated each of the outcome measures as normally distributed and pooled the mean differences for each of the comparison and reported 95% confidence intervals. We also pooled, for each of the comparison, the trial data regarding adverse events.
In the meta-analyses, we applied the random-effects model using Mantel-Haenszel method. We examined heterogeneity by Cochran’s Q test and I2 statistic. Where possible, we conducted, for each meta-analysis, a pre-defined subgroup analysis by fracture types (stable fractures vs. unstable fractures by AO/OTA classification) to explore source of heterogeneity.
We performed sensitivity analyses by using alternative pooling methods (Peto method vs. Mantel-Haenszel method applicable to dichotomous data) and alterative statistical model (random vs. fixed effect). We performed the data analysis by the RevMan 5.3.
Results
Characteristics of included studies
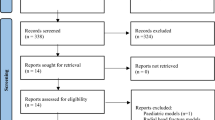
The search yielded 3,397 potential relevant reports. After screening of titles and abstracts, 234 records were retrieved for judging final eligibility. We eventually included 43 RCTs involving 6911 patients (Fig. 1). These trials were conducted in 18 countries, of which 4 were international trials. The sample sizes ranged from 40 to 600 and the length of follow up from 6 to 40 months.
Flaw diagram of study selection based on the eligibility criteria.
We initially searched 8937 reports and 3397 potential relevant reports were included in title and abstract screening after duplication (n = 5540). After screening of titles and abstracts, 234 records were retrieved for judging final eligibility. We eventually included 43 RCTs after full text reviewing.
Among those trials, 52.4% (3625/6911) of the participants were female; the mean age ranged from 53.9 to 84.3 years; 30 trials (69.8%) recruited both stable and unstable fractures (n = 5010), 12 trials explored the effects of devices on unstable patients (n = 1695) and 1 trial did not report the fracture type of participants (n = 206) (Table 1).
Those 43 trials investigated 11 internal fixation treatments, including GN, ACE nail, holland nail, PFN, PFNA, IMHS, SHS, PCCP, Medoff sliding plate, Targon proximal femoral and LISS. The types of implants under assessment varied considerably across studies; few studies tested a same comparison. The details of comparisons were presented in Table 1.
Among those 43 trials, 14 (32.5%) adequately generated random sequences; 9 (20.9%) adequately concealed allocation; none blinded patients and surgeons; 10 (21.7%) blinded outcome assessors; 28 (65.1%) reported more than 80% of patients with completed follow up; only 1 (2.2%) did not report their pre-defined outcomes; 7 trials (15.2%) explicitly stated that surgeons standardized their operations by manuals or guidelines; and 31 (67.4%) referred that surgeons were experienced with trial operations (appendix 2).
Effects on quality of life and functional measures
Quality of life scores (QoL scores)
Four trials (n = 420) used EuroQol 5D (EQ-5D) and Short Form (36) Health Survey (SF-36) to measure the effects of internal fixation treatments on the quality of life17,18,19,20, all of which were small in sample sizes. Two studies provided the baseline and follow up date on quality of life17,19; compared to the baseline, the scores at the end of follow up decreased. In the comparison of the data at the end of follow up, GN group had a significant higher score than SHS group in one trial reporting EQ-5D (MD: 0.12, 95%CI 0.02 to 0.22); in another small trial, no significant difference in the SF-36 was present between GN and PFNA (Table 2). The other two studies neither provided the data regarding standard deviation nor baseline.
Functional scores
Sixteen trials (n = 1467), consisting of 10 comparisons, reported functional status by 6 scores, including Parker-and-Palmer mobility score, Harris hip score, Jensen social-function score, Merle d’aubigne hip score, Geriatric hip fracture recovery scores and Barthei index17,19,21,22,23,24,25,26,27,28,29,30,31,32. Ten studies provided the baseline and follow up date, all of which showed that functional scores decreased at the end of follow up17,22,23,24,26,27,29,30,33,34.
