Abstract
Parasitism is a successful survival strategy across all kingdoms and has evolved repeatedly in angiosperms. Parasitic plants obtain nutrients from other plants and some are agricultural pests. Obligate parasites, which cannot complete their lifecycle without a host, may lack functional photosystems (holoparasites), or have retained photosynthesis (hemiparasites). Plastid genomes are often reduced in parasites, but complete mitochondrial genomes have not been sequenced and their mitochondrial respiratory capacities are largely unknown. The hemiparasitic European mistletoe (Viscum album), known from folklore and postulated therapeutic properties, is a pest in plantations and forestry. We compare the mitochondrial genomes of three Viscum species based on the complete mitochondrial genome of V. album, the first from a parasitic plant. We show that mitochondrial genes encoding proteins of all respiratory complexes are lacking or pseudogenized raising several questions relevant to all parasitic plants: Are any mitochondrial gene functions essential? Do any genes need to be located in the mitochondrial genome or can they all be transferred to the nucleus? Can parasitic plants survive without oxidative phosphorylation by using alternative respiratory pathways? More generally, our study is a step towards understanding how host- and self-perception, host integration and nucleic acid transfer has modified ancestral mitochondrial genomes.
Similar content being viewed by others
Introduction
Mitochondria, originally captured α-proteobacteria, produce energy through respiration and are thought to be required for eukaryote complexity and the evolution of multi-cellularity. Mitochondria house key metabolic pathways (e.g., the tricarboxylic acid cycle (TCA), Fe-S-protein biogenesis, co-enzyme production) and mediate forms of programmed cell-death, anti-microbial defense and stress responses. Mitochondrial genomes vary remarkably in size and structural heterogeneity, particularly in protists, lower metazoans and plants and there are even reports of eukaryotes in which the mitochondrial genome is almost certainly absent1. While the animal mitochondrial genome size of 15–18 kb is almost constant, that of seed plants varies from 208 kb to more than 11 mb2,3. A significant part of the increase in size in plants is due to inter-organellar transfer4,5,6 and, at least in some cases, exogenous DNA2,7. However, imported genes usually degrade into pseudogenes8. For these reasons ~5150 complete animal mitochondrial genomes have been sequenced and assembled, but only 58 from seed plants. The differences in size are not reflected in numbers of protein-coding genes. For example, the Arabidopsis mitochondrial genome is 22 times larger than that of humans but only encodes 2.5 times as many proteins (13 and 31, respectively)9 and even the smallest and largest genomes of nonparasitic seed plants contain almost the same number of genes. The smallest number of protein-coding mitochondrial genes hitherto reported in a seed plant is 2510. Though complete mitochondrial genomes from holoparasitic species of Rafflesiaceae have not been assembled, sequencing data do not suggest gene loss11,12.
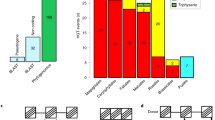
The hemiparasitic genus mistletoe (Viscum) consists of ~100 species parasitic on a large variety of hosts (Fig. 1). While it is known that the chloroplast genomes of parasites may be reduced together with the loss of photosynthetic activity13, we show here that the number of proteins encoded by the mitochondrial genomes of three Viscum species is significantly smaller than seen in any other seed plant (Fig. 2). This indicates that the chloroplast and mitochondrial genomes of parasitic plants share parallel fates and raises questions about the functions of their mitochondria.
Viscum minimum parasitizing succulent Euphorbia.
(a) The Viscum seedling to the left has established contact with the host through a haustorium. Endophytic growth of the parasite is visible to the right just beneath the host, Euphorbia horrida, epidermis; (b) Mature, green shoots of Viscum minimum on Euphorbia mammillaris. (Photo: Gitte Petersen).
Overview of gene content in the mitochondrial genome of Viscum and 29 other seed plants.
Dark grey fields indicate presence of the gene. Light grey fields indicate pseudogenes or gene fragments. The rrn18 genes are scored as functional with doubt. See Extended Data Table 1 for details of the genes (including tRNAs) found in Viscum.
