Abstract
Capsular outcomes of anterior/posterior capsulorhexis opening (ACO/PCO) are essential for performing a secondary in-the-bag intraocular lens implantation. To compare the capsular outcomes with different primary capsulorhexis sizes, Thirty-eight eligible patients (45 eyes) were randomly assigned to three groups by anterior capsulorhexis diameter (Group A: 3.0–3.9, Group B: 4.0–5.0 and Group C: 5.1–6.0 mm). The areas of ACO/PCO and posterior capsule opening opacity (PCOO) as primary outcomes, while, the incidence of visual axis opacity (VAO) as secondary outcome were measured at follow-up visits. Among the thirty eyes included in the final analysis, the mean area of the ACO decreased significantly, whereas the PCO enlarged with time. Group A had the highest anterior capsule constriction and percentage reduction, which increased with time. There were significant differences in the percentage reductions at 6 months and 1 year compared to 1 month in Group A and B. Group C had the highest posterior capsule enlargement. The percentage of PCOO to PCO area and the incidence of VAO was highest in Group A and lowest in Group C. Thus, Capsulorhexis diameter of 4.0–5.0 mm may yield better capsular outcomes, considering moderate contraction of ACO, moderate enlargement of PCO and lower percentage of PCOO and VAO.
Similar content being viewed by others
Introduction
Pediatric cataract is one of the common cause of childhood blindness and are responsible for approximately 10–20% of blindness in children worldwide1. Despite the rapid developments in recent years of techniques for pediatric cataract surgery2,3, many children experience limited vision improvement after intervention, due to various capsular complications. Visual axis opacity (VAO), anterior and posterior capsule fibrosis and capsule contraction are common capsular complications that result from the high proliferative capacity of the lens epithelium and severe postoperative inflammation. To reduce the high incidence of capsular complications and refractive uncertainties of early intraocular lens (IOL) placement techniques, many surgeons choose to leave infantile cataract patients aphakic before mature and stable development of the eye ball4,5,6.
It is well known that the pediatric eye is more reactive after cataract surgery7. An increasing number of pediatric ophthalmologists have recognized that the ideal site for IOL implantation is in the capsular bag, because in-the-bag implantation sequesters the IOL from the highly reactive uveal tissue and maintains better IOL centration8,9,10. In our previous study, we found that, prior to performing a secondary in-the-bag IOL implantation, an ideal capsular outcome for the anterior/posterior capsulorhexis opening (ACO/PCO) should be achieved after the primary surgery11. Although many studies of the impact of capsulorhexis diameter, location and shape on the development of posterior capsule opacification after age-related cataract surgeries have been conducted12,13,14, there are no reports investigating the relationship between primary capsulorhexis size and capsular outcome after pediatric cataract surgery. Therefore, the aim of the current study was to prospectively evaluate the capsular outcomes of three controlled groups of patients with different anterior capsulorhexis diameters (3.0–3.9, 4.0–5.0 and 5.1–6.0 mm).
Results
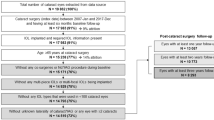
In total, 8 patients dropped out after inclusion. Three patients (4 eyes) who were unable to complete the designed follow-up, 4 patients (6 eyes) whose retroillumination images could not be analyzed due to the small size of their pupils or iris synechiae and 1 patient (1 eye) with a high intraocular pressure after surgery were excluded from the analysis. Additionally, 2 eyes in Group A, 1 eye in Group B and 1 eye in Group C received Nd: YAG laser capsulotomy due to serious VAO during the follow-up (Fig. 1). Therefore, 26 patients (30 eyes) were available for the final analysis. There were 7 eyes in Group A, 10 eyes in Group B and 13 eyes in Group C. The mean patient age was 6 ± 2.76 months (range, 3–16 months) and was comparable in the three groups. The follow-up time was 18 ± 2.49 months (range, 13–24 months) and was comparable in the three groups.