Eight trials compared GN versus 3 other devices (SHS, ACE nail and PFNA); 4 trials compared SHS versus other 4 devices (IMHS, PFN, PFNA, Medoff sliding plate); and 4 trials compared PFNA versus other 3 implants (LISS, Targon PF, PCCP) (appendix Fig. 1).
The comparison of means at the end of follow up showed that patients at GN group had a higher score compared to those at SHS group (SMD: 0.23, 95%CI 0.01 to 0.46, I2 = 22%), but had a lower score than PFNA group (SMD: −0.99, 95%CI −1.39 to −0.60, I2 = 66%). SHS had a higher score than IMHS (SMD: 0.43, 95%CI 0.03 to 0.83), but a lower score than PFNA (SMD: −0.73, 95%CI −1.18 to −0.29) (Table 3). No statistically significant differences were presented in other comparisons.
Adverse events
Mortality
Thirty-three trials (n = 5940) reported mortality, all of which were at high risk of bias17,18,20,21,22,24,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53. The mortality data were collected during the follow up of 6 to 40 months after operations. 14 trials compared GN versus 4 other implants (SHS, ACE nails, PFN, PCCP); 4 trials compared PFNA versus 3 other implants (LISS, Tragon PF, PCCP); and 17 trials compared SHS versus 7 other implants (appendix Figs 2–4). None of the comparisons showed statistically significant differences, likely due to the very small number of events and sample sizes across all the trials (Table 4).
Cut out
Twenty-nine trials (n = 3960)13,14,15,16,17,18,19,21,22,23,24,25,26,28,34,35,36,37,41,42,44,46,47,48,49,52,54,55,56 reported cut out data. Of those trials, 5 reported no event during the course of study (6–40 months)17,26,30,38,57. A total of 141 events were reported from 3692 patients (3.5%). Eighteen trials reported the comparison of GN versus 5 other implants (SHS, ACE nails, PFN, PFNA, PCCP); 11 trials compared SHS versus 6 other implants (appendix Figs 5–7); and 1 trial compared PFNA versus Targon PF.
The pooling of the trials showed that GN increased the risk of cut out compared to SHS (43/802 vs. 23/830; OR: 1.87, 95%CI 1.08 to 3.21, I2 = 0%). No statistically significant differences were found in other comparisons, largely because of the small number of events and sample sizes (Table 4).
Non-union
Data regarding non-union were available in 29 trials (n = 3795), among which 17 reported 90 non-union events during the follow up (2.37%)17,19,24,26,27,28,29,30,34,35,36,37,38,39,40,41,42,44,47,48,49,50,51,52,53,54,57,58,59. Fifteen trials compared GN with other 4 implants (SHS, ACE nails, PFN, PFNA); 12 tested the effect of SHS and other 7 devices; 2 compared PFNA with LISS and Targon PF (appendix Figs 8–10).
The pooling analysis suggested that SHS was associated with a lower risk of non-union compared to IMHS (1/228 vs. 9/246; OR: 0.15, 95%CI 0.03 to 0.87, I2 = 0%), but did not found statistically significant differences in other comparisons, again mostly because of the small number of events and sample sizes (Table 4).
Re-operation
Thirty-one trials (n = 4506) reported re-operation. A total of 248 re-operation events occurred in 4506 participants (5.5%)17,18,21,22,26,27,29,30,32,33,34,35,36,37,38,39,40,41,42,44,45,46,48,49,50,51,53,54,56,57,59. Sixteen trials compared GN with other 3 implants (SHS, PFN, PFNA); 14 compared SHS with other 7 implants; and 3 compared PFNA with PFN, LISS and Targon PF, respectively (appendix Figs 11–13).
Most of comparisons were made with few trials. The pooling of trials showed that GN increased the risk of re-operation compared to SHS (53/907 vs. 35/939; OR: 1.61, 95%CI 1.02 to 2.53, I2 = 0%) (Table 4). No statistically significant differences were found between the other comparisons.