Roughly half the proteins encoded in the mitochondria of seed plants are involved in the respiratory chain and tricarboxylic (TCA) acid cycle and are therefore usually considered essential5. Proteins of the respiratory chain belong to five enzymatic complexes. Complexes I, III and IV function in proton translocation and are probably tightly associated in a supramolecular assembly, whereas complex II conveys electrons to the respiratory chain and complex V uses the proton gradient to phosphorylate ADP to ATP14.
Complex I, NADH-ubiquinone oxidoreductase, is the largest complex in the respiratory chain. It is responsible for the first steps in electron transport which lead to the translocation of four protons between the matrix and the intermembrane space, though further enzymatic activities have also been postulated15. In Arabidopsis, complex I is composed of 48 subunits16. Typically, nine of them are encoded in the mitochondrial genome17 (nad1, 2, 3, 4, 4L, 5, 6, 7, 9) and are invariably present in nonparasitic seed plants4 (Fig. 2). However, none of these genes are present in the mitochondrial genome of the three Viscum species although a few smaller, degenerated fragments may be identified (Extended Data Table 1).
Complex II, succinate dehydrogenase (SDH), is the only enzyme with a role in both the TCA cycle and electron transport. In Arabidopsis it is composed of eight subunits, four of them plant specific. Two of the subunits are encoded in the mitochondrial genome and the function of four of the remaining subunits remains unknown18. Whereas the amino acid sequences of SDH1 and SDH2 are fairly well conserved across kingdoms, those of the membrane anchor proteins SDH3 and SDH4 are very diverse. Only sdh3 and sdh4 are found in mitochondrial genomes of seed plants, though they are often missing or pseudogenized (Fig. 2). A short fragment of sdh3 is found in all three Viscum species, but only V. album includes a fragment of sdh4.
Complex III, the cytochrome bc1 complex, consists of 10–11 subunits19 of which only one is encoded in the mitochondrial genome17. It oxidizes the ubiquinol produced by complex I and II by transferring electrons from ubiquinol to cytochrome c and pumping protons across the membrane. Apocytochrome b (cob) is without exception found in all seed plant mitochondrial genomes and is present in the three Viscum species (Fig. 2), but highly divergent from other seed plant cob sequences (Extended Data Fig. 1).
Complex IV, cytochrome c oxidase, is encoded by 11 genes, three encoded in the mitochondria17. It catalyzes the complete reduction of oxygen to water and promotes proton translocation across membranes to help form the electrochemical gradient. The cox1, cox2 and cox3 genes are found in all seed plants except in certain legumes20 in which a functional cox2 copy is transferred to the nuclear genome21 (Fig. 2). The three Viscum species all have the three cox-genes, but like cob these genes are highly divergent (Extended Data Fig. 2). Whereas cox2 in other seed plants include zero to two cis-spliced introns, the first intron in V. album is trans-spliced and both introns appear trans-spliced in V. crassulae and V. minimum.
Complex V, ATP or F1F0-ATP synthase, is encoded by 16 genes, five of which are mitochondrial in Arabidopsis17 and all other seed plants (Fig. 2) with one possible exception4. It uses the proton gradient produced by complexes I, III and IV to synthesize ATP from ADP22. Two of the five genes of complex V encoded in the mitochondria of all other known seed plants are missing in the three Viscum species and the remaining genes are highly divergent (Extended Data Fig. 3).
Apart from the respiratory chain genes, seed plant mitochondrial genomes all include four genes related to cytochrome biogenesis (ccmB, C, Fc and Fn) and a maturase-related protein gene (matR). Whereas all three Viscum species have retained matR, they have lost one or two of the cytochrome biogenesis genes (Fig. 2). Most seed plant mitochondria also include a membrane transport protein gene (mttB). This is pseudogenized in the Viscum species, is missing in other seed plants (Fig. 2, Extended Data Table 1) and appears to be non-essential. In addition, most seed plants include a variable number of ribosomal protein genes (up to 15), but none of them are ubiquitous5. We found a single gene in V. album (rps12), but the absence of all genes in the other two species of Viscum is remarkable. So far, ribosomal RNA genes have been found in the mitochondrial genomes of all seed plants (Fig. 2) and cotranscription of the 18S-5S rRNA cluster has been considered indispensable23. While seed plant mitochondrial genomes never have all 20 tRNAs necessary for protein synthesis, the presence of only 3–4 tRNA genes in the Viscum mitochondrial genomes is highly unusual3 (Extended Data Table 1).
