Abstract
The CD44 is cellular surface adhesion molecule that is involved in physiological processes such as hematopoiesis, lymphocyte homing and limb development. It plays an important role in a variety of cellular functions including adhesion, migration, invasion and survival. In bone tissue, CD44 is widely expressed in osteoblasts, osteoclasts and osteocytes. However, the mechanisms underlying its role in bone metabolism remain unclear. We found that CD44 expression was upregulated during osteoclastogenesis. CD44 deficiency in vitro significantly inhibited osteoclast activity and function by regulating the NF-κB/NFATc1-mediated pathway. In vivo, CD44 mRNA levels were significantly upregulated in osteoclasts isolated from the hindlimb of tail-suspended mice. CD44 deficiency can reduce osteoclast activity and counteract cortical bone loss in the hindlimb of unloaded mice. These results suggest that therapeutic inhibition of CD44 may protect from unloading induced bone loss by inhibiting osteoclast activity.
Similar content being viewed by others
Introduction
CD44 participates in diverse signaling pathways ranging from growth factor-induced signaling to its ligand mediated pathways. Increasing evidence demonstrates that CD44 acts as a signaling hub controlling cell surface receptors of very diverse structure and function1,2. These receptors, through interactions with their principal ligands, provide bone cells with the ability to sense changes in the extracellular environment3,4,5,6. The macromolecules hyaluronan (HA), osteopontin (OPN), fibronectin and collagen I can bind to CD44 and activate intracellular signaling7,8,9,10. These ligands are important regulators of bone remodeling. OPN knockout (KO) mice are resistant to hindlimb unloading and ovariectomy-induced bone loss11,12. However, the Roles of CD44 in the regulation of bone homeostasis remain unclear.
CD44 plays diverse roles in promoting pre-osteoclast fusion13 and specific CD44 antibody inhibits osteoclast formation14,15. The fusion of macrophages is inhibited by the binding of CD44 ligands OPN and HA16,17,18. CD44 is activated by MMP9, which leads to proteolytic cleavage of CD44 and produces an intracytoplasmic domain called CD44-ICD19,20, which binds to Runx2 and activates the expression of many genes. This domain can also promote the fusion of macrophages13,21. Galectin-9 induces osteoblast differentiation through the CD44/Smad signaling pathway22. Osteoclasts express CD44 and the interplay of CD44 with extracellular matrix proteins such as OPN may regulate osteoclast function8,10,23,24,25,26. However, little is known regarding to the mechanism underlying CD44-mediated osteoclast activity. CD44-deficient mice are viable without obvious developmental defects and show no overt abnormalities27. The changes in bone phenotype of CD44 KO mice under hindlimb-unloading conditions have not been previously reported.
In this study, we found that CD44 expression was clearly up-regulated during M-CSF and RANKL-induced osteoclastogenesis. The activity and function of osteoclasts were significantly reduced in CD44-deficient mice via downregulation of the NF-κB/NFATc1 pathway. In addition, CD44 mRNA levels were specifically upregulated in osteoclasts from hindlimb-unloaded mice and cortical bone loss was ameliorated in CD44 KO mice in this model, via downregulation of osteoclast function rather than by changes in osteoblast function.
Results
CD44 deficiency inhibits osteoclastogenesis
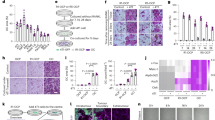
Bone marrow monocytes (BMMs) isolated from bone marrow cells were induced into osteoclasts in the presence of M-CSF (30 ng/mL) and RANKL (50 ng/mL) (Fig. 1A). To investigate the potential role of CD44 in this process, we examined the changes of its mRNA and protein levels during osteoclastogenesis and found that they progressively increased during this process. Specifically, CD44 mRNA levels increased 6-fold on day 3 after induction and reached 17-fold on day 5 compared to day 0 (Fig. 1B). CD44 protein levels were also significantly increased during osteoclastogenesis (Fig. 1C, see Supplementary Fig. S1A online). Immunofluorescence for CD44 showed the same results (Fig. 1D). When comparing the osteoclast differentiation potential of BMMs from wild-type (WT) and CD44-deficient (KO) mice, the expression levels of molecular marker genes for osteoclast function, including Clc7, Trap, CathepsinK and Mmp9, were significantly reduced during osteoclast differentiation (Fig. 1E–H) and the transcription factor NFATc1, which plays a critical role in osteoclast differentiation, was also decreased (Fig. 1I). Western blotting results also revealed a much lower levels of NFATc1 and TRAP5 in osteoclasts from CD44 KO mice than that from WT mice (see Supplementary Fig. S1B online). These results indicate that CD44 plays an important role in the process of osteoclastogenesis.