Intra-operative fracture
A total of 17 trials (n = 2661) reported intra-operative fracture data17,21,22,27,29,30,34,36,37,38,39,40,41,42,47,49,54. Of those, 59 intra-operative fractures occurred during the follow up (2.21%). Five comparisons were made among those trials, including the comparisons between GN versus SHS, GN versus PFN, SHS versus IMHS, SHS versus PFN and SHS versus PFNA (appendix Figs 14,15).
Most of the comparisons did not demonstrated statistically significant differences because of the small number of events. However, the pooling of the trials showed increased risk of intra-operative fracture in patients receiving GN versus those with SHS (22/861 vs. 5/861; OR: 3.14, 95%CI 1.34 to 7.35, I2 = 0%) and in patients with GN versus with PFN (19/125 vs. 5/125; OR: 4.30, 95%CI 1.55 to 11.92) (Table 4).
Later fracture
Twenty-six trials, totaling 3508 patients and 42 events, reported data on later fracture rate17,19,21,22,26,29,30,33,34,36,37,38,39,40,41,42,44,47,50,51,53,54,56,57,58,59. Sixteen trials compared GN with other 3 implants; 9 compared SHS with other 6 devices; 1 compared PFNA with PFN; and the other 1 compared PFNA with Targon PF (appendix Figs 16–18).
Again, most of the comparisons did not show statistically significant differences between the alternative surgical options. Pooling of the trials comparing GN versus SHS, however, showed that GN was associated with increased risk of later fractures (18/703 vs. 2/704; OR 3.67, 95%CI 1.37 to 9.83, I2 = 0%) (Table 4).
Wound infection
Thirty trials reported wound infections (superficial infections and deep infections) (n = 4265)18,19,21,22,23,24,25,26,27,29,30,32,33,34,36,38,39,40,41,42,45,46,48,50,51,53,54,57,58,59. Among those, a total of 147 patients (3.4%) reported to have wound infection events. Eighteen trials compared GN with other 4 implants (SHS, ACE nails, PFN, PFNA); 11 compared SHS with 7 devices; and 2 compared PFNA with LISS and Targon PF (appendix Figs 19–21). None of the comparisons showed statistically significant differences in the risk of wound infection (Table 4).
Embolism
A total of 21 trials (n = 2655) provided data on embolism (deep venous embolism and pulmonary embolism) and 88 embolic events occurred in 2657 participants (3.3%)21,22,23,24,27,29,31,33,36,37,41,42,45,46,48,50,54,59. Eleven trials reported the comparative outcomes of GN and 3 other implants, 7 trials tested the effect of SHS and other 5 devices; and one trial compared PFNA with Targon PF (appendix Figs 22–24). Of 9 comparative groups, SHS increased the risk of embolism compared to PCCP (11/87 vs. 4/92; OR: 3.40, 95%CI 1.02 to 11.26, I2 = 0%) (Table 4).
Procedure measures
Operative time (min)
Differences between internal fixation treatments on operation duration were reported in 34 trials including 5692 patients[17–19,22–26,29,30,32,34–36,38–42,46–53,55–59. Heterogeneity across the studies were high in some comparisons. 15 trials compared GN with other 4 implants (SHS, ACE nails, PFN, PFNA); 16 trials compared SHS with 7 other devices; and 3 trials compared PFNA with 3 implants (appendix Figs 25–27). We found a substantial difference on operative time among varied comparisons, likely due to different definitions in original trials. Overall, PFNA were associated with less operative time than other internal fixation treatments (PFNA vs. GN: (MD:−4.45, 95%CI −5.17 to −3.73, I2 = 0%); PFNA vs. LISS: (MD: −26.78, 95%CI −32.8 to −20.75, I2 = 0%); PFNA vs. Targon PF: (MD: −18.5, 95%CI −30.63 to −6.37); PFNA vs. PCCP: (MD:13.5, 95%CI 7.54 to19.46)) (Table 5).