Mean ACO and PCO Areas
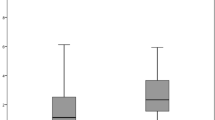
The mean areas of the ACO in Group A were 12.16 ± 0.71, 10.09 ± 0.43 (p = 0.009 compared with baseline), 9.08 ± 0.53 (p = 0.005) and 9.00 ± 0.85 (p = 0.001) mm2 at baseline and postoperative 1 month, 6 months and 1 year, respectively. The ACOs of Group B were 15.22 ± 1.18, 13.43 ± 0.97 (p = 0.007), 12.79 ± 1.00 (p = 0.003) and 12.41 ± 1.09 (p = 0.002) mm2, respectively; those of Group C were 22.92 ± 1.45, 20.86 ± 0.87 (p = 0.028), 20.49 ± 1.04 (p = 0.014) and 19.88 ± 0.81 (p = 0.020) mm2, respectively. For all three groups, the mean area of the ACO decreased significantly postoperatively (all p < 0.05, as above) (Fig. 2).
Mean area of the ACO and PCO at baseline and at the postoperative visits.
The area of the ACO decreased and the area of the PCO increased postoperatively. There was a significant difference in the area of the ACO between the baseline and the postoperative visits (*p < 0.05). However, for the area of the PCO, the only significant differences occurred in Groups B and C between the baseline and at 6 and 12 months postoperatively (#p < 0.05). Notes: ACO = anterior capsular opening; PCO = posterior capsular opening; mo = month.*p < 0.05, #p < 0.05.
The mean areas of the PCO in Group A were 7.55 ± 0.49, 7.64 ± 0.78 (p = 0.790), 8.00 ± 0.66 (p = 0.185) and 8.22 ± 0.74 (p = 0.101) mm2 at baseline and postoperative 1 month, 6 months and 1 year, respectively. The PCOs of Group B were 7.26 ± 1.21, 7.75 ± 1.35 (p = 0.078), 8.29 ± 1.21 (p = 0.045) and 8.53 ± 1.25 (p = 0.033) mm2, respectively and those of Group C were 10.37 ± 2.91, 11.29 ± 2.49 (p = 0.086), 12.07 ± 2.86 (p = 0.001) and 12.46 ± 2.95 (p = 0.005) mm2, respectively. For all three groups, the mean area of the PCO increased postoperatively (Fig. 2).
Area Change in ACO and PCO
The percentage decreases of the ACO in Group A at 1 and 6 months and 1 year after surgery were 16.97 ± 1.93, 25.26 ± 2.19 and 26.04 ± 2.85%, respectively; in Group B, the respective values were 12.92 ± 1.73, 15.95 ± 0.60 and 17.74 ± 1.09%; and in Group C, the respective values were 8.88 ± 2.17, 10.55 ± 1.66 and 13.18 ± 2.60%. Group A had the highest capsule constriction and there was a significant difference among the three groups at the various time points (p = 0.007, 0.000 and 0.001, respectively). The percentage reduction in the ACO area increased with time and the differences between 6 months, 1 year and 1 month were significant in Group A (p = 0.008 and 0.010, respectively) and Group B (p = 0.045 and 0.015, respectively). The differences among the various visits in Group C were not significant (p = 0.351 and 0.093, respectively) (Fig. 3).
The percentage increases in the PCO in Group A at 1 and 6 months and 1 year after surgery were 1.12 ± 6.63, 5.93 ± 5.13 and 8.80 ± 4.98, respectively; the respective values in Group B were 6.67 ± 3.35, 14.52 ± 5.95 and 17.84 ± 6.24; and the respective values in Group C were 11.94 ± 5.44, 17.59 ± 6.26 and 21.45 ± 6.73. Group C had the highest posterior capsule enlargement compared with the other two groups, although the differences did not reach statistical significance (p = 0.119, 0.110 and 0.098, respectively). Furthermore, there was no significant difference among the various postoperative visits in any of the groups (Fig. 3).
Overall, Group A had the highest capsule constriction and percentage reduction, which increased with time. There was a significant difference in the percentage reduction between 6 months, 1 year and 1 month in Group A and Group B (*p < 0.05). Group C had the highest posterior capsule enlargement, although the difference did not reach statistical significance.