CD44 deficiency inhibits the osteoclast differentiation of BMMs in vitro.
(A) Schematic presentation of BMMs cultures, BMMs from two-month-old WT and CD44 KO mice were cultured in medium with M-CSF (30 ng/ml) and RANKL (50 ng/ml) for 5 days. (B) The CD44 mRNA level and (C) protein level was determined in the process of osteoclast differentiation of WT BMMs by qPCR. The expression level was normalized to Gapdh. Data are the mean ± SEM. n=3; **p < 0.01, compared to 0 day. (D) Immunofluorescence for CD44 (green) in the process of osteoclastogenesis on day 0, 3 and 5. (E-I) The mRNA levels of Clc7, Trap, CathepsinK, Mmp9 and NFATc1 in WT and CD44 KO BMMs were analyzed by qPCR. The transcripts levels were normalized to Gapdh. All data are the mean ± SEM. n=3, *p < 0.05, **p < 0.01, compared to WT.
Loss of CD44 decreases osteoclast function
To investigate the effect of CD44 deficiency on osteoclast function, we compared the changes of osteoclast fusion and bone resorption ability of osteoclasts with CD44 KO or not. BMMs were cultured in the presence M-CSF (30 ng/mL) and RANKL (50 ng/mL) for 5 days (Fig. 1A), after which the number of TRAP-positive, multinucleated osteoclasts per well were counted. The number of multinucleated osteoclasts was remarkably decreased by nearly 50% in the CD44 KO group (Fig. 2A,B). Mature osteoclasts can absorb bone surface. When we cultured these osteoclasts on bovine bone slice for 2 days, we observed pit formation by toluidine blue staining. Consistent with the result of TRAP staining, the number of pits and the eroded area of bone resorption were significantly decreased in the bovine bone slices cultured with CD44 KO osteoclasts (Fig. 2C,D,E). These results demonstrate that CD44 deficiency inhibits osteoclasts function.
CD44 deficiency inhibits osteoclastogenesis.
(A) BMMs were isolated from WT and CD44 KO mice, cultured in the presence of M-CSF (30 ng/ml) and RANKL (50 ng/ml) for 5 days. TRAP-stained cells show the fusion of BMMs on day 5. (B) The numbers of TRAP+ OCs with three or more nucleis are shown. To quantify the number of TRAP-stained cells in BMMs, at least 3 wells of each experiment per group were captured with a digital camera and counted. The number of experiment per group is 3. *p < 0.05, **p < 0.01, compared to WT. **p < 0.01, compared to WT. (C) Toluidine blue staining shows the eroded area of bone resorption. WT and CD44 KO BMMs were cultured in OC medium for 5 days. Then OCs were cultured on bovine bone slices with OC medium for 2 days. The pits formation (arrows in red) was shown. (D) The numbers and (E) areas of bone resorption pits on bovine bone slices were measured by image analysis. To quantify the number and areas of bone resorption pits in bovine bone slices, 10 fields at 100 × magnification of each slices were counted and at least 3 slices of each group were captured with a digital camera and analyzed using Image Pro 405 Plus 6.2 software (Media Cybernetics Inc. USA). *p < 0.05, **p < 0.01, compared to WT.
CD44 regulates osteoclast differentiation through the NF-κB signaling pathway
The NF-κB signaling pathway plays an essential role in osteoclast differentiation, function and survival28,29,30,31. After binding to RANKL, RANK recruits TRAF6 to activate signaling cascades controlling osteoclastogenesis32,33,34, which include the phosphorylation of Src and Akt and the activation of NF-κB. The phosphorylation of Src can also enhance NF-κB activity via stimulation of Akt and IκB kinase activity. To investigate the regulation of CD44 on the signaling pathway related to osteoclast differentiation, BMMs from WT and CD44 KO cells were cultured with M-CSF and RANKL for 1, 3 and 5 days, after which western blotting was used to analyze the expression of Src, Akt and NFκB expression during osteoclast differentiation. As shown in Fig. 3A,B and see Supplementary Fig. S2A online, both p-Src (Tyr416) and p-Akt (Ser473) levels in WT BMM were higher than those in CD44 KO mice. After induction with M-CSF and RANKL, p-IκB-α levels were increased during osteoclastogenesis, but significantly decreased in CD44 KO cells. NF-κB protein levels peaked at day 5 and were much higher than those in CD44 KO cells (Fig. 3C and see Supplementary Fig. S2A online). Next, we examined the protein levels of the osteoclastogenic transcription factor NFATc1, which was downstream of NF-κB and found they were upregulated during osteoclast differentiation. However, its levels were significantly decreased in CD44 KO cells (Fig. 3D and see Supplementary Fig. S2A online). During RANKL-induced osteoclastogenesis, TRAF6, can be directly recruited into RANK cytoplasmic domains and triggers downstream signaling molecules for the activation of Src, Akt and NF-κB. To explore whether CD44 can influence the interaction of TRAF6 with RANK, we performed coimmunoprecipitation experiment in RANKL-induced WT and CD44 KO BMMs. The results showed that CD44 could promote the interaction between TRAF6 and RANK (Fig. 3E and see Supplementary Fig. S2B online). We also found the CD44 ligand, HA or OPN, could not activate the Src, Akt and NFκB signal in these cells (see Supplementary Fig. S2C, D online). These results demonstrate that the NF-κB signaling pathway is inhibited during osteoclast differentiation of CD44 KO BMMs and suggest that CD44 regulates osteoclast differentiation through regulating RANKL-RANK mediated NF-κB signaling pathway.