However, we were unable to locate 5S rRNA in any of the three Viscum species and the recovered 26S rRNA-like sequences are so divergent that the genes are unlikely to be functional. The identified sequences of the 18S rRNA genes are also very different from those of all other seed plants suggesting that they too are not functional. Additionally a number of ribosomal proteins are usually encoded in the plant mitochondrial DNA, but in Viscum album only one complete ribosomal gene and one fragment is found and none in the other two Viscum species. While seed plant mitochondrial genomes never have all 20 tRNAs necessary for protein synthesis, the presence of only 3–4 tRNA genes in the Viscum mitochondrial genomes is highly unusual3 (Extended Data Table 1). Thus, the three Viscum mitochondrial genomes are not only exceptionally gene poor, but those present are almost all extremely divergent from those of other seed plants (Fig. 3, Extended Data Figs 1–3). Nonetheless, a phylogenetic analysis of 10 of the protein-coding genes places the three Viscum species in their traditional position sister to Caryophyllales and the asterids, but on an exceptionally long branch reflecting the highly divergent sequences of the genes (Fig. 3). Individual gene trees (Extended Data Figs 1–4) reveal that only two genes (matR and ccmB), not part of the respiratory chain, are not unexpectedly divergent. This may indicate that they are the only truly functional genes in their mitochondrial genomes. In general, it appears that only respiratory complexes III and IV are fully encoded in the mitochondrial genomes of the three Viscum species, but their potentially pseudogenized state and the paucity of the remaining respiratory genes is a strong indication that none of them are functional. And even if they are functional, Viscum mitochondria would be unable to perform protein biosynthesis unless all the missing rRNA, ribosomal proteins and tRNAs are imported.
Phylogenetic tree based on sequence data from 10 mitochondrial genes.
The position of Viscum is in agreement with models of angiosperm phylogeny, but the long branch indicates that the Viscum gene sequences are extremely divergent compared to other plants. See Extended Data Figs 1–4 for individual gene trees. Scale bar, substitutions per site.
If legumes have successfully transferred the cox2 gene to the nucleus20,21, is it then possible that Viscum has transferred a whole suite of genes and retained a fully functional electron transport chain and oxidative phosphorylation? This would explain how the seedlings survive prior to establishing physical contact with a host, which would otherwise be very difficult, but our sequencing depth was insufficient to decisively detect nuclear transfers. These questions will be pursued in transcriptome and proteome studies possibly coupled with deeper sequencing of Viscum nuclear genomes, though the latter may be impeded by their enormous size24.
The alternative to the transfer of most or all the mitochondrial genes to the nucleus is that Viscum mitochondria have experienced a reductive evolution similar to that observed for hydrogenosomes and mitosomes in anaerobic ciliates and fungi25. The mitosomes appear to lack the ATP-generating pathways and have a simpler structure as they lack cristae. Nonetheless they have been retained as independent organelles probably to provide the cell with important metabolic intermediates, iron-sulfur clusters, coenzymes, etc. In this scenario, Viscum cells would obtain their ATP from photosynthesis (which starts immediately following germination in Viscum album seeds26) and eventually from glycolytic degradation of sugars imported from the host after the establishment of the haustorium. In the aerobic Viscum cells, cytosolic NADH and ATP could then be used to fuel respiratory metabolism, for instance by energizing the inner mitochondrial membrane, which is necessary to ensure a controlled exchange of metabolites between the matrix and the cytosol.