Ratio of Opacity Accounting for PCO
The percentage of PCOO was highest in Group A compared with Groups B and C (p = 0.001 and 0.001, respectively). There was a significant difference between 6 months and 1 year after surgery in Groups A, B and C (p = 0.005, 0.043 and 0.002, respectively; Fig. 4). In total, 2 eyes in Group A, 1 eye in Group B and 1 eye in Group C underwent Nd: YAG laser capsulotomy due to serious VAOs from 1 month to 16 months. The incidences of serious VAOs were 22.25% (2/9), 9.1% (1/11) and 7.1% (1/14) in Groups A, B and C, respectively. Group A had the highest rate of serious VAOs, although there was no significant difference among the three groups (p = 0.519).
Proportion of opacity accounting for the PCO at 6 months and 1 year after surgery.
Notes: PCO = posterior capsular opening; mo = month.*p < 0.05. The percentage of participants with PCOO was the highest in Group A, compared with the two other groups. There was a significant difference between 6 months and 1 year after surgery in all of the groups (*p < 0.05).
Discussion
This randomized controlled trial (RCT) investigated the capsular outcomes of pediatric cataract surgery performed with different anterior capsulorhexis sizes. We found that a diameter of 4.0–5.0 mm yielded optimal capsular outcomes, based on its relatively moderate contraction of ACO, moderate enlargement of PCO and lower percentage of PCOO and VAO. To the best of our knowledge, this is the first RCT to investigate the relationship between the primary capsulorhexis size and capsular outcomes after pediatric cataract surgery.
Currently, primary anterior and posterior capsulectomy and anterior vitrectomy are the main surgical interventions for pediatric cataracts, particularly among young children15. The decision regarding whether to implant an IOL at the time of cataract surgery in infants, or leave the child aphakic with the secondary IOL implantation later in childhood, is controversial. However, the study of the Infant Aphakia Treatment Study (IATS) showed that for infants, cataract surgery without IOL implantation is an optimal choice, as it decreases the incidence of VAO and the need for second interventions, as well as decreasing costs4,5. Furthermore, ideal capsular outcomes are attained and secondary in-the-bag IOL implantation can retain the normal anatomic position of the IOL while reducing uveal irritation and chronic inflammation8,9. For age-related cataracts, many studies have clarified that the optimal anterior capsulorhexis diameter is slightly smaller than the diameter of the simultaneously implanted IOL optic surface, with 0.5–1.0 mm capsulorhexis edges covering the IOL optic surface16. However, the optimal size of the anterior capsulorhexis that should be used to obtain superior capsular outcomes for secondary IOL implantation in primary pediatric cataract surgery has not yet been reported.
Several devices have been reported to control the diameter and shape of capsulorhexis, such as capsulotomy diameter marks, ring-shaped calipers, or the data-injection system17,18,19,20. Some of these devices require specific microscopes and a sophisticated dedicated instrument. In this study, we designed a needle with 1-mm scale laser markers as a calibration tool to verify the diameter of the capsulorhexis. Our needles are made from viscoelastic needles that have been processed with lasers and that can be autoclaved for repeated usage. During the surgery, we used the needle before and after the capsulorhexis to confirm the capsulorhexis size. Compared with the other devices, this self-made needle calibration system is inexpensive, convenient and effective. Moreover, an available method for objective quantification of capsular outcomes during follow-up is another important issue. Therefore, we also developed the imaging software ECO, a custom image-analysis algorithm using MATLAB (The MathWorks, Inc., Natick, MA, USA) to quantitatively measure capsular outcomes, which was determined to be user-friendly in our previous study and the current study. As is known that the pediatric capsule is highly elastic, making it difficult to perform capsulorhexis with a bent needle or forceps. Therefore we used radiofrequency diathermy to open the anterior and posterior capsule, obtaining a success rate greater than 95%. This is a simple, effective and excellently safe procedure. Burkhard Dick et al.21 recently reported the safety of femtosecond laser-assisted capsulotomy in pediatric cataract surgery. However, it is not to date sufficiently inexpensive for clinical use in developing countries. Many factors can affect the constriction of the ACO after cataract surgery22. In our study, Group A (3.0–3.9 mm anterior capsulorhexis in diameter) had the most pronounced ACO constriction and there were significant differences among the three groups at their postoperative visits. The constriction process of the ACO began postoperatively at 1 month and continued until 1 year. In general, Davison suggested that the capsule contraction was caused by an imbalance between the centrifugal and centripetal forces acting on the capsular bag23. However, the associations between the initial ACO size and the anterior capsule constriction remain unclear. Joo et al.24 reported that the initial ACO size might play a role in capsule contraction. However, Cochener et al.25 found insignificant relationships between ACO size and postoperative constriction, partly due to the small variation in their observed ACO sizes. These conclusions were drawn only from studies of age-related cataracts.