CD44 regulates osteoclast differentiation through NF-κB signaling pathway.
BMMs from two-month-old WT and CD44 KO mice were cultured with M-CSF (30 ng/ml) and RANKL (50 ng/ml) for 1, 3 and 5 days. Cell lysates were subjected to western blot analysis using specific antibodies. Representative western blot of p-Src (Tyr416) and c-Src (A), p-Akt (S473) and Akt (B), p-IκBα, IκBα and NF-κB (C), NFATc1 (D) were shown. GAPDH was used as internal control. (E) The effect of CD44 on the interaction between TRAF6 and RANK. BMMs from two-month-old WT and CD44 KO mice were cultured with M-CSF (30 ng/ml) and RANKL (50 ng/ml) for 5 days, the cell lysate was immunoprecipitated by TRAF6 antibody, followd by RANK detection with anti-RANK antibody.
CD44 deficiency suppresses hindlimb unloading-induced cortical bone loss
To investigate the regulation of CD44 on osteoclast function in vivo, we examined the effect of CD44 deficiency on the decrease in bone formation in hindlimb-unloaded mice. The results of micro CT revealed that WT and CD44 KO control mice showed a similar bone phenotype (Fig. 4A). In hindlimb-unloaded mice, trabecular bone volume (BV/TV) (Fig. 4B), trabecular thickness (Tb.Th) (Fig. 4C) and trabecular number (Tb.N*) (Fig. 4D) were decreased in WT and CD44 KO mice. Cortical bone area and thickness were significantly decreased in WT mice after hindlimb unloading, however, there were no obvious changes in CD44 KO mice (Fig. 4E,F). These results suggest that hindlimb unloading-induced changes in cortical bone were efficiently attenuated in CD44 KO mice.
CD44 deficiency suppresses hindlimb unloading-induced bone loss.
(A) The 4-month-old WT and CD44 KO mice were subjected to hindlimd unloading through tail suspension for 28 days, μCT images of proximal femurs from WT-control (WT-Ctrl, n=6), WT-hindlimb-unloading (WT-HS, n=6), CD44 KO-control (KO-Ctrl, n=8) and KO-hindlimb-unloading (KO-HS, n=8) mice were shown. Trabecular bone volume per total volume (BV/TV %) (B), Trabecular thickness (C) and Trabecular number (D) Cortical wall thickness (E-F) were shown. All data are the mean ± SEM.*p < 0.05.
CD44 inhibits osteoclast but not osteoblast function in hindlimb-unloading induced bone loss
To assess the significance of CD44 in osteoclast or osteoblast function in the hindlimb-unloading mice model, we first analyzed changes in CD44 mRNA expression levels in osteoblasts (Alp+) and osteoclast (Oscar+) cells from the hindlimb of tail-suspended and control mice. The results showed that CD44 mRNA and protein levels increased in whole bone tissues and by 50% in osteoclasts after hindlimb-unloading, but there were no obvious changes in osteoblasts (Fig. 5A–D). The expression levels of osteoblast markers Alp, Collagen-1 and Bglap were all significantly decreased in bone tissues from WT and CD44 KO mice 28 days after hindlimb unloading (Fig. 5E–G). However, the mRNA levels of osteoclast markers Mmp9 and Trap had lower increase in the CD44 KO group compared to WT mice (Fig. 5H, I). These results indicate that CD44 deficiency inhibits cortical bone loss by inhibiting osteoclast function in the hindlimb unloading model.
CD44 deficiency reduces osteoclast funtion but not osteoblast in hindlimb-unloaded mice.
