Abstract
The aim of this study was to investigate the neural substrate underlying quality of life (QoL) and to demonstrate the microstructural abnormalities associated with impaired QoL in a large sample of patients with schizophrenia, using magnetisation transfer imaging. A total of 81 right-handed men with a diagnosis of schizophrenia and 25 age- and sex-similar healthy controls were included and underwent a 3T MRI with magnetization transfer ratio (MTR) to detect microstructural abnormalities. Compared with healthy controls, patients with schizophrenia had grey matter (GM) decreased MTR values in the temporal lobe (BA21, BA37 and BA38), the bilateral insula, the occipital lobe (BA17, BA18 and BA19) and the cerebellum. Patients with impaired QoL had lower GM MTR values relative to patients with preserved QoL in the bilateral temporal pole (BA38), the bilateral insula, the secondary visual cortex (BA18), the vermis and the cerebellum. Significant correlations between MTR values and QoL scores (p < 0.005) were observed in the GM of patients in the right temporal pole (BA38), the bilateral insula, the vermis and the right cerebellum. Our study shows that QoL impairment in patients with schizophrenia is related to the microstructural changes in an extensive network, suggesting that QoL is a bio-psychosocial marker.
Similar content being viewed by others
Introduction
Schizophrenia is a chronic, severe and disabling psychiatric disorder that affects approximately 0.7% to 1% of the general population1. Although reducing the severity of symptoms is an important goal for treating patients with schizophrenia, it is well recognised that reducing the symptoms does not entail managing all of the facets that patients consider to be important in their life2. The quality of life (QoL) measurements are considered to be increasingly important with regard to evaluating disease progression, treatment and the management of care for patients with schizophrenia3. In particular, QoL has been reported to be an independent predictor for long-term symptomatic remission, functional recovery and disability4,5.
Despite the acknowledged need to consider QoL issues in clinical practice and research, QoL measurement has not been routinely implemented6. Among the various reasons for this lack of implementation discussed in the literature7,8, one concern expressed by clinicians is that they did not feel comfortable with the concept of QoL, or interpreting and including QoL data into medical strategies9,10. Because QoL is construed historically and traditionally as a psychosocial subjective construct6,11, many clinicians do not understand the connection between QoL in terms of psychosocial data and the biomedical aspects of patient care. Therefore, a new understanding of the QoL concept provided by building bridges between QoL and neural entities6 is necessary to enhance the implementation of QoL data in clinical practice12.
Several studies have investigated the neural substrate of QoL in patients with schizophrenia13,14,15. Thus far, only one structural imaging study explored the volumetric abnormalities associated with impaired QoL, using voxel-based morphometry and revealed grey matter (GM) reductions in the right dorsolateral prefrontal cortex, the left superior frontal sulcus, the left parahippocampal gyrus and the left inferior temporal gyrus13. However, recent magnetic resonance imaging (MRI)-based analytical approaches, more sensitive to microstructural changes, have not yet been applied to study the neurobiological substrate of QoL in patients with schizophrenia. In particular, the magnetisation transfer imaging (MTI) has the potential for providing more neuropathological information in vivo to subtle or early neuropathological changes than volumetric MRI16,17. The MT ratio (MTR) provided by MTI allows the accurate estimates of structural abnormalities in the normal-appearing brain tissue, as well as in white matter (WM) and GM16,18. Moreover, the MTI is a valuable brain-imaging tool that can be used in various psychiatric disorders, such as schizophrenia16,19,20.
The aim of the present study was to investigate the neural substrate underlying QoL by quantifying subtle microstructural abnormalities associated with impaired QoL in a large sample of patients with schizophrenia, using MTI.
Methods
Participants
This study was conducted in the Center for Mental Health and Addiction at Conception University Hospital and in the Neuroimaging Department (CEMEREM) at Timone University Hospital (Marseille, France) from April 2011 to December 2013.