As opposed to the ACO, for the initial PCO, a 3.0-mm diameter capsulorhexis was well accepted and had a tendency to widen. Michael et al.26 demonstrated that the PCO was enlarged in a group undergoing silicone IOL implantation. We also noted the PCO enlargement after pediatric cataract surgery, even without IOL implantation in this pediatric study population. The PCO change in Group A was relatively minimal, whereas Group C had the highest posterior capsule enlargement, although the difference did not attain statistical significance. The reason for this finding may lie in the reduced PCO centrifugal force due to the serious constriction and fibrosis of the anterior capsule and the fusion of the anterior and posterior capsules, which reflect the dynamics of the capsular bag. The percentage of PCOO and the incidence of a second intervention due to VAO was highest in Group A compared with the other two groups. This finding may be due to the higher rate of residual lens epithelial cells (LECs) in the group with the small capsulorhexis opening. Group A had the highest rate of VAO, although no significant difference was found among the three groups due to the small number of patients enrolled in our study.
The results and interpretation of the current study must be understood within the context of its strengths and limitations. The strengths of the study include its randomized controlled design, the performance of the surgeries by a single experienced pediatric cataract surgeon using radiofrequency diathermy with a self-made needle calibration that ensured accuracy of the three controlled groups at baseline and the investigation’s adherence to a unified and strict postoperative regimen and follow-up protocol within the same study group (CCPMOH). The weaknesses of the study must also be acknowledged. The sample size of the study groups was relatively small and the results were most likely slightly biased by the exclusion of patients with serious iris synechiae and patients whose pupils could not dilate after surgery. This study only evaluated aphakic rather than pseudophakic eyes and our findings could not be applied to pseudophakic eyes. However, the aim of this paper is to investigate the relationship between primary capsulorhexis size and capsular outcome of aphakic patients and eventually provide the theoretical basis to the successful in-the-bag IOL implantation at the second surgery. We employed a self-made calibrated needle in the chamber to measure the size of the capsule openings during surgery, but these measurements were made externally and postoperatively. Because of corneal magnification, immediate postoperative readings would be larger than intraoperative readings, yielding another potential bias. Despite these limitations, this study remains one of the first to investigate the relationship between the primary capsulorhexis size and capsular outcomes after pediatric cataract surgery.
There are important potential implications for our present findings. Our research has demonstrated that an ideal capsulorhexis size for pediatric cataracts not only would decrease the rates of capsular complications, including capsule contraction and VAO, but also would increase the probability of successful in-the-bag IOL implantation at the second surgery. We are currently following the pediatric patients from our study to assess their rates of accurate in-the-bag IOL implantation.
Methods
Patients
Forty-one congenital cataract patients <2 years old were recruited from Zhongshan Ophthalmic Center (ZOC) between March 2011 and November 2012 as registered members of the Childhood Cataract Program of the Chinese Ministry of Health (CCPMOH)27. The research protocol was approved by the Institutional Review Board/Ethics Committee of the Sun Yat-sen University. For more details, refer to Protocol S1 and Checklist S1 in the supplementary information. Informed written consent was obtained from at least one parent of each participating child and the tenets of the Declaration of Helsinki were followed throughout this study. To make the Evaluation of Capsular Outcomes (ECO) software used in this study confidential, this study was registered with the Clinical Research Internal Management System of ZOC before participant enrollment began. Consequently, the study was registered after the enrollment of participants started, with Clinical-Trials.gov, (https://www.clinicaltrials.gov, registration number: NCT02158325, First received date: June 4, 2014). The authors confirm that all ongoing and related trials for this study are registered. Patients with glaucoma, ocular trauma, corneal disorders, persistent hyperplastic primary vitreous, rubella, Lowe syndrome, capsular fibrosis, or surgical complications, as well as those whose pupils could not dilate normally postoperation or who could not complete the follow-up, were excluded. Three patients were excluded and thirty-eight patients (45 eyes) were included in this study (Fig. 1).