To speculate further, cytosolic NADH (or NADPH) could potentially be oxidized by one of the alternative NAD(P)H dehydrogenases located on the outer surface of the inner mitochondrial membrane and the electrons via ubiquinone go to the alternative oxidase, which reduces oxygen on the inner surface. All of the alternative NAD(P)H dehydrogenases as well as the alternative oxidase are nuclear-encoded so they would be unaffected by the loss of virtually the full mitochondrial genome. There is co-expression evidence to suggest that each external NAD(P)H dehydrogenase in plant mitochondria is associated with a particular alternative oxidase isoform, although evidence for a specific functional interaction is still lacking27. The net result would be a transfer of two electrons per NAD(P)H molecule from the outside to the inside of the mitochondrion, which would create a membrane potential. At the same time a proton will be released on the outer surface and two protons consumed on the inner surface setting up a proton gradient with a more basic matrix. Thus, an electrochemical proton gradient across the inner mitochondrial membrane is formed similar to that formed by the complete electron transport chain. This alternative mechanism of inner membrane energization would be less efficient because only two protons are transferred per two electrons compared to six protons when the electrons from external NADH pass through complexes III and IV27, but it would be able to energize metabolite exchange across the inner membrane (e.g., import of ATP). In contrast, it would not be coupled to ATP synthesis because of the absence of the ATP synthase (Complex V). Similarly, but without energization of the inner membrane, NADH produced in the matrix by the TCA cycle could be reoxidized by an internal alternative NADH dehydrogenase linked to another alternative oxidase isoform, thus allowing the continued function of the TCA cycle. Note that in the scenario we are considering here, the five respiratory complexes are missing including the TCA cycle enzyme succinate dehydrogenase (Complex II). The TCA cycle would therefore have to use alternative flux modes28 to produce all the important metabolic intermediates.
Interestingly, parallel reductions in the plastid gene complements of parasitic plants have been observed, most drastically in Rafflesiaceae where the entire plastome may be lost12, pointing at a correlation between diminished photosynthetic capacity and different degrees of nutritional dependence upon the host13. Surprisingly, our data from the plastid genome of Viscum e.g, showing loss of all NADH dehydrogenase genes, are in line with this trend29.
In general our study raises a series of challenges faced by any parasitic plant related to recognizing self and non-self, host integration, alternative respiratory pathways and the extent of ancestral nucleic acid transfer in the seed plant tree of life.
Methods Summary
DNA from intact mitochondria isolated from Viscum album leaves was extracted using a CTAB procedure and sequenced using 454 GS FLX Titanium technology. The complete mitochondrial genome was assembled using Newbler and bb.454contignet software and coding regions identified by BLAST searches against GenBank and local data bases. From two other Viscum species, V. crassulae and V. minimum, total genomic DNA was extracted and sequenced using Illumina/HiSeq technology. Sequence reads were used to extract mitochondrial gene sequences only. Sequences from 10 mitochondrial genes identified in all or just one species of Viscum were included in a phylogenetic analysis together with sequences from other seed plants from which whole mitochondrial genome sequences are available. Individual gene trees were also constructed.
Methods
Plant material
Fresh material of three species of Viscum L. was collected from specimens grown in the Botanical Garden, Natural History Museum of Denmark, University of Copenhagen, Denmark. In the garden Viscum album L. is a parasite on Malus Mill. sp. and the two endemic, South African species V. crassulae Eckl. & Zeyh. and V. minimum Harv. are parasites on Portulacaria afra Jacq. and Euphorbia mammillaris L., respectively. Voucher specimens are deposited in the herbarium (C) of the Natural History Museum of Denmark (V. album: C2546, V. crassulae: C2553, V. minimum: C2884).
DNA extraction and sequencing
Green leaves were used for DNA extraction of V. album. Intact mitochondria were isolated by centrifugation following a modified protocol30 and using DNAase I to digest nuclear and other DNA contaminants. Mitochondrial DNA was extracted using CTAB and a regular chloroform-isoamyl alcohol DNA isolation protocol. Whole genome amplifications were carried out using repli-g kit (Qiagen) after the manufacturer’s protocol. A GS FLX Titanium (Roche, USA) shot gun library was constructed and sequenced at the National High-throughput DNA Sequencing Centre, U. Copenhagen in a quarter of a GS PicoTiterPlate according to the manufacturer’s instructions.