Blood loss (mL)
Data on blood loss were available in 19 trials (n = 3475)20,21,25,26,27,30,31,32,33,35,39,46,47,48,50,52,54,55,57. Heterogeneity across the studies were high in some comparisons. 7 trials compared GN with other 4 implants (SHS, ACE nails, PFN, PFNA); 9 trials compared SHS with 5 other devices; and 3 trials compared PFNA with LISS and PCCP (appendix Figs 28–30). Overall, patients who underwent SHS had more blood loss than those who were treated with other internal fixation implants (SHS vs. IMHS: (MD: 62.42, 95%CI 26.28 to 98.56, I2 = 0%); SHS vs. PFNA: (MD: 253.86, 95%CI 237.47 to 270.25, I2 = 0%); SHS vs. Holland nail: (MD: 82.0, 95%CI 37.81 to 126.91) (Table 5).
Hospital stay (days)
A total of 22 trials (n = 3705) reported the duration of hospital stay18,19,26,29,30,31,32,33,34,35,37,38,39,40,43,45,46,47,48,51,52,54. 11 trials compared GN with other 4 implants (SHS, ACE nails, PFN, PCCP); 9 trials compared SHS with 6 other devices; and 3 trials compared PFNA with LISS and PCCP (appendix Figs 31–33). One comparison revealed that PFN had longer hospital stay compared to GN (MD: 2.7, 95%CI 2.44 to 2.96) and no significant differences were found in other 11 comparisons of devices (Table 5).
Sensitivity analysis
The sensitivity analyses using alternative analysis methods (Peto method vs. Mantel-Haenszel method) and considerations of heterogeneity (random-effects vs. fixed-effect) did not show important changes in the pooled effects for these outcomes.
Discussion
Our study has identified a wide variety of internal fixation implants for patients with intertrochanteric fracture, among which sliding hip screw (SHS) and gamma nail (GN) were the most commonly investigated treatment options, as evident from the trials. The other implants, including percutaneous compression plate (PCCP), proximal femoral nail antirotation (PFNA) and proximal femoral nail (PFN), also are often investigated.
The findings from those trials suggested that substantial uncertainty regarding the relative effects – both benefits and harms – remain among those alternative internal fixation strategies, except only a few comparisons, because of the small number of sample sizes with the vast majority of trials and the serious limitations that threat the validity (e.g. failure to conceal treatment allocation).
The quality of life and functional status are of important interest to surgical treatment and are often used in orthopedics surgical trials60,61,62,63,64. However, our review identified under-reporting of these outcomes. In the trials having reported these two outcomes, the completeness of data remains less satisfied in most circumstances – many trials failed to report the baseline data and the change from baseline; even if reported, the standard deviations were not available. All those limitations have made fair comparison of alternative internal fixation strategies less likely. Given the current body of evidence, it is uncertain if the quality of life would be improved after the surgical interventions and which of surgical treatment would achieve better quality of life. In our analyses of the functional scores, the findings similarly suggested a lack of evidence and no definitive conclusions can be made for most of the comparisons. However, it seems from the analyses that patients receiving PFNA might achieve better functional status after surgery than those receiving GN or SHS. This finding was preliminary given the limitations of the included trials.
The trials extensively reported complications of internal fixation treatments. However, due to the small sample sizes and methodological limitations, the current body of evidence is inadequate to draw clear conclusion for most of the comparisons. The analyses suggested substantial uncertainty of relative effects on complications between internal fixation strategies. However, a number of trials compared GN and SHS. The analyses consistently suggested that patients receiving GN may have significantly higher risk of complications than those SHS, including the risk of cut out, operations, intra-operative fracture and later facture. The consistency of findings across studies and outcomes and the relatively large magnitude of effect increases the credibility of this finding. A few other studies also suggested that SHS might have a lower risk of non-union, but have a higher risk of embolism than PCCP. These findings were, however, fragile given the small number of events and sample sizes, as well as the potential risk of bias those studies pose.
A number of trials also reported procedure measures (operation time, blood loss and hospital stay). The findings were however inconsistent across studies, which resulted in substantial heterogeneity. The presence of the varying procedural outcomes across studies may represent the differential levels of expertise among the surgeons participating in those trials. Overall, the results suggested patients undergoing PFNA may have shorter operative time and patients undergoing SHS may have more blood loss than other extramural implants.