Randomization Grouping
Preoperatively, all patients were randomly divided into 3 groups (Group A had 13 patients and 15 eyes, Group B had 13 patients and 15 eyes and Group C had 12 patients and 15 eyes) using a random number chart and underwent cataract surgery without IOL implantation. The patients in Group A received a small anterior capsulorhexis that was 3.0 mm to 3.9 mm in diameter, Group B received a medium capsulorhexis that was 4.0 mm to 5.0 mm in diameter and group C received a large capsulorhexis that was 5.1 to 6.0 mm in diameter.
Surgical Technique
All patients underwent cataract surgery performed by a single experienced pediatric cataract surgeon (YZL) using a technique that has been previously described28 and is summarized briefly below. After a conjunctival peritomy was performed, a superior scleral tunnel incision was created using a 3.2-mm keratome. An anterior central curvilinear capsulotomy was made using radiofrequency diathermy. The anterior capsulorhexis size differed among the three groups. Before and after capsulorhexis, a self-made calibrated needle with a laser marker (1-mm scale) (Xinkeling, Inc., Shanghai, China) was used to measure and confirm the capsulorhexis diameters (Fig. 5). The nucleus and cortex were removed using the irrigation/aspiration device of the Infiniti Vision System (Alcon Laboratories, Fort Worth, TX, USA). A posterior central curvilinear capsulotomy incision that was 3.0-mm in diameter was made, also using radiofrequency diathermy. Then, a limited anterior vitrectomy was performed using the Infiniti Vision System vitrectomy instrument. The tunnel incision was sutured with 10–0 nylon sutures. All patients received subconjunctival dexamethasone (2 mg) during surgery and all surgeries were performed under general anesthesia.
The self-made needle with laser marker (1-mm scale) used to measure and confirm the capsulorhexis diameter during surgery.
(a) The eight laser markers can clearly be seen on the needle. (b) The entire needle with laser marker is shown. (c) A 5.0-mm capsulorhexis was completed and confirmed with the laser-marker needle. (d) A 6.0-mm capsulorhexis was completed and confirmed with the laser-marker needle. Notes: D = Diameter.
Postoperative Regimen and Follow-up Protocol
Postoperatively, Tobradex eye drops (tobramycin 0.3%, dexamethasone 0.1%, Alcon) were used 6 times per day and Tobradex eye ointment (tobramycin 0.3%, dexamethasone 0.1%, Alcon) was applied once per night for 2 weeks. From 2 weeks to 1 month postoperatively, the eye drops were used 4 times per day. For the second postoperative month, the patient switched to pranoprofen eye drops 4 times per day (Senju Pharmaceutical Co., Ltd., Osaka, JP). This follow-up protocol was also used in our previous study. All patients were examined at 1 week, 1 month, 3 months, 6 months, 1 year, 18 months and two years after surgery. Patients were followed for two years or until the development of severe VAO requiring Nd: YAG laser capsulotomy, whichever came first. The examinations consisted of visual acuity, IOP measurement by a Tonopen tonometer (Reichert, Inc., Seefeld, Germany), fundoscopy, an assessment by high-resolution digital retroillumination imaging (detailed protocol presented below).
Slit-Lamp-Adapted Anterior-Segmental Photography
All of the postoperative pediatric patients underwent pupil dilation with compound tropicamide eye drops (tropicamide 0.3%, phemylephrine hydrochloride 0.3%, Zhongshan Ophthalmic Center, Guangzhou, China) 3–5 times (once every 10 minutes) until the pupil size was larger than 6 mm. Then, the children underwent slit-lamp-adapted anterior-segmental photography (BX900, Haag-Streit AG, Köniz, Switzerland) for each operated eye, including one digital coaxial retroillumination photograph (DCRP), one diffuse light photo and one slit-light photo across the central visual axis. For uncooperative children, a set of assistance procedures for slit-lamp-adapted photography was developed to instruct the parents to help prepare the child’s head position and to help the child open his or her eyes as described in our previous studies11,28. In brief, we developed a transformable bed for assisting pediatric ophthalmic examination (US Patent No.US 9,015,882 B2). We performed examinations after administering a sedative drug (i.e., chloral hydrate as a sleep aid) or under general anesthesia (if chloral hydrate was contraindicated or unacceptable) for children who were uncooperative to allow for regular examinations in the clinic.