The CD44 mRNA level (A) and protein level (B) in whole bone tissues collected from the hindlimb-unloaded and age-matched control mice were determined. qPCR analysis of CD44 mRNA levels in Alp+ (C) and Oscar+ (D) cells isolated by FACS from bone marrow stromal cells in bilateral tibias and femurs of hindlimb-unloaded and control mice. Real-time PCR analysis the expression of osteoblast marker genes, Alp (E), Collagen I (F) and Bglap (G) and osteoclast marker genes, Mmp9 (H) and Trap (I)mRNA levels in tibias and femurs collected from WT-control (WT-Ctrl, n=6), WT-hindlimb-unloaded (WT-HS, n=6), CD44 KO-control (KO-Ctrl, n=8) and KO-hindlimb-unloaded (KO-HS, n=8) mice. All data are the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
We demonstrated that the expression of CD44 is upregulated during M-CSF- and RANKL-induced osteoclastogenesis. CD44 KO resulted in a much lower expression of genes related to osteoclast differentiation and function in vitro. Osteoclasts induced from bone marrow-derived monocytes isolated from CD44 KO mice exhibited reduced activity and function by TRAP staining and bone resorption measurement. We also found that CD44 was involved in the regulation of osteoclastogenesis through NF-κB-mediated signaling pathway. CD44 enhanced the interaction between RANK and TRAF6 and faciliates its down-stream signaling. In the hindlimb unloading model, the expression of CD44 was significantly upregulated in the hindlimb bone. CD44 KO could protect from hindlimb unloading-induced cortical bone loss, whereby the downregulation of osteoclasts rather than osteoblasts contributed to this process. A model of the CD44-mediated pathway in osteoclast differentiation and activity is shown in Fig. 6.
Model of CD44-mediated pathway in osteoclast differentiation and activity.
After RANKL stimulation, the CD44 expression in osteoclast cells was upregulated. CD44 could increase the interaction between RANK and TRAF6, then it would activate its downstream signaling molecules, lead the phosphorylation of Src or Akt, which phosphorylates IκB-α and promotes the expression of NFATc1. NFATc1 induces the expression of genes related to the function and activity of osteoclast.
CD44 is widely expressed in many tissues and cell types35. However, its expression and roles in skeletal tissues remain unclear. In this study, we found that the expression of CD44 was progressively up-regulated during M-CSF and RANKL-induced osteoclastogenesis. Osteoclasts originate from hematopoietic precursors of a monocyte/macrophage lineage and differentiate into multinucleated giant cells specialized to resorb bone by fusion of mononuclear progenitors36. RANKL interacts with the osteoclast cell surface receptor RANK, which in turn recruits TNF receptor associated factors (TRAFs) and plays a crucial role in osteoclast differentiation axis37. Our results demonstrated that CD44, as a membrane receptor, plays an important role in this process. Without CD44, the differentiation of BMMs into osteoclasts was greatly retarded and the function of osteoclasts was weakened.
Specific antibodies against CD44 inhibit osteoclast formation in vitro, thereby blocking the signal transduction of CD44 from extracellular matrix to intracellular regions. CD44 ligands such as HA and OPN inhibit BMM fusion in vitro23. The expression of NFATc1 is downregulated when HA binds to CD44, which leads to the downregulation of MMP-9, cathepsin K and TRAP expression, as well as impairment of osteoclast migration and resorption activity38. In this study, we found that RANKL could induce the expression of CD44 during osteoclastogenesis. CD44 promotes the activation of RANKL-RANK-NF-κB-mediated signaling pathway by increasing the interaction between RANK and TRAF6.
The modulating role of CD44 in osteoclast formation depends on the microenvironment39. It has been reported that cancellous bone volume in the metaphysis of WT and CD44 KO mice is normal. However, cortical thickness is increased and the medullary area is decreased in CD44 KO mice27. In our experiments, we did not observe a difference in cortical thickness between WT and CD44 KO mice. However, in the hindlimb unloading model, cortical bone loss was obviously alleviated in CD44 KO mice. The expression of CD44 was significantly increased in the hindlimb bone of tail-suspended mice, which mainly resulted from the upregulation of CD44 in osteoclasts. After hindlimb unloading, the activity of osteoclast from CD44 KO mice was much lower than that from WT mice. We also found that CD44 expression is also up-regulated in femurs from ovariectomy (OVX) mice compared with control mice (see Supplementary Fig. S3A-C online). These results suggest that therapeutic inhibition of CD44 may protect from osteoporosis by inhibiting osteoclast activity.