Eighty-one right-handed consecutive patients (mean age = 30.0 years, SD = 7.5) with a diagnosis of schizophrenia were included in the study. The inclusion criteria were as follows: being male; aged from 18 years to 45 years; having a diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV-TR) criteria21, confirmed by the Structural Clinical Interview (SCID) for DSM-IV-TR22, speaking French as the native language; providing informed consent to participate in the study; having a stable disease (no need for hospitalisation at the time of inclusion in the study and no major change in the patient’s condition for 2 months before inclusion in the study)23 and being an outpatient. The exclusion criteria were as follows: having a psychiatric diagnosis other than schizophrenia on Axis I of DSM-IV-TR; having mental retardation; and fulfilling any contraindication for MRI examination.
Twenty five healthy right-handed controls (mean age = 29.2 years, SD = 4.8), who were age- and gender-similar to the patients, were included in the study. The controls were free from psychiatric disease on the basis of the SCID22 and had no history of neurological or psychiatric disease and no first-degree relatives with psychotic episodes.
The local ethics committee approved the investigations on patients (clinicalTrials.gov identifier: NCT01295411; Comité de Protection des Personnes Sud-Méditerranée V: 2010-A01421-38; AFSSAPS : 11.003) and on controls (Local Ethics Committee for human experimentation, La Timone University Hospital, Marseille, France). Our research was conducted in accordance with the Declaration of Helsinki and French good clinical practices24,25. In particular, both patients and controls received an explanation of the study and gave their written, informed consent after a standardised and structured clinical interview.
Data collection
On the same day the MRI was performed, each patient completed the QoL questionnaire and received a standardised psychiatric assessment performed by a psychiatrist using face-to-face interview, clinical examination and standardised tools.
The following data were collected:
-
1
QoL measurement: QoL was assessed using the S-QoL 18 questionnaire, which is a self-administered, multidimensional instrument developed and validated for specifically assessing QoL in patients with schizophrenia26,27,28. Because the accuracy and completeness of answers in QoL questionnaires may depend on the questionnaires’ difficulty and length27, we have chosen the S-QoL 18, which presents several important properties: the S-QoL 18 is a well-validated questionnaire based exclusively on the patients’ perspectives, ensuring a more appropriate content than the questionnaires based on experts’ determinations29. The items of the S-QoL 18 refer to the present time with a one response option, which may be easier for individuals with schizophrenia to understand. Finally, because of its short format and because of the difficulties in concentration and perception characteristic of patients with deficit syndrome or thought disorders, the S-QoL 18 appears to be better adapted to the populations with schizophrenia27,30. A global score (the index) is calculated whereby the range is from 0, which indicates the lowest QoL, to 100, which indicates the highest QoL.
-
2
Socio-demographic information: Gender, age and educational level (primary school or middle school vs. high school) were documented.
-
3
Clinical characteristics: Duration of disease (defined as time from first contact with mental services) and symptom severity were rated using the Positive and Negative Syndrome Scale (PANSS)31. The three subscales of the PANSS (positive, negative and general psychopathology) are divided into five factors from which hostility-excitation, cognitive and depression factors are of specific interest in our study because of their potential link to QoL32.
-
4
Drug information: Antipsychotic medication (first-generation antipsychotics, FGAs; second-generation antipsychotics, SGAs), chlorpromazine equivalent daily dose and medications (antidepressants, anxiolytics and hypnotics) were documented.
MRI exploration
All of the participants (patients and healthy controls) were examined using an identical MR protocol. All data were obtained using a 3T Magnetom Verio MR Scanner (Siemens, Erlangen, Germany) equipped with a 12-channel head coil. The MR imaging protocol included localiser scout imaging, sagittal 3D FLAIR (fluid attenuated inversion recovery) sequence (TI/TE/TR, 1800 ms/395 ms/5000 ms; 160 contiguous slices; isotropic voxel size, 1 × 1 × 1 mm3; bandwidth, 781 Hz/pixel) and sagittal 3D MPRAGE (Magnetization Prepared Gradient Echo) T1-weighted images (TI/TE/TR, 900 ms/2.92 ms/1900 ms; flip angle, 9°; 176 contiguous slices; isotropic voxel size, 1 × 1 × 1 mm3; bandwidth, 200 Hz/pixel) and transverse proton, density-weighted, spoiled gradient-echo sequences (750/4.5 ms [TR/TE], 44 contiguous slices; 3-mm section thickness; 30° flip angle; 320 mm FOV, 256 × 256 matrix) performed without and with magnetisation transfer (MT) saturation (1.5-kHz off-water resonance, pulse duration 500 μ).