Division and Definition of ACO, PCO, PCOO and VAO
The postoperative capsular DCRPs were divided and defined as follows: area of the ACO, area of the PCO and area of the posterior capsule opening opacity (PCOO) (Fig. 6). The ACO and PCO are self-explanatory. PCOO indicates lens epithelium proliferation within the posterior capsule opening area causing prominent opacity. VAO is defined as opacity of the anterior and/or posterior capsule or lens epithelium proliferation within a 3-mm pupil size, which completely occludes light transmission across the pupil. If the fundus cannot be clearly viewed through the visual axis, the incidence of VAO is identified and Nd: YAG laser is prescribed to eliminate the opacity of visual axis.
Division and definition of different capsule areas on standardized DCRPs.
(a) Contour of different capsule areas in the retroillumination image, including the ACO (blue area), PCO (red area) and PCOO (black area). (b) The anterior capsule opening (ACO) area, (c) Posterior capsule opening (PCO) area, (d) Posterior capsule opening opacity (PCOO) area were traced by the ECO software. (Notes: DCRPs = digital coaxial retroillumination photographs; ACO = anterior capsular opening; PCO = posterior capsular opening; PCOO = posterior capsular opening opacification).
Measurement of Outcomes
The primary outcomes were the areas of the ACO and PCO at different postoperative follow-up visits. The secondary outcomes were the percent reduction or increase in the ACO, PCO and PCOO at different postoperative follow-up visits and the total incidence of VAO. We also assessed surgical complications, such as capsular tears, inflammation, corneal edema, glaucoma, iris synechiae and postoperative macular edema. The surgery was video recorded and pictures of the completed ACO and PCO were taken intraoperatively. Both the intraoperative and postoperative pictures were standardized and documented on an analysis computer using our ECO software, a custom image analysis algorithm developed by our team using MATLAB (The MathWorks, Inc., Natick, MA, USA). The contours of the ACO, PCO and PCOO were traced by hand using the ECO image processing software. Next, the capsule opening area, capsulorhexis diameter and opacity area were measured by computer. Two researchers independently used the software to quantify the values of the ACO, PCO and PCOO and the results are the mean values determined by the two researchers. The mean values of the ACO, PCO and PCOO of each capsular type were calculated. The mean areas of the PCOO were analyzed at 6 months and 1 year. The percentage of PCOO was calculated as follows: PCOO area × 100/PCO area at the same time. If the patient underwent the Nd: YAG laser capsulotomy or a secondary surgical intervention due to serious VAO, the percentage of PCOO was recorded as 100%.
Data Analysis and Statistics
The SPSS software package (version 17.0, SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. The sample size calculation was based on power analysis. Power analysis adopts a hypothesis-testing method to determine the sample size according to several parameters, which include the pre-specified significance level, desired power level and expected effect size. Assuming a two-tailed alpha of 0.05, a probability of 0.2 for beta error (80% power) and an effect size of 0.28 after calculating with respect to the same primary outcome measure (area of the ACO) from the result of our preliminary research, 10 participants per group were required. A paired T test was used to compare the areas of the ACO and PCO between the postoperative visits and baseline. ANOVA was used for changes in the ACO and PCO areas among the three groups at different postoperative visits. Due to the non-normal distribution, Kruskal-Wallis nonparametric tests were used to compare the area percentage of PCOO among the three groups and the Wilcoxon signed rank test was used to compare the difference between the postoperative visits. All statistical tests were two-tailed, with α = 0.05. A p value of <0.05 was considered statistically significant.
Additional Information
How to cite this article: Lin, H. et al. Capsular Outcomes Differ with Capsulorhexis Sizes after Pediatric Cataract Surgery: A Randomized Controlled Trial. Sci. Rep. 5, 16227; doi: 10.1038/srep16227 (2015).