Compared to the Cochrane systematic review that compared intramedullary nails with SHS for extracapsular hip fractures14, we excluded subtrochanteric fractures and assessed more extramedullary implants. The Cochrane Review conducted in 2010 included 43 RCTs that set no limit regarding the length of follow up. They found that the SHS was a better fixation device for the intertrochanteric fractures than nails. They also suggested intramedullary nails have advantages over extramedullary plates/screws for some unstable intertrochanteric fractures. Considering a wide variety of implants with inconsistent outcomes and low precision of estimate effects, we suggest that cautions need to be taken in drawing any definite conclusions.
We conducted a comprehensive systematic review using rigorous methods. However, there are a few limitations. First, because of the limited availability of data, we compared means of functional scores at the end of follow up between treatment groups. We assumed that the data at baseline were well balanced between groups. However, this assumption may not always be hold. Second, the trials we included in the analysis suffered from important methodological limitations, as many other surgical trials. The potential risk of bias that those trials poses has weakened our inference of the treatment effects. Third, most of the trials included in our analyses were small in sample sizes. This has resulted in imprecise estimation of effects and definitive conclusion is unlikely for most of the comparisons. Fourth, due to the limited evidence with different types of fractures (69.7% of studies did not take into account of the fracture stability), we were unable to explore if the treatment effects might differ by fracture types.
In conclusion, due to the small number of events and sample sizes and serious limitations that those trials pose, the current body of evidence is inadequate to establish the relative effects – including quality of life, functional scores and complications – of all of the alternative internal fixation strategies. However, the evidence suggests that patients undergoing GN may have a higher risk of complications than those receiving SHS. Future trials that are adequately powered and well designed and conducted are warranted to fairly test the effects of the different surgical treatments. Observational studies that are carefully collect and analyze the data may also provide important insights regarding the effects of the surgical treatments.
Additional Information
How to cite this article: Yu, J. et al. Internal fixation treatments for intertrochanteric fracture: a systematic review and meta-analysis of randomized evidence. Sci. Rep. 5, 18195; doi: 10.1038/srep18195 (2015).
References
Gullberg, B., Johnell, O. & Kanis, J. A. World-wide projections for hip fracture. Osteoporosis Int. 7, 407–13 (1997).
Miyamoto, R. G. et al. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 16, 596–607 (2008)
Kaplan, K. et al. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 16, 665–73 (2008)
Kaufer, H. Mechanics of the treatment of hip injuries. Clin Orthop. 146, 53–60 (1980)
Panula, J. et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 12, 105 (2011)
Richmond, J. et al. Mortality risk after hip fracture. J Orthop Trauma. 17, 163–6 (2003)
Kenzora, J. E. et al. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery and complications. Clin Orthop Relat Res. 186, 45–56 (1984)
Fung, W. et al. Classifying intertrochanteric fractures of the proximal femur: does experience matter? Med Princ Pract 16, 198–202 (2007)
Bhandari, M. et al. Gamma nails revisited: gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma 23, 460–4 (2009)
Audigé, L., Hanson, B. & Swiontkowski, M. F. Implant-related complications in the treatment of unstable intertrochanteric fractures: meta-analysis of dynamics screw-plate versus dynamic screw-intramedullary nail devices. Int Orthop 27, 197–203 (2003)
Ma, J. et al. The percutaneous compression plate versus the dynamic hip screw for treatment of intertrochanteric hipfractures: a systematic review and meta-analysis of comparative studies. Orthop Traumatol Surg Res. 98, 773–83 (2012)
Norris, R., Bhattacharjee, D. & Parker, M. J. Occurrence of secondary fracture around intramedullary nails used for trochanteric hip fractures: a systematic review of 13,568 patients. Injury 43, 706–11 (2012)
Huang, X. et al. Proximal femoral nail versus dynamic hip screw fixation for trochanteric fracture: a meta-analysis of randomized controlled trials. Scientific World Journal. 2013, 805 (2013)
Parker, M. J. & Handoll, H. H. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 9, CD000093 (2010).