Image processing
MTR maps were calculated using a semi-automated method on a voxel-by-voxel basis, according to the following equation: MTR (%) = (M0 – Mmt)/M0, where M0 and Mmt are the images obtained without and with MT saturation pulse, respectively. The MTR maps were co-registered onto the corresponding T2-weighted images of each subject. The MTR maps were spatially normalised into the Montreal Neurologic Institute (MNI) space using the T1 anatomic template provided in the SPM8 software (Wellcome Institute, London, UK). After segmentation of the normalised MTR maps using voxel intensities and prior knowledge procedures (SPM8), three maps representing fractions of GM, WM and CSF were obtained. Pixels with a percentage of tissue (GM + WM) more than 90% were used to mask the normalised MTR map. After normalisation, brain tissue MTR maps were smoothed using a 6-mm Gaussian filter.
Statistical mapping analysis
First, the microstructural abnormalities of patients with schizophrenia were determined on a voxel-by-voxel basis by comparing the MTR maps of 81 patients with 25 healthy controls using Student’s t test (P < 0.005; FDR was corrected at a cluster level of P < 0.05). The clusters were located on a Talairach atlas33 after MNI coordinates were transformed into Talairach coordinates, using a non-linear transform34 to infer their architectonic locations in GM (Brodmann Area, BA).
Second, the patients were grouped into preserved and impaired QoL groups, using the population norm of the S-QoL 18 index as the cut-off 26. The patients’ characteristics were compared across both groups using Student’s t test or the Mann-Whitney U test for continuous variables and a chi-squared test or Fisher’s exact test for frequencies. One-way analysis of variance (ANOVA) and Bonferroni’s post hoc comparison were performed to assess significant differences in MTR maps between patients with impaired QoL, patients with preserved QoL and healthy controls (P < 0.005; extent threshold k = 20; FDR corrected at cluster level, P < 0.05). Age, PANSS score, disease duration and chlorpromazine equivalent daily dose were considered to be confounding variables.
Finally, correlations were computed between MTR values and S-QoL 18 index scores using a multiple regression model, considering age, disease duration, chlorpromazine equivalent daily dose and PANSS score to be confounding variables (P < 0.005; k = 20). The raw data from the surviving clusters were extracted and the correlations from the S-QoL 18 index were confirmed using Spearman rank correlation tests.
Results
Clinical and MRI characteristics of the entire group of patients
Table 1 shows clinical characteristics for the 81 patients included in the present study. There was no patient with schizoaffective disorder.
Compared to the healthy controls, the entire group of patients had low GM MTR values in the right middle temporal gyrus (BA21), the left temporal pole (BA38), the bilateral insula, the right fusiform gyrus (BA37), the right cuneus (BA17), the right secondary visual cortex (BA18), the right associative visual cortex (BA19) and the right cerebellum. Compared to the control group, the patients exhibited no increase in MTR values in WM and GM and no decrease in the WM (Fig. 1).
Clinical and MRI differences of patients according to QoL levels
Among the 81 patients with schizophrenia, 44 (54.3%) had a preserved QoL level (Table 1).
Compared to healthy controls, the patients with preserved QoL levels had significant low GM MTR values in the right middle temporal gyrus (BA21), the bilateral insula, the fusiform gyrus (BA37), the right cuneus (BA17), the right secondary visual cortex (BA18), the right associative visual cortex (BA19) and the right cerebellum (Fig. 2a). Compared to healthy controls, the patients with impaired QoL levels had low GM MTR values in the right dorsolateral prefrontal cortex (BA9), the right middle temporal gyrus (BA21), the left superior temporal gyrus (BA22), the bilateral temporal pole (BA38), the bilateral insula, the left dorsal posterior cingulate (BA31), the right inferior temporal gyrus (BA37), the right cuneus (BA17), the bilateral secondary visual cortex (BA18) and the right cerebellum (Fig. 2b).