Green leaves were also used for DNA extraction of V. crassulae, but to minimize the risk of host tissue contamination, green seeds were used for DNA extraction of the minute V. minimum. In both cases ca. 100 mg of fresh tissue was used for total genomic DNA extractions using a standard CTAB method31. Illumina short-insert, paired-end libraries with average insert sizes of 500 bp were constructed and each sample was run in 1/16 of a lane on an Illumina HiSeq 2500.
Sequence analysis
A total of 60,389 sequences (average size 339 nt) from V. album were assembled using Newbler 2.3 (454 Life Sciences Corp, CT, USA) using default settings. A total of 38 contigs >100 nt were obtained, with the largest contig of 116,034 nt. The bb.454contignet software32 was used to visualize the contig connections and join the contigs. Contig connections were also checked against raw reads. The total assembled genome has a size of 565,432 nt and was assembled into a linear structure. An alternative assembly would break the genome into two submolecules, one circular and the other linear. Average sequence coverage of the mitochondrial genome was 18×. The genome sequence is deposited in GenBank under accession number KJ129610.
Coding regions were identified by BLASTX searches performed against a local database including nucleotide sequences for all coding genes from 20 plant mitochondrial genomes available in GenBank. The exact gene and exon boundaries were determined by alignment of homologous genes from available annotated plant mitochondrial genes. rRNA and tRNA genes were identified by BLASTN searches against a local database including all rRNA genes and a database including all tRNA genes from 20 available land plant mitochondrial genomes. tRNAs were annotated using tRNAscan-SE33,34.
A total of 24 and 40 million sequences of length 101 nt were obtained from V. crassulae and V. minimum, respectively. Average coverage of mitochondrial sequences was ca. 35× for both samples. Using the complete mitochondrial genome of V. album as a reference, the sequences from V. crassulae and V. minimum were mapped in Geneious ver. 6 using the Map to Reference function with Medium-Low sensitivity and 5 times iteration. Sequences mapping to identified, complete or partial genes were extracted, reassembled in individual loci and extended again with the Map to Reference function. A data base of genes from other completely assembled mitochondrial genomes was used as reference sequences to search for genes not present in V. album mitochondrial loci. Identified complete and partial genes and flanking sequence are deposited in GenBank under accession numbers KJ146758-KJ146774 (V. crassulae) and KJ146758-KJ146774 (V. minimum) (see Extended Data Table 1 for further details).
Phylogenetic analysis
Sequences from 10 protein coding genes (atp1, atp6, atp9, ccmB, ccmC, cob, cox1, cox2, cox3, matR) present in full length in at least one species of Viscum were used for a phylogenetic analysis including sequences from 28 other seed plants for which complete mitochondrial genomes are available in GenBank. Alignments were constructed manually using Mesquite ver. 2.7535 taking codon and amino acid sequence conservation into account. Due to alignment ambiguity two genes present in all or just one species of Viscum (ccmFn and rps12) were not included in the phylogenetic analyses. For the same reason we did not attempt to use the ribosomal RNA gene sequences. Maximum likelihood (ML) analyses of individual genes were done at the Cyperinfrastructure for Phylogenetic Analysis (CIPRES; www.phylo.org) running RAxML-HPC Blackbox ver. 8.0.036. Default options were used, except that the GTR plus GAMMA + I model was applied to each gene when run individually. A combined ML analysis of all genes was performed as above except that the input file was treated both as unpartitioned and as divided into 10 partitions with very similar results. The ML trees were edited using the program FigTree ver. 1.4.0 (tree.bio.ed.ac.uk/software/figtree).
Additional Information
Accession codes: The complete mitochondrial genome sequence of V. album is deposited at GenBank under accession number KJ129610 and sequences of genes and named gene fragments from V. crassulae and V. minimum under accession numbers KJ146758-KJ146774 and KJ146758-KJ146774, respectively. http://www.nature.com/srep
How to cite this article: Petersen, G. et al. Massive gene loss in mistletoe (Viscum, Viscaceae) mitochondria. Sci. Rep. 5, 17588; doi: 10.1038/srep17588 (2015).