References
Johar, S. R., Savalia, N. K., Vasavada, A. R. & Gupta, P. D. Epidemiology based etiological study of pediatric cataract in western India. Indian J Med Sci 58, 115–121 (2004).
Lin, A. A. & Buckley, E. G. Update on pediatric cataract surgery and intraocular lens implantation. Curr Opin Ophthalmol 21, 55–59, 10.1097/ICU.0b013e32833383cb (2010).
Nihalani, B. R. & VanderVeen, D. K. Technological advances in pediatric cataract surgery. Semin Ophthalmol 25, 271–274, 10.3109/08820538.2010.518836 (2010).
Plager, D. A., Lynn, M. J., Buckley, E. G., Wilson, M. E. & Lambert, S. R. Complications, adverse events and additional intraocular surgery 1 year after cataract surgery in the infant Aphakia Treatment Study. Ophthalmology 118, 2330–2334, 10.1016/j.ophtha.2011.06.017 (2011).
Carrigan, A. K., DuBois, L. G., Becker, E. R. & Lambert, S. R. Cost of intraocular lens versus contact lens treatment after unilateral congenital cataract surgery: retrospective analysis at age 1 year. Ophthalmology 120, 14–19, 10.1016/j.ophtha.2012.07.049 (2013).
Lambert, S. R. et al. A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Archives of ophthalmology 128, 810–818, 10.1001/archophthalmol.2010.101 (2010).
Kleinmann, G. et al. Postoperative surface deposits on intraocular lenses in children. J Cataract Refract Surg 32, 1932–1937, 10.1016/j.jcrs.2006.06.035 (2006).
Mura, J. J. et al. Ultrasound biomicroscopic analysis of iris-sutured foldable posterior chamber intraocular lenses. Am J Ophthalmol 149, 245–252 e242, 10.1016/j.ajo.2009.08.022 (2010).
Vasavada, A. R., Raj, S. M., Karve, S., Vasavada, V. & Theoulakis, P. Retrospective ultrasound biomicroscopic analysis of single-piece sulcus-fixated acrylic intraocular lenses. J Cataract Refract Surg 36, 771–777, 10.1016/j.jcrs.2009.11.027 (2010).
Apple, D. J. et al. A comparison of ciliary sulcus and capsular bag fixation of posterior chamber intraocular lenses. Journal - American Intra-Ocular Implant Society 11, 44–63 (1985).
Luo, L. et al. In-the-bag intraocular lens placement via secondary capsulorhexis with radiofrequency diathermy in pediatric aphakic eyes. PLoS One 8, e62381, 10.1371/journal.pone.0062381 (2013).
Langwinska-Wosko, E., Broniek-Kowalik, K. & Szulborski, K. The impact of capsulorhexis diameter, localization and shape on posterior capsule opacification. Med Sci Monit 17, CR577–582 (2011).
Aykan, U., Bilge, A. H., Karadayi, K. & Akin, T. The effect of capsulorhexis size on development of posterior capsule opacification: small (4.5 to 5.0 mm) versus large (6.0 to 7.0 mm). Eur J Ophthalmol 13, 541–545 (2003).
Ravalico, G., Tognetto, D., Palomba, M., Busatto, P. & Baccara, F. Capsulorhexis size and posterior capsule opacification. J Cataract Refract Surg 22, 98–103 (1996).
Guo, S., Wagner, R. S. & Caputo, A. Management of the anterior and posterior lens capsules and vitreous in pediatric cataract surgery. J Pediatr Ophthalmol Strabismus 41, 330–337 quiz 356-337 (2004).
Hollick, E. J., Spalton, D. J. & Meacock, W. R. The effect of capsulorhexis size on posterior capsular opacification: one-year results of a randomized prospective trial. Am J Ophthalmol 128, 271–279 (1999).
Wallace, R. B., 3rd . Capsulotomy diameter mark. J Cataract Refract Surg 29, 1866–1868 (2003).
Jasinskas, V., Galdikas, A. & Zemaitiene, R. Novel capsulotomy diameter marking capability. J Cataract Refract Surg 31, 1675, 10.1016/j.jcrs.2005.07.027 (2005).