Compared to patients with preserved QoL levels, the patients with impaired QoL levels had lower MTR values in the GM of the bilateral temporal pole (BA38), the bilateral insula, the left secondary visual cortex (BA18), the vermis and the right cerebellum (Fig. 2c). No other significant difference was found between impaired and non-impaired patients.
No differences between the groups were observed in WM.
Correlation between MTR values and S-QoL 18 index scores
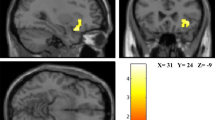
Significant correlations between MTR values and S-QoL 18 index scores (p < 0.005) were observed in the GM of patients (n = 81) within the right temporal pole (BA38) (r = 0.21; P < 0.0001), the left insula (r = 0.19; P < 0.0005), the right insula (r = 0.20; P < 0.0005), the vermis (r = 0.18; P < 0.0005) and the right cerebellum (r = 0.14; P < 0.005) (Fig. 3).
Discussion
The present magnetisation transfer imaging study investigates the neural substrate underlying QoL in patients with schizophrenia. Our study first reports significant decreases in GM MTR values that reflect schizophrenia-related microstructural changes compared to healthy controls, confirming the existence of GM microstructural alterations in patients with schizophrenia35,36. Above all, our study reveals that independent of the usual clinical indicators, the GM MTR decreases were more important in patients with impaired QoL. Our findings, controlled for age, disease duration, symptomatology and medications, obtained by comparing two groups of patients discriminated only by the level of QoL and secondarily confirmed using a correlation analysis in the entire group of patients, highlight the involvement of the temporal pole, the insula and the cerebellum.
Our initial findings report a pattern of GM MTR value decreases in patients with chronic schizophrenia compared to healthy controls, i.e., the temporal lobe (BA21, BA37 and BA38), the bilateral insula, the occipital lobe (BA17, BA18 and BA19) and the cerebellum. These findings using MTI, a sensitive method for evaluating demyelination and axonal loss with a better spatial resolution and less geometric distortions than diffusion tensor imaging, are consistent with previous morphometric studies using region of interest or whole-brain voxel-based morphometry analyses in first-episode and chronic schizophrenia that have reported the existence of GM loss in multiple brain structures36. Decreased GM volume in the temporal regions is the most common finding in the comparative studies of patients with schizophrenia and healthy controls37,38. To the extent that the symptoms can be localised, the temporal region abnormalities have been linked to auditory hallucinations, thought disorder and memory dysfunction39,40. GM microstructure impairment within the insula, usually bilaterally, as observed in our study, is also consistent with GM decreases commonly observed in schizophrenia41, associated with psychotic symptoms42 and cognitive impairments43. Cerebellar atrophy44, particularly in the vermis45, has also been described, affecting cognitive and emotional processing46. Finally, although less implicated in the pathophysiology of schizophrenia, a decreased GM volume in the occipital lobe has been detected47 supporting dysfunctions of cognitive or visual processing48. Altogether, our results demonstrate MTR GM decreases in crucial brain regions that involve several functions that are altered in schizophrenia. Unlike previous studies, our results failed to identify WM MTR value decreases17,49. This discrepancy may be explained by the heterogeneity of chronic schizophrenia population (e.g., duration of illness, medications).
Second, a neural understanding of QoL may address the concerns of healthcare professionals regarding the meaning of this measure, its relevance and its usefulness in clinical practice.