It has been reported that OPN is regulated by mechanical stress in vivo and in vitro40,41. However, its mechanisms remain unclear. Our data demonstrated that CD44 is required for unloading-induced bone resorption in vivo, thereby suggesting that CD44 plays a key role in conveying the effect of mechanical stress to osteoclasts. Additional experiments are being performed to explore the OPN/CD44-mediated pathway in the regulation of osteoclast function under unloading-induced bone boss.
Materials and Methods
Animals
All WT and CD44 knockout (KO) mice used in the experiments were bred and maintained at the SPF Animal Research Building of China Astronaut Research and Training Center (12-h light, 12-h dark cycles, temperature controlled for 23 °C and free access to food and water). Animals were fed with standard maintenance rodent diet (Beijing KEAO XIELI FEED Co. LTD, China). The mice used on this study were 4 month old males and in a C57BL/6J background. Mice were euthanized for dissecting bilateral femurs and tibias by injection with Avertin (2.5% 2,2,2-tribromoethanol; Sigma, USA). CD44 KO mice were endowed by Dr. Li Tang from the Academy of Military Medical Sciences. The experimental procedures were approved by the Animal Care and Use Committee of China Astronaut Research and Training Center and all animal studies were performed according to approved guidelines for the use and care of live animals.
Micro-computed tomography (Micro-CT) analysis
High-resolution micro-CT analyses were performed on the distal femurs using a model of μ 40 scanco (Switzerland). In the femurs, the trabecular bone proximal to the distal growth plate was selected for analyses within a conforming volume of interest (cortical bone excluded) commencing at a distance of 840 μm from the growth plate and extending a further longitudinal distance of 1680 μm in the proximal direction. Cortical measurements were performed in the diaphyseal region of the femur starting at a distance of 3.57 mm from the growth plate and extending a further longitudinal distance of 210 μm in the proximal direction.
Cell culture and osteoclast formation assay
Mouse bone marrow cells were isolated from the femur and tibia of 2-month-old mice. Briefly, bone marrow cells were flushed, collected and washed twice with α-MEM. Cells were then cultured with complete α-MEM medium in the presence of M-CSF (10 ng/ml, R&D, USA) for 1 day. Suspension cells were collected for osteoclast generation. Cells were cultured in complete medium with 30 ng/ml M-CSF and 50 ng/ml RANKL (R&D, USA) for 5 days. Tartaric acid phosphatase (TRAP) staining was according to the protocol of Acid Phosphatase kit (Sigma, USA). The TRAP positive multinuclear cells were recorded using inverted microscope (Nikon, Japan).
Immunofluorescence
For immunostaining assay, mouse BMMs were cultured in complete medium with 30 ng/ml M-CSF and 50 ng/ml RANKL for 0, 3, 5 days. Then cells were washed three times with cold PBS and fixed in 4% Paraformaldehyde (Sigma, USA) for 30 min. After being washed three times with cold PBS, the cells were blocked at 37 °C for 1 h in 5% goat serum. Cells were incubated with anti-CD44 antibody (Abcam, USA) at room temperature for 2 h. After being washed three times with cold TBST (TBS, 0.1%Tween 20), the cells were incubated in goat anti-rabbit IgG/FITC at room temperature for 40 min. At last, the cells were incubated with DAPI (Roche, USA) for 15 min and then analyzed by confocal microscopy (Leica, Germany).
RNA extraction and qPCR
Total RNA was extracted from cultured cells or bone tissue using RNAiso Plus reagent (Takara, China). The RNA was reverse transcribed into cDNA and qPCR was performed using a SYBR Green PCR kit (Takara, China) in a Light Cycler (Eppendorf, Germany). The expression level of each gene was normalized to that of Gapdh, which served as an internal control. Primers (synthesized by Sunbiotech Co, China) for CD44, Gapdh, CathepsinK, MMP9, Trap, CLC7, NFATc1, Alp, Bglap and Collagen1 were as follows:

Pit formation assay
Mouse BMMs were obtained as described previously, for pit formation assay, BMMs (5 × 105 cells/well) were seeded on bovine bone slices in 24-well plates in proliferation medium for 1 day and switched to differentiation medium for 3 days. Bovine bone slices were ultrasonicated in 1 mol/L NH4OH to remove adherent cells and stained with 0.1% toluidine blue solution42. Pit area was measured using Image Pro 405 Plus 6.2 software (Media Cybernetics Inc. USA).