Tassignon, M. J., Rozema, J. J. & Gobin, L . Ring-shaped caliper for better anterior capsulorhexis sizing and centration. J Cataract Refract Surg 32, 1253–1255, 10.1016/j.jcrs.2006.02.067 (2006).
Dick, H. B., Pena-Aceves, A., Manns, M. & Krummenauer, F. New technology for sizing the continuous curvilinear capsulorhexis: prospective trial. J Cataract Refract Surg 34, 1136–1144, 10.1016/j.jcrs.2008.03.025 (2008).
Dick, H. B., Schelenz, D. & Schultz, T. Femtosecond laser-assisted pediatric cataract surgery: Bochum formula. J Cataract Refract Surg 41, 821–826, 10.1016/j.jcrs.2014.08.032 (2015).
Hayashi, K. & Hayashi, H. Intraocular lens factors that may affect anterior capsule contraction. Ophthalmology 112, 286–292, 10.1016/j.ophtha.2004.11.013 (2005).
Davison, J. A. Capsule contraction syndrome. J Cataract Refract Surg 19, 582–589 (1993).
Joo, C. K., Shin, J. A. & Kim, J. H. Capsular opening contraction after continuous curvilinear capsulorhexis and intraocular lens implantation. J Cataract Refract Surg 22, 585–590 (1996).
Cochener, B., Jacq, P. L. & Colin, J. Capsule contraction after continuous curvilinear capsulorhexis: poly(methyl methacrylate) versus silicone intraocular lenses. J Cataract Refract Surg 25, 1362–1369 (1999).
Georgopoulos, M. et al. Posterior continuous curvilinear capsulorhexis with hydrogel and silicone intraocular lens implantation: development of capsulorhexis size and capsule opacification. J Cataract Refract Surg 27, 825–832 (2001).
Lin, H. et al. Effectiveness of a short message reminder in increasing compliance with pediatric cataract treatment: a randomized trial. Ophthalmology 119, 2463–2470, 10.1016/j.ophtha.2012.06.046 (2012).
Lin, H. et al. Ocular hypertension after pediatric cataract surgery: baseline characteristics and first-year report. PLoS One 8, e69867, 10.1371/journal.pone.0069867 (2013).
Acknowledgements
This clinical study was supported by the Ministry of Science and Technology of China Grants (973 program, 2015CB964600), the Key Projects for Hospital Clinical Disciplines of Ministry of Health of China in 2010–2012 (Project No.175 in Document 439 of the Planning and Finance Secretary of Ministry of Health), the Pearl River Science and Technology New Star (Grant No. 2014J2200060) Project of Guangzhou City, the Guangdong Provincial Natural Science Foundation for Distinguished Young Scholars of China (Grant No. 2014A030306030), Youth Science and Technology Innovation Talents Funds in Special Support Plan for High Level Talents in Guangdong Province (Grant No. 2014TQ01R573), the Cultivation Projects (12ykpy61) and Intensive Cultivation Projects (2015ykzd11) for Young Teaching Staff at Sun Yat-sen University from the Fundamental Research Funds for the Central Universities and Fundamental Research Funds of State Key Laboratory of Ophthalmology (Grant No. 2015QN01). The sponsors of the study played no role in the study protocol design, data collection, data analysis, data interpretation, manuscript preparation, or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
H.T.L., X.H.T., W.R.C. and Y.Z.L. were involved in the study conception, design and funding; H.T.L., X.H.T., J.J.C. and L.X.L. conducted the study; H.T.L., X.H.T., J.J.C. and Z.L.L. collected the data; H.T.L., X.H.T., J.J.C., L.X.L., W.R.C. and Y.Z.L. analyzed the data; H.T.L., X.H.T., J.J.C., L.X.L., Z.L.L., X.H.W., E.P.L., W.R.C. and Y.Z.L. reviewed the manuscript; and H.T.L., X.H.T., J.J.C., L.X.L., Z.L.L., X.H.W., E.P.L., W.R.C. and Y.Z.L. gave final approval of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lin, H., Tan, X., Lin, Z. et al. Capsular Outcomes Differ with Capsulorhexis Sizes after Pediatric Cataract Surgery: A Randomized Controlled Trial. Sci Rep 5, 16227 (2015). https://doi.org/10.1038/srep16227
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16227
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.