An impaired QoL was associated with more microstructural changes in distributed GM areas that correspond to particular functional networks. These findings implies that QoL is influenced by a neurological background, suggesting that QoL could be considered to be a bio-psychosocial construct rather than only a psychosocial construct6. The brain areas involved in the QoL levels (i.e., the temporal pole, the insula and the cerebellum) provide information concerning what is measured using a QoL questionnaire. Our findings suggest that QoL is closely linked to a functional brain network regulating emotional behaviour. On the one hand, the insula integrates perceptions, emotions and thoughts into a subjective word50 and has been involved in linking emotion signals with other sensory information. Interestingly, a dopaminergic abnormality is likely to be associated with this brain area in patients with schizophrenia51 and dopamine has been implicated in the mediation of pleasurable experiences52. From a network perspective, insula plays a key role in the salience network and especially in the switching between the default mode network and the central executive network51,53,54,55,56. The anterior frontal region involved in significant MTR decreased in impaired patients compared to non-impaired could also be part of the salience network. On the other hand, the right temporal pole has been associated with metacognition (i.e., the ability to attribute mental states, in terms of beliefs and goals, to oneself and others)57, which plays a central role in the understanding, learning and regulation of emotions58. Moreover, neural connections have been reported between this temporal area and the insula59. Finally, the cerebellum and particularly the vermis, is also involved in emotional behaviour60. The involvement of all these brain areas is also consistent with a recent neuroimaging studies in fibromyalgia in which QoL was associated with right medial temporal metabolism within the limbic system, which is involved in the affective and emotional domains61. Another study found that in schizophrenia, QoL was associated with the superior temporal sulcus involved in metacognition12. Considering all of these points, we may hypothesise that QoL provides information regarding the emotional experience and social interactions of individuals.
The persistency of the link between QoL and the microstructural changes in the GM after adjustment for the usual clinical indicators suggests that QoL adds information to the data traditionally collected and used in psychiatry. QoL should not be considered to be a derivative of symptomatology and other “objective” factors (e.g., functioning, socio-economic factors) although moderate links exist62,63 but rather a unique and relevant perspective of the patient with regard to his health.
Finally, the neural underpinnings of QoL could open interesting perspectives for using QoL as a bio-psychosocial marker in the evolution of schizophrenia. QoL has been reported to be an independent predictor for the long-term prognosis of schizophrenia and other chronic diseases, sometimes more than the severity of symptoms5,64,65. In our study, the association of QoL impairment with microstructural changes in core regions of functional networks involved in emotional and social processing suggests that QoL may precociously capture progressive brain tissue loss involved in the prognosis of schizophrenia66,67. This hypothesis presents the likelihood that QoL could guide individual therapeutic strategy, especially to identify early those patients with more important progressive brain loss and thus with poorer long-term prognosis. The early detection of these patients may allow proposing more adapted treatment. This hypothesis should be explored and confirmed in future longitudinal studies.
Several limitations of the study should be carefully considered. First, the sample may not be representative of the entire population of patients with schizophrenia. All patients were males, had a moderate illness, were middle-aged and had more than 5 years of disease duration. Additional exploration on more diverse and larger groups of patients is therefore required to corroborate our findings. Second, the relatively small sample size for the control group can be a limitation. However, their characteristics were similar to those of the patients. Third the cross-sectional design of our study precludes any conclusions regarding the directionality of the association between QoL and GM abnormalities in schizophrenia. Future longitudinal studies should specifically explore this issue. Fourth, our findings could have been blurred by the impact produced by the antipsychotic medications for the likely effects on the structure or functioning of the brain. However, because the patient groups were for the most part comparable, the results observed should be interpreted in terms of differences in QoL levels. Last, we chose to explore the neural substrate underlying QoL by focusing on the microstructural abnormalities using MTI. It would be interesting to study the functional neural substrate underlying QoL using resting state functional MRI approach and connectivity analysis.
Conclusion
Our study shows that QoL impairment in patients with schizophrenia is related to microstructural changes in distributed areas usually participating to functional networks involved in emotional and social interactions processes, suggesting that QoL provides unique information concerning the emotional and social experience of individuals who are not available for traditional assessments. Additionally, this study could present interesting perspectives for using QoL as a bio-psychosocial marker in the evolution of schizophrenia.
Additional Information
How to cite this article: Faget-Agius, C. et al. Neural substrate of quality of life in patients with schizophrenia: a magnetisation transfer imaging study. Sci. Rep. 5, 17650; doi: 10.1038/srep17650 (2015).