Higgins J. P. T., Green S. eds. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. available at http://www.cochrane-handbook.org. (accessed: 5th Oct 2014)
Thoma, A. et al. Users’ guide to the surgical literature. How to assess a randomized controlled trial in surgery. Can J Surg. 47, 200–8 (2004).
Aktselis, I. et al. Prospective randomised controlled trial of an intramedullary nail versus a sliding hip screw for intertrochanteric fractures of the femur. Int Orthop. 38, 155–61 (2014).
Barton, T. M. et al. A comparison of the long gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 92, 792–8. (2010).
Vaquero, J. et al. Proximal Femoral Nail Antirotation versus Gamma3 nail for intramedullary nailing of unstable trochanteric fractures. A randomised comparative study. Injury. 43, S47–54 (2012)
Yang, E., Qureshi, S., Trokhan, S. & Joseph, D. Gotfried percutaneous compression plating compared with sliding hip screw fixation of intertrochanteric hip fractures: a prospective randomized study. J Bone Joint Surg Am. 93, 942–7 (2011).
Adams, C. I. et al. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 15, 394–400 (2001).
Utrilla, A. L. et al. Trochanteric gamma nail and compression hip screw for trochanteric fractures: a randomized, prospective, comparative study in 210 elderly patients with a new design of the gamma nail. J Orthop Trauma. 19, 229–33 (2005).
Efstathopoulos, N. E., Nikolaou, V. S. & Lazarettos, J. T. Intramedullary fixation of intertrochanteric hip fractures: a comparison of two implant designs. Int Orthop. 31, 71–6 (2007).
De Grave, P. W. et al. Intramedullary fixation of intertrochanteric hip fractures: a comparison of two implant designs. A prospective randomised clinical trial. Acta Orthop Belg. 78, 192–8 (2012).
Vidyadhara, S. & Rao, S. K. One and two femoral neck screws with intramedullary nails for unstable trochanteric fractures of femur in the elderly-randomised clinical trial. Injury. 38, 806–14 (2007).
Xu, Y. et al. Treatment of unstable proximal femoral fractures: comparison of the proximal femoral nail antirotation and gamma nail 3. Orthopedics. 33, 473 (2010).
Hardy, D. C. R. et al. Use of an intramedullary hip-screw compared with a compression hip—screw with a plate for intertrochanteric femoral fractures: A prospective, randomized study of one hundred patients. Journal of Bone and Joint Surgery—Series A. 80, 618–30 (1998).
McCormack, R. et al. A multicentre, prospective, randomised comparison of the sliding hip screw with the Medoff sliding screw and side plate for unstable intertrochanteric hip fractures. Injury. 44, 1904–9 (2013).
Saudan, M. et al. Pertrochanteric fractures: is there an advantage to an intramedullary nail? a randomized, prospective study of 206 patients comparing the dynamic hip screw and proximal femoral nail. J Orthop Trauma. 16, 386–93 (2002).
Xu, Y. Z. et al. A comparison of the proximal femoral nail antirotation device and dynamic hip screw in the treatment of unstable pertrochanteric fracture. J Int Med Res. 38, 1266–75 (2010).
Tao, R. et al. Internal fixation of intertrochanteric hip fractures: a clinical comparison of two implant designs. Scientific World Journal. 834–825 (2013).
Zhou, Z. et al. Minimally invasive versus conventional dynamic hip screw for the treatment of intertrochanteric fractures in older patients. Orthopedics. 35, e244–9 (2012).
Schipper, I. B., Marti, R. K. & van der Werken, C. Unstable trochanteric femoral fractures: extramedullary or intramedullary fixation. Review of literature. Injury. 35, 142–51 (2004).
Harrington, P. et al. Intramedullary hip screw versus sliding hip screw for unstable intertrochanteric femoral fractures in the elderly. Injury. 33, 23–8 (2002).