Change history
14 January 2016
The version of this paper was updated shortly after publication, following a technical error that resulted in the link to the Supplementary Information file being omitted. This has now been corrected in the PDF and HTML versions of the paper.
References
van der Giezen, M. & Tovar, J. Degenerate mitochondria. EMBO rep. 6, 525–530 (2005).
Logan, D. C. Plant mitochondrial dynamics. Biochim. Biophys. Acta., Mol. Cell Res. 1763, 430–441 (2006).
Sloan, D. B. et al. Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLOS 10, e1001241 (2012).
Adams, K. L., Qiu, Y. L., Stoutemyer, M. & Palmer, J. D. Punctuated evolution of mitochondrial gene content: high and variable rates of mitochondrial gene loss and transfer to the nucleus during angiosperm evolution. Proc. Natl Acad. Sci. USA 99, 9905–9912 (2002).
Adams, K. L. & Palmer, J. D. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet. Evol. 29, 380–395 (2003).
Keeling, P. J. & Palmer, J. D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9, 605–618 (2008).
Bergthorsson, U., Adams, K. L., Thomason, B. & Palmer, J. D. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 424, 197–201 (2003).
Mower, J. et al. Horizontal acquisition of multiple mitochondrial genes from a parasitic plant followed by gene conversion with host mitochondrial genes. BMC Biology 8, 150 (2010).
Marienfeld, J. R., Unseld, M. & Brennicke, A. The Mitochondrial genome of Arabidopsis is composed of both native and immigrant infromation. Trends Plant Sci. 4, 495–502 (1999).
Sloan, D., Alverson, A., Storchova, H., Palmer, J. & Taylor, D. R. Extensive loss of translational genes in the structurally dynamic mitochondrial genome of the angiosperm Silene latifolia. BMC Evol. Biol. 10, 274 (2010).
Zhenxiang, X. et al. Massive mitochondrial gene transfer in a parasitic flowering plant clade. PLoS Genetics 9, 1–10 (2013).
Molina, J. et al. Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesia lagascae (Rafflesiaceae). Mol. Biol. Evol. 31, 793–803 (2014).
Krause, K. Plastid Genomes of Parasitic Plants: A Trail of reductions and losses. In Organelle Genetics (ed. Bullerwell, C. E. ) 79–103 (Springer Berlin Heidelberg, 2012).
Welchen, E., Klodmann, J. & Braun, H.-P. Biogenesis and supramolecular organization of oxidative phosphorylation system in plants. In Plant Mitochondria (ed. Kempken, F. ) (Springer Science, New York, Dordrecht, Heidlberg, London, 2011).
Remacle, C., Barbieri, M. R., Cardol, P. & Hamel, P. P. Eukaryotic complex I: functional diversity and experimental systems to unravel the assembly process. Mol. Genet. Genomics 280, 93–110 (2008).
Cardol, P. Mitochondrial NADH: Ubiquinone oxidoreductase (complex I) in eukaryotes: A highly conserved subunit. BNA-Bioenergetics 1807, 1390–1397 (2011).
Giegé, P. Mitochondrial respiratory complex biogenesis: communicatoin, gene expression and assembly. In Plant Mitochodria (ed. Logsdon, J.M., Jr. ) 141–170 (Blackwell, Oxford, 2007).
Huang, S. & Millar, A. H. Succinate dehydrogenase: the complex roles of a simple enzyme. Curr. Opin. Plant Biol. 16, 344–349 (2013).
Heinemeyer, J., Dudkina, N. V., Boekema, E. J. & Braun, H.-P. Supramolecular structure of the oxidative phosphorylation system in plants. In Plant Mitochondria (ed. Logan, D. C. ) 171–184 (Blackwell Publishing, Oxford, 2007).
Daley, D. O. et al. Gene transfer from mitochondrion to nucleus: novel mechanisms for gene activation from Cox2. Plant Journ. 30, 11–21 (2002).