Change history
15 February 2016
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
McGrath, J., Saha, S., Chant, D. & Welham, J. Schizophrenia: A Concise Overview of Incidence, Prevalence and Mortality. Epidemiol. Rev. 30, 67–76 (2008).
Boyer, L. et al. Measuring quality of life in patients with schizophrenia:an overview. Expert Rev. Pharmacoecon. Outcomes Res. 13, 343–349 (2013).
Awad, A. G., Voruganti, L. N. & Heslegrave, R. J. Measuring quality of life in patients with schizophrenia. PharmacoEconomics 11, 32–47 (1997).
Lambert, M., Karow, A., Leucht, S., Schimmelmann, B. G. & Naber, D. Remission in schizophrenia: validity, frequency, predictors and patients’ perspective 5 years later. Dialogues Clin. Neurosci. 12, 393–407 (2010).
Boyer, L. et al. Quality of life is predictive of relapse in schizophrenia. BMC Psychiatry 13, 15 (2013).
Awad, A. G. & Voruganti, L. N. P. Measuring quality of life in patients with schizophrenia: an update. PharmacoEconomics 30, 183–195 (2012).
Boyer, L. & Auquier, P. The lack of impact of quality-of-life measures in schizophrenia: a shared responsibility? PharmacoEconomics 30, 531–532; author reply 532–533 (2012).
Boyer, L., Baumstarck, K., Guedj, E. & Auquier, P. What’s wrong with quality-of-life measures? A philosophical reflection and insights from neuroimaging. Expert Rev. Pharmacoecon. Outcomes Res. 14, 767–769 (2014).
Halyard, M. Y., Frost, M. H., Dueck, A. & Sloan, J. A. Is the use of QOL data really any different than other medical testing? Curr. Probl. Cancer 30, 261–271 (2006).
Halyard, M. Y., Frost, M. H. & Dueck, A. Integrating QOL assessments for clinical and research purposes. Curr. Probl. Cancer 30, 319–330 (2006).
Gill, T. M. & Feinstein, A. R. A critical appraisal of the quality of quality-of-life measurements. JAMA 272, 619–626 (1994).
Boyer, L. et al. Functional involvement of superior temporal sulcus in quality of life of patients with schizophrenia. Psychiatry Res. 202, 155–160 (2012).
Ubukata, S. et al. Regional gray matter reduction correlates with subjective quality of life in schizophrenia. J. Psychiatr. Res. 47, 548–554 (2013).
Higuchi, Y. et al. Electrophysiological basis for the ability of olanzapine to improve verbal memory and functional outcome in patients with schizophrenia: a LORETA analysis of P300. Schizophr. Res. 101, 320–330 (2008).
Pu, S. et al. Association between subjective well-being and prefrontal function during a cognitive task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr. Res. 149, 180–185 (2013).
Foong, J. et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain J. Neurol. 124, 882–892 (2001).
Bagary, M. S. et al. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch. Gen. Psychiatry 60, 779–788 (2003).
Ranjeva, J.-P. et al. Local tissue damage assessed with statistical mapping analysis of brain magnetization transfer ratio: relationship with functional status of patients in the earliest stage of multiple sclerosis. AJNR Am. J. Neuroradiol. 26, 119–127 (2005).
Price, G. et al. Brain pathology in first-episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. NeuroImage 49, 185–192 (2010).
Bohner, G. et al. MTR abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Schizophr. Res. 137, 85–90 (2012).
American Psychiatric Association. In Diagnostic and statistical manual of mental disorders, (4th edition, text revised), 343–399 (APA, 2000).
First Michael, B., Spitzer Robert, L. & Gibbon Miriam, W. J. B. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute. (SCID-I/P) New York: Nov, 2002).
Appelberg, B., Tuisku, K. & Joffe, G. Is it worth while changing clinically stable schizophrenic out-patients with mild to moderate residual symptoms and/or side effects from conventional to atypical antipsychotics? A prospective, randomised study with olanzapine. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 19, 516–518 (2004).
CNIL. Act n°78-17 of 6 January 1978 on Data processing on information technology, data files and civil liberties. French Legislation.