Ahrengart, L. et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 401, 209–22 (2002).
Bridle, S. H. et al. Fixation of intertrochanteric fractures of the femur. A randomised prospective comparison of the gamma nail and the dynamic hip screw. J Bone Joint Surg Br. 2, 330–4 (1991).
Hoffman, C. W. & Lynskey, T. G. Intertrochanteric fractures of the femur: a randomized prospective comparison of the Gamma nail and the Ambi hip screw. Aust N Z J Surg. 66, 151–5 (1996).
Kukla, C. et al. Gamma nail vs. Dynamic Hip Screw in 120 patients over 60 years—A randomized trial. Acta Chirurgica Austriaca. 29, 290–3 (1997).
Leung, K. S. et al. Gamma nails and dynamic hip screws for peritrochanteric fractures. A randomised prospective study in elderly patients. J Bone Joint Surg Br. 74, 345–51 (1992).
Ovesen, O. et al. The trochanteric gamma nail versus the dynamic hip screw: A prospective randomised study. One-year follow-up of 146 intertrochanteric fractures. HIP International. 16, 293–8 (2006).
Radford, P. J., Needoff, M. & Webb, J. K. A prospective randomised comparison of the dynamic hip screw and the gamma locking nail. J Bone Joint Surg Br. 75, 789–93 (1993).
Herrera, A. et al. A comparative study of trochanteric fractures treated with the Gamma nail or the proximal femoral nail. Int Orthop. 26, 365–9 (2002).
Varela-Egocheaga, J. R. et al. Minimally invasive osteosynthesis in stable trochanteric fractures: a comparative study between Gotfried percutaneous compression plate and Gamma 3 intramedullary nail. Arch Orthop Trauma Surg. 129, 1401–7 (2009).
Janzing, H. M. et al. The Gotfried PerCutaneous Compression Plate versus the Dynamic Hip Screw in the treatment of pertrochanteric hip fractures: minimal invasive treatment reduces operative time and postoperative pain. J Trauma. 52, 293–8 (2002).
Kosygan, K. P., Mohan, R. & Newman, R. J. The Gotfried percutaneous compression plate compared with the conventional classic hip screw for the fixation of intertrochanteric fractures of the hip. J Bone Joint Surg Br. 84, 19–22 (2002).
Peyser, A. et al. A prospective, randomised study comparing the percutaneous compression plate and the compression hip screw for the treatment of intertrochanteric fractures of the hip. J Bone Joint Surg Br. 89, 1210–7 (2007).
Baumgaertner, M. R., Curtin, S. L. & Lindskog, D. M. Intramedullary versus extramedullary fixation for the treatment of intertrochanteric hip fractures. Clin Orthop Relat Res. 348, 87–94 (1998).
Lunsjo, K. et al. Extramedullary fixation of 569 unstable intertrochanteric fractures: a randomized multicenter trial of the Medoff sliding plate versus three other screw-plate systems. Acta Orthop Scand. 72, 133–40 (2001)
Garg, B. et al. Outcome of short proximal femoral nail antirotation and dynamic hip screw for fixation of unstable trochanteric fractures. A randomised prospective comparative trial. Hip Int. 21, 531–6 (2011).
Little, N. J. et al. A prospective trial comparing the Holland nail with the dynamic hip screw in the treatment of intertrochanteric fractures of the hip. J Bone Joint Surg Br. 90, 1073–8 (2008).
Parker, M. J., Bowers, T. R. & Pryor, G. A. Sliding hip screw versus the Targon PF nail in the treatment of trochanteric fractures of the hip: a randomised trial of 600 fractures. J Bone Joint Surg Br. 94, 391–7 (2012).
Guo, Q. et al. Percutaneous compression plate versus proximal femoral nail antirotation in treating elderly patients with intertrochanteric fractures: a prospective randomized study. J Orthop Sci. 18(6), 977–86 (2013)
Wild, M., Jungbluth, P., Thelen, S. et al. The dynamics of proximal femoral nails: a clinical comparison between PFNA and Targon PF. Orthopedics. 2010;33(8).