Western blot analysis
BMM cells were cultured with differentiation medium for 1, 3, 5 days. Cells were washed with cold PBS twice and then lysed in lysis buffer (50 mM Tris, pH7.5, 250 mM NaCl, 0.1% SDS, 2 mM dithiothreitol, 0.5% NP-40, 1 mM PMSF and protease inhibitor cocktail) on ice for 15 min. Cell extracts were collected by centrifugation at 15,00 0 g at 4 °C for 30 min, applied to 8–10% SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes by electroblotting. The membranes were blocked for 1 hour in a blocking buffer containing 5% powdered milk in TBST. The membranes were incubated with primary antibody overnight at 4 °C followed by incubation with a secondary antibody conjugated to horseradish peroxidase (HRP) and visualized using an chemiluminescence kit (Thermo Pierce, No.32 109). Specific antibodies to p-Src (Cell Signaling Technology, #6943), Src (Cell Signaling Technology, #12945), p-Akt (Cell Signaling Technology, #9272), Akt (Cell Signaling Technology, #9271), p-IκBα (Cell Signaling Technology, #2859), IκBα (Cell Signaling Technology, #4814), NFATc1 (Cell Signaling Technology, #8032), NFκB (Santa Cruz Biotechnology, sc-7178), RANK (Santa Cruz Biotechnology, sc-9072), GAPDH (Santa Cruz Biotechnology, sc-25778) were used to detect protein levels.
Immunoprecipitation
WT and CD44 knockout mouse BMMs were cultured in complete medium with 30 ng/ml M-CSF and 50 ng/ml RANKL for 5 days and cells were harvested in in HEPES lysis buffer (20 mM HEPES pH 7.2, 50 mM NaCl, 0.5% Triton X-100, 1 mM NaF, 1 mM dithiothreitol) supplemented with protease inhibitor cocktail (Roche, Indianapolis, Indiana, USA) for immunoprecipitation. The cell lysates were transferred to a new fresh tube. Next 5 μg/ml rabbit polyclonal anti-TRAF6 (Abcam, USA) were added for 3 h and incubated the mixture with proteins A/G PLUS-agarose beads (Santa Cruz Biotechnology, USA) over night at 4 °C. Immune complexes were washed with cold lysis buffer for three times. After the final wash, we aspirated and discarded the supernatant and resuspended the pellet in 1X electrophoresis sample buffer and boiled it for 10 min.
Cell sorting with flow cytometry
The bone marrow cells and bone marrow stromal cells were collected from the femur and tibia of 2-month-old WT and CD44 KO mice. Antibody to mouse Alp (R&D systems, Minneapolis, USA) and antibody to mouse Oscar (Santa Cruz Biotechnology, USA) antibodies were used for FACS according to the following protocol. After washed by PBS and 1% BSA, the cells were directly stained with antibody to Alp (1:50, R&D systems, Minneapolis, USA) and then stained with goat anti-mouse IgG-FITC (1:100, R&D systems, Minneapolis, USA) or were incubated with antibody to Oscar (1:40, Santa Cruz Biotechnology, sc-34237) and then stained with donkey anti-goat IgG-PE (1:100, R&D systems, Minneapolis, USA). After that, stained cell populations were used for FACS. The obtained selected Alp+ and Oscar+ cell populations were used for total RNA extraction and qPCR analysis.
Hindlimb-unloading model
The hindlimb-unloading procedure was achieved by tail suspension, as described previously43. Briefly, the 4-month-old mice were individually caged and suspended by the tail using a strip of adhesive surgical tape attached to a chain hanging from a pulley. The mice were suspended at a 30° angle to the floor with only the forelimbs touching the floor, which allowed the mice to move and access food and water freely. The mice were subjected to hindlimb unloading through tail suspension for 28 d. After euthanasia, bilateral femurs and tibiae were dissected and processed for microCT examination and real-time PCR analysis.
Statistical analysis
Data are presented as mean ± SEM per experimental condition. Considering the possibility of unequal variance for the data, we first test the equality of variances across groups. If it shows that the variances are unequal, we then use the Welch t test for 1-way analysis or mixed model with heterogeneous variances for 2-way analysis. Otherwise, we use the Student’s t test or the regular linear model. Bonferroni adjustment was used for multiple comparisons. p < 0.05 is considered statistically significant. p < 0.01 is considered very significant. All the statistical tests are analyzed by Prism software (Graphpad prism for windows, version 5.01) and SPSS (Version 14.0 for windows).
Additional Information
How to cite this article: Li, Y. et al. CD44 deficiency inhibits unloading-induced cortical bone loss through downregulation of osteoclast activity. Sci. Rep. 5, 16124; doi: 10.1038/srep16124 (2015).
References
Orian-Rousseau, V. CD44 Acts as a Signaling Platform Controlling Tumor Progression and Metastasis. Front Immunol 6, 154, doi: 10.3389/fimmu.2015.00154 (2015).