Adams, K. L. et al. Intracellular gene transfer in action: Dual transcription and multiple silencings of nuclear and mitochondrial cox2 genes in legumes. Proc. Natl Acad. Sci. USA 96, 13863–13868 (1999).
Wittig, I. & Schägger, H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. BBA-Bioenergetics 1787, 672–680 (2009).
Gagliardi, D. & Binder, S. Expression of the plant mitochondrial genome. In Plant Mitochondria (ed. Logan, D. C. ) 50–96 (Blackwell Publishing, Oxford, 2007).
Zonneveld, K. New record holders for maximum genome size in eudicots and monocots. J. Bot. 2010, ID 527357 (2010).
Hjort, K., Goldberg, A. V., Tsaousis, A. D., Hirt, R. P. & Embley, T. M. Diversity and reductive evolution of mitochondria among microbial eukaryotes. Phil. Trans. R. Soc. of Lond. B: Biol. Sci. 365, 713–727 (2010).
Heide-Jørgensen, H. S. Parasitic flowering plants (Koninklijke Brill NV, Leiden, 2008).
Rasmusson, A. G. & Møller, I. M. Mitochondrial electron transport and plant stress. In Plant Mitochondria (ed. Kempken, F. ) 357–381 (Springer Science, New York, Dordrecht, Heidelberg, London, 2011).
Sweetlove, L. J., Beard, K. F. M., Nunes-Nesi, A., Fernie, A. R. & Radcliffe, R. G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 15, 462–470 (2010).
Petersen, G., Cuenca, A. & Seberg, O. Plastome evolution in hemiparasitic mistletoes. Genome Biol. Evol. 7, 2520–2532 (2015).
Triboush, S. O., Danilenko, N. G. & Davydenko, O. G. A method for isolation of chloroplast DNA and mitochondrial DNA from sunflower. Plant Mol. Biol. Rep. 16, 183 (1998).
Doyle, J. J. & Doyle, J. F. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Iorizzo, M. et al. De novo assembly of the carrot mitochondrial genome using next generation sequencing of whole genomic DNA provides first evidence of DNA transfer into an angiosperm plastid genome. BMC Evol. Biol. 12, 61 (2012).
Lowe, T. M. & Eddy, S. R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25, 0955–0964 (1997).
Schattner, P., Brooks, A. N. & Lowe, T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, W686–W689 (2005).
Maddison, W. P. & Maddison, D. R. Mesquite: a modular system for evolutionary analysis. Version 2.75. 2014.
Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (2006).
Acknowledgements
We wish to thank Charlotte Hansen for assistance with DNA extraction and staff at the National High-throughput DNA Sequencing Centre, University of Copenhagen, for library construction and next-generation sequencing. We thank John Mundy for comments on the manuscript. Funding was provided by the Danish Council for Independent Research | Natural Sciences grant no. 12-126713.
Author information
Authors and Affiliations
Contributions
G.P. and O.S. conceived the study, A.C. and G.P. generated the data and all authors analysed the data. G.P. and O.S. wrote the manuscript with contributions from A.C. and I.M.M. All authors read and approved the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Petersen, G., Cuenca, A., Møller, I. et al. Massive gene loss in mistletoe (Viscum, Viscaceae) mitochondria. Sci Rep 5, 17588 (2015). https://doi.org/10.1038/srep17588
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep17588
This article is cited by
-
Factors contributing to mitogenome size variation and a recurrent intracellular DNA transfer in Melastoma
BMC Genomics (2023)
-
Both Conifer II and Gnetales are characterized by a high frequency of ancient mitochondrial gene transfer to the nuclear genome
BMC Biology (2021)
-
Mitochondrial genomes of two parasitic Cuscuta species lack clear evidence of horizontal gene transfer and retain unusually fragmented ccmFC genes
BMC Genomics (2021)
-
Multichromosomal structure and foreign tracts in the Ombrophytum subterraneum (Balanophoraceae) mitochondrial genome
Plant Molecular Biology (2020)
-
Mitochondrial genomes of the early land plant lineage liverworts (Marchantiophyta): conserved genome structure, and ongoing low frequency recombination
BMC Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.