O’Brien, P. J. et al. Fixation of intertrochanteric hip fractures: gamma nail versus dynamic hip screw. A randomized, prospective study. Can J Surg. 38, 516–20 (1995).
Watson, J. T. et al. Comparison of the compression hip screw with the Medoff sliding plate for intertrochanteric fractures. Clin Orthop Relat Res. 348, 79–86 (1998).
Park, J. H. et al. A comparative study of screw and helical proximal femoral nails for the treatment of intertrochanteric fractures. Orthopedics. 33, 81–5. (2010).
Zou, J., Xu, Y. & Yang, H. A comparison of proximal femoral nail antirotation and dynamic hip screw devices in trochanteric fractures. J Int Med Res. 37, 1057–64 (2009).
Park, S. R. et al. Treatment of intertrochanteric fracture with the Gamma AP locking nail or by a compression hip screw—a randomised prospective trial. International orthopaedics. 22, 157–60 (1998).
Papasimos, S. et al. A randomised comparison of AMBI, TGN and PFN for treatment of unstable trochanteric fractures. Arch Orthop Trauma Surg. 125, 462–8 (2005).
Wood-Dauphinee, S. Quality of life assessment: Recent trends in surgery. Can J Surg 39, 368–72 (1996)
Soulez, G. et al. Pain and quality of life assessment after endovascular versus open repair of abdominal aortic aneurysms in patients at low risk. J Vasc Interv Radiol 16, 1093–100 (2005)
Ethgen, O. et al. Health-related quality of life in total hip and knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 86-A, 963–74 (2004)
Mangione, C. M. et al. Health-related quality of life after elective surgery: measurement of longitudinal changes. J Gen Intern Med. 12, 686–97(1997)
Thoma, A. et al. Evidence-Based Surgery. Users’ guide to the surgical literature: how to assess an article on health-related quality of life. Can J Surg. 51(3), 215–24 (2008)
Acknowledgements
The study was supported by Young Investigator Award, Sichuan University (project No 2013SCU04A37). The funders had no role in study design, data collection and analysis, manuscript drafting or decision to publish.
Author information
Authors and Affiliations
Contributions
Y.J. conceived and designed this study, searched the literature, extracted data, synthesized data and developed the first draft of the manuscript; L.L. and T.L. carefully checked studies and extracted data; Z.C., Z.X. and T.L. synthesized data; S.X., K.J. and L.Y. provided critical methodological guidance; X.L. provided clinical guidance. All authors critically revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yu, J., Zhang, C., Li, L. et al. Internal fixation treatments for intertrochanteric fracture: a systematic review and meta-analysis of randomized evidence. Sci Rep 5, 18195 (2016). https://doi.org/10.1038/srep18195
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18195
This article is cited by
-
Complication rates after proximal femoral nailing: does level of training matter?
Journal of Orthopaedics and Traumatology (2023)
-
Intramedullary Femur Nailing in Intertrochanteric Fractures: Postoperatively Do Helical Blades Migrate More Than Lag Screws? A Randomized Controlled Trial
Indian Journal of Orthopaedics (2023)
-
Letter to the editor regarding “Comparison of a twin interlocking derotation and compression screw cephalomedullary nail (InterTAN) with a single screw derotation cephalomedullary nail (proximal femoral nail antirotation): a systematic review and meta-analysis for intertrochanteric fractures”
Journal of Orthopaedic Surgery and Research (2022)
-
In reply to the letter to the editor regarding “Comparison of a twin interlocking derotation and compression screw cephalomedullary nail (InterTAN) with a single screw derotation cephalomedullary nail (proximal femoral nail antirotation): a systematic review and meta-analysis for intertrochanteric fractures”
Journal of Orthopaedic Surgery and Research (2022)
-
Comparison of clinical outcomes with proximal femoral nail anti-rotation versus bipolar hemiarthroplasty for the treatment of elderly unstable comminuted intertrochanteric fractures
BMC Musculoskeletal Disorders (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.