WMA. Declaration of Helsinki, ethical principles for medical research involving human subjects. (2008).
Boyer, L. et al. The development of the S-QoL 18: a shortened quality of life questionnaire for patients with schizophrenia. Schizophr. Res. 121, 241–250 (2010).
Baumstarck, K. et al. Self-reported quality of life measure is reliable and valid in adult patients suffering from schizophrenia with executive impairment. Schizophr. Res. 147, 58–67 (2013).
Auquier, P. et al. Toward meeting the needs of homeless people with schizophrenia: the validity of quality of life measurement. PloS One 8, e79677 (2013).
Cramer, J. A. et al. Quality of life in schizophrenia: a comparison of instruments. Department of Veterans Affairs Cooperative Study Group on Clozapine in Refractory Schizophrenia. Schizophr. Bull. 26, 659–666 (2000).
Baumstarck, K. et al. Quantification of relevance of quality of life assessment for patients with cognitive impairment: the suitability indices. BMC Neurol. 14, 78 (2014).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Lançon, C., Auquier, P., Nayt, G. & Reine, G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS). Schizophr. Res. 42, 231–239 (2000).
Talairach, J. & Tournoux, P. In Co-planar stereotaxic atlas of the human brain, Vol. 3 (eds Thieme-Stratton Corp) (1998).
Brett, M., Leff, A. P., Rorden, C. & Ashburner, J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage 14, 486–500 (2001).
Gupta, C. N. et al. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-analysis. Schizophr. Bull. 10.1093/schbul/sbu177 (2014).
Goodkind, M. et al. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry 10.1001/jamapsychiatry.2014.2206 (2015).
Honea, R., Crow, T. J., Passingham, D. & Mackay, C. E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 162, 2233–2245 (2005).
Witthaus, H. et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 173, 163–169 (2009).
Horn, H. et al. Gray matter volume differences specific to formal thought disorder in schizophrenia. Psychiatry Res. 182, 183–186 (2010).
Tang, J. et al. Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PloS One 7, e40247 (2012).
Wylie, K. P. & Tregellas, J. R. The role of the insula in schizophrenia. Schizophr. Res. 123, 93–104 (2010).
Roper, S. N., Lévesque, M. F., Sutherling, W. W. & Engel, J. Surgical treatment of partial epilepsy arising from the insular cortex. Report of two cases. J. Neurosurg. 79, 266–269 (1993).
Crespo-Facorro, B. et al. Neural basis of novel and well-learned recognition memory in schizophrenia: a positron emission tomography study. Hum. Brain Mapp. 12, 219–231 (2001).
Weinberger, D. R., Torrey, E. F. & Wyatt, R. J. Cerebellar atrophy in chronic schizophrenia. Lancet 1, 718–719 (1979).
Nopoulos, P. C., Ceilley, J. W., Gailis, E. A. & Andreasen, N. C. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol. Psychiatry 46, 703–711 (1999).
Andreasen, N. C. & Pierson, R. The Role of the Cerebellum in Schizophrenia. Biol. Psychiatry 64, 81–88 (2008).
Cascella, N. G. et al. Gray-matter abnormalities in deficit schizophrenia. Schizophr. Res. 120, 63–70 (2010).
Qiu, L. et al. Neuroanatomical circuitry associated with exploratory eye movement in schizophrenia: a voxel-based morphometric study. PloS One 6, e25805 (2011).
Kanaan, R. et al. White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br. J. Psychiatry J. Ment. Sci. 194, 236–242 (2009).
Kurth, F. et al. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain structure & function. 214, 5–6 (2010).
Palaniyappan, L. & Liddle, P. F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. JPN 37, 17–27 (2012).
Adinoff, B. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry 12, 305–320 (2004).
Goulden, N. et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage 99, 180–190 (2014).
Jilka, S. R. et al. Damage to the Salience Network and interactions with the Default Mode Network. J. Neurosci. Off. J. Soc. Neurosci. 34, 10798–10807 (2014).
Sidlauskaite, J. et al. Anticipatory processes in brain state switching - evidence from a novel cued-switching task implicating default mode and salience networks. NeuroImage 98, 359–365 (2014).
Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 105, 12569–12574 (2008).
Andreasen, N. C., Calarge, C. A., Calage, C. A. & O’Leary, D. S. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr. Bull. 34, 708–719 (2008).
Lysaker, P. H. et al. Capacities for theory of mind, metacognition and neurocognitive function are independently related to emotional recognition in schizophrenia. Psychiatry Res. 219, 79–85 (2014).
Almashaikhi, T. et al. Functional connectivity of insular efferences. Hum. Brain Mapp. 35, 5279–5294 (2014).
Strata, P. The Emotional Cerebellum. Cerebellum Lond. Engl. 10.1007/s12311-015-0649-9 (2015).
Boyer, L. et al. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology 82, 1231–1238 (2014).
Chou, C.-Y., Ma, M.-C. & Yang, T.-T. Determinants of subjective health-related quality of life (HRQoL) for patients with schizophrenia. Schizophr. Res. 154, 83–88 (2014).
Fervaha, G., Agid, O., Takeuchi, H., Foussias, G. & Remington, G. Clinical determinants of life satisfaction in chronic schizophrenia: data from the CATIE study. Schizophr. Res. 151, 203–208 (2013).
Baumstarck, K., Pelletier, J., Boucekine, M. & Auquier, P. & MusiQoL study group. Predictors of quality of life in patients with relapsing-remitting multiple sclerosis: A 2-year longitudinal study. Rev. Neurol. (Paris) 171, 173–180 (2015).
Maione, P. et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 23, 6865–6872 (2005).
Andreasen, N. C. et al. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol. Psychiatry 70, 672–679 (2011).
Andreasen, N. C., Liu, D., Ziebell, S., Vora, A. & Ho, B.-C. Relapse duration, treatment intensity and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am. J. Psychiatry 170, 609–615 (2013).
Acknowledgements
The authors are indebted to Thérèse Auriol-Vigne for her assistance with the preparation data-base gathering information belonging to subjects included in this study. Funding: This work was supported, by institutional grants (grant number: NCT01295411) from the 2010 Hospital Program of Clinical Research and the French Ministry of Health (General Direction of Health).
Author information
Authors and Affiliations
Contributions
C.F.A.: Drafting/revising the manuscript for content, including medical writing for content; Analysis or interpretation of data; Statistical analysis; Study supervision or coordination. L.B.: Drafting/revising the manuscript for content, including medical writing for content; Analysis or interpretation of data; Statistical analysis. J.W.: Analysis or interpretation of data; Acquisition of data. J.P.R.: Drafting/revising the manuscript for content, including medical writing for content; Study concept or design. R.R.: Study supervision or coordination; Analysis or interpretation of data. E.S.: Analysis or interpretation of data; Acquisition of data. S.C.-G.: Analysis or interpretation of data; Acquisition of data. P.A.: Drafting/revising the manuscript for content, including medical writing for content; Study concept or design; Analysis or interpretation of data; Statistical analysis; Obtaining funding. M.G.: Drafting/revising the manuscript for content, including medical writing for content; Study concept or design; Analysis or interpretation of data; Statistical analysis. C.L.: Drafting/revising the manuscript for content, including medical writing for content; Study concept or design; Analysis or interpretation of data; Acquisition of data; Statistical analysis; Study supervision or coordination; Obtaining funding.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Faget-Agius, C., Boyer, L., Wirsich, J. et al. Neural substrate of quality of life in patients with schizophrenia: a magnetisation transfer imaging study. Sci Rep 5, 17650 (2015). https://doi.org/10.1038/srep17650
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17650
This article is cited by
-
Magnetization transfer imaging alterations and its diagnostic value in antipsychotic-naïve first-episode schizophrenia
Translational Psychiatry (2022)
-
Differences on Quality of Life of Patients with Schizophrenia: A Multicentric Study from Three Latin-America Countries
Culture, Medicine, and Psychiatry (2019)
-
Caregiver’s quality of life and its positive impact on symptomatology and quality of life of patients with schizophrenia
Health and Quality of Life Outcomes (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.