Schmitt, M., Metzger, M., Gradl, D., Davidson, G. & Orian-Rousseau, V. CD44 functions in Wnt signaling by regulating LRP6 localization and activation. Cell Death Differ 22, 677–689, doi:10.1038/cdd.2014.156 (2015).
Nakamura, H. & Ozawa, H. Cell-cell and cell-matrix interaction in bone remodeling. Nihon Seikeigeka Gakkai zasshi 70, 740–750 (1996).
Paiva, K. B. & Granjeiro, J. M. Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch Biochem Biophys 561, 74–87, doi: 10.1016/j.abb.2014.07. 034 (2014).
Sims, N. A. & Vrahnas, C. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch Biochem Biophys 561, 22–28, doi: 10.1016/j.abb.2014.05.015 (2014).
Nakashima, T. Coupling and communication between bone cells. Clin Calcium 24, 853–861, doi: CliCa1406853861 (2014).
Spessotto, P. et al. Hyaluronan-CD44 interaction hampers migration of osteoclast-like cells by down-regulating MMP-9. J Cell Biol 158, 1133–1144, doi: 10.1083/jcb.200202120 (2002).
Chellaiah, M. A. et al. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell 14, 173–189, doi: 10.1091/mbc.E02-06-0354 (2003).
Nakamura, H., Kenmotsu, S., Sakai, H. & Ozawa, H. Localization of CD44, the hyaluronate receptor, on the plasma membrane of osteocytes and osteoclasts in rat tibiae. Cell Tissue Res 280, 225–233 (1995).
Chellaiah, M. A. & Hruska, K. A. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int 72, 197–205, doi: 10.1007/s00223-002- 1025-6 (2003).
Ishijima, M. et al. Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. J Bone Miner Res 17, 661–667, doi: 10.1359/jbmr.2002.17.4.661 (2002).
Yoshitake, H., Rittling, S. R., Denhardt, D. T. & Noda, M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci USA 96, 8156–8160 (1999).
Cui, W. et al. The intracellular domain of CD44 promotes the fusion of macrophages. Blood 107, 796–805, doi: 10.1182/blood-2005-05-1902 (2006).
Chang, E. J. et al. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci 120, 166–176, doi: 10.1242/jcs.03310 (2007).
Ariyoshi, W., Okinaga, T., Knudson, C. B., Knudson, W. & Nishihara, T. High molecular weight hyaluronic acid regulates osteoclast formation by inhibiting receptor activator of NF-kappaB ligand through Rho kinase. Osteoarthritis Cartilage 22, 111–120, doi: 10.1016/j.joca.2013.10. 013 (2014).
Yasuda, T. Hyaluronan inhibits prostaglandin E2 production via CD44 in U937 human macrophages. Tohoku J Exp Med 220, 229–235, doi: JST.JSTAGE/tjem/220.229 [pii] (2010).
Zhu, B. et al. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol 198, 155–167, doi: 10.1002/jcp.10394 (2004).
Ruffell, B. et al. Differential use of chondroitin sulfate to regulate hyaluronan binding by receptor CD44 in Inflammatory and Interleukin 4-activated Macrophages. J Biol Chem 286, 19179–19190, doi: 10.1074/jbc.M110.200790 (2011).
Miletti-Gonzalez, K. E. et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem 287, 18995–19007, doi: 10.1074/jbc.M111.318774 (2012).
Chellaiah, M. A. & Ma, T. Membrane localization of membrane type 1 matrix metalloproteinase by CD44 regulates the activation of pro-matrix metalloproteinase 9 in osteoclasts. Biomed Res Int 2013, 302392, doi: 10.1155/2013/302392 (2013).
Okamoto, I. et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 155, 755–762, doi: 10.1083/jcb.200108159 (2001).
Tanikawa, R., Tanikawa, T., Hirashima, M., Yamauchi, A. & Tanaka, Y. Galectin-9 induces osteoblast differentiation through the CD44/Smad signaling pathway. Biochem Biophys Res Commun 394, 317–322, doi: 10.1016/j.bbrc.2010.02.175 (2010).
Suzuki, K. et al. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion and resorption in osteoclasts. J Bone Miner Res 17, 1486–1497, doi: 10.1359/jbmr.2002.17.8.1486 (2002).
Oh, Y., Oh, I., Morimoto, J., Uede, T. & Morimoto, A. Osteopontin has a crucial role in osteoclast-like multinucleated giant cell formation. J Cell Biochem 115, 585–595, doi: 10.1002/jcb.24695 (2014).
Tanabe, N. et al. Osteopontin signals through calcium and nuclear factor of activated T cells (NFAT) in osteoclasts: a novel RGD-dependent pathway promoting cell survival. J Biol Chem 286, 39871–39881, doi: 10.1074/jbc.M111.295048 (2011).
Walker, C. G., Dangaria, S., Ito, Y., Luan, X. & Diekwisch, T. G. Osteopontin is required for unloading-induced osteoclast recruitment and modulation of RANKL expression during tooth drift-associated bone remodeling, but not for super-eruption. Bone 47, 1020–1029, doi: 10. 1016/j.bone.2010.08.025 (2010).
Cao, J. J. et al. Hyaluronan increases RANKL expression in bone marrow stromal cells through CD44. J Bone Miner Res 20, 30–40, doi: 10.1359/JBMR.041014 (2005).
Jimi, E. et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med 10, 617–624, doi: 10.1038/nm1054 (2004).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342, doi: 10.1038/nature01658 (2003).
Yao, Z., Xing, L. & Boyce, B. F. NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest 119, 3024–3034, doi: 10.1172/JCI38716 (2009).
Xing, L. et al. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J Bone Miner Res 17, 1200–1210, doi: 10.1359/jbmr.2002.17.7.1200 (2002).
Yamashita, T. et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem 282, 18245–18253, doi: 10.1074/jbc.M610701200 (2007).
Asagiri, M. & Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 40, 251–264, doi: 10.1016/j.bone.2006.09.023 (2007).
Ikeda, F. et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest 114, 475–484, doi: 10.1172/JCI19657 (2004).
Zoller, M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 11, 254–267, doi: 10.1038/nrc3023 (2011).
Udagawa, N. et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA 87, 7260–7264 (1990).
Regmi, A. et al. Suramin interacts with RANK and inhibits RANKL-induced osteoclast differentiation. Bone 36, 284–291, doi: 10.1016/j.bone.2004.09.022 (2005).
Pivetta, E. et al. Blood-derived human osteoclast resorption activity is impaired by Hyaluronan-CD44 engagement via a p38-dependent mechanism. J Cell Physiol 226, 769–779, doi: 10.1002/jcp.22398 (2011).
Yaccoby, S. et al. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol 116, 278–290 (2002).
Morinobu, M. et al. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res 18, 1706–1715, doi: 10.1359/jbmr.2003.18.9.1706 (2003).
Fujihara, S. et al. Function and regulation of osteopontin in response to mechanical stress. J Bone Miner Res 21, 956–964, doi: 10.1359/jbmr.060315 (2006).
Zhao, C. et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA biology 12, 343–353, doi: 10.1080/15476286.2015.1017205 (2015).
Wang, X. et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med 19, 93–100, doi: 10.1038/nm.3026 (2012).
Acknowledgements
We thank Dr. Li Tang (Academy of Military Medical Sciences) for endowing the CD44 knock out mice and providing some valuable advices. This work was supported by National Natural Science Foundation of China Project (31325012, 31170811, 31271225 and 31340064), Advanced Space Medico-engineering Research Project of China (SJ2012SY54B1602) and State Key Lab of Space Medicine Fundamentals and Application Grant (SMFA15B03).
Author information
Authors and Affiliations
Contributions
Study design: Y.X.L. and S.K.L. Study conduct: Y.H.L., G.H.Z., W.J.S., C.Y.Z., D.S.Z., S.K.L., Q.L., J.P.S. Data collection: J.P.S. and Q.L. Data analysis: S.K.L. and G.H.Z. Drafting manuscript: P.F.Z. and W.J.S. Revising manuscript content: S.K.L. and Y.H.L. S.K.L. and Y.X.L. take responsibility for the integrity of the data analysis. All authors have read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, Y., Zhong, G., Sun, W. et al. CD44 deficiency inhibits unloading-induced cortical bone loss through downregulation of osteoclast activity. Sci Rep 5, 16124 (2015). https://doi.org/10.1038/srep16124
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16124
This article is cited by
-
Hyaluronidase 1 deficiency decreases bone mineral density in mice
Scientific Reports (2022)
-
ELMO1 signaling is a promoter of osteoclast function and bone loss
Nature Communications (2021)
-
Cellular and molecular actors of myeloid cell fusion: podosomes and tunneling nanotubes call the tune
Cellular and Molecular Life Sciences (2021)
-
Identification of suitable reference gene and biomarkers of serum miRNAs for osteoporosis
Scientific Reports (2016)
-
Inhibition of Osteoclast Differentiation and Bone Resorption by Bisphosphonate-conjugated Gold Nanoparticles
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.