Abstract
The lack of effective and accurate diagnostic tools contributes to the high prevalence of tuberculosis (TB) worldwide. The current serodiagnostics for TB are inadequate mainly due to lack of TB-specific antigens with highly accurate diagnosis. In the current study, we aimed to identify novel diagnostic antigens using glutathione S-transferase (GST)-fusion protein technique. We determined the reactivity of these recombinant proteins arrayed in solution and on GSH-immobilized microplates with TB patient sera. Of 409 TB proteins produced, ninety-two yielded seropositive reactions, fourteen including eight novel proteins showed strong immunoreactivity. Further, six were selected and constructed as a multiple-antigen combination set through analysis of various combinations. A comparative study of the multiple-antigen combination set and a commercially available kit revealed that the combination set showed 66.3% (95% CI 60.5–71.8) sensitivity, which was significantly higher than that of the commercial kit [31.6% (95% CI 26.3–37.3)]. The specificity of both methods was similar at 89.6% (95% CI 83.3–95.4) and 90.6% (95% CI 83.0–95.6), respectively. This study provides a set of novel diagnostic protein markers with great potential for the development of novel diagnostic tools for active TB.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is a chronic infectious disease caused by infection with Mycobacterium tuberculosis. It remains a serious global health problem with 8.6 million new cases and 1.3 million deaths reported in 20121. Moreover, nearly one-third of the world’s population is latently infected with M. tuberculosis, of which approximately 10% develop active TB in their lifetime2. In addition, the emergence and spread of the multidrug-resistant TB is threatening the global control program3,4,5.
The lack of effective diagnostic methods is a major contributing factor in the high disease prevalence. The sputum smear and culture tests are the traditional gold standard methods for TB diagnosis; however, the former exhibits low sensitivity particularly in children and immunocompromised individuals, while the latter requires a very long cultivation period (6–8 weeks)6. The tuberculin skin test (TST) is the only screening test for latent M. tuberculosis infection, but it exhibits extremely low specificity and high cross-reactivity with other species of mycobacteria7.
TB diagnosis has been improved by the recent development of methods such as nucleic acid amplification (NAA) tests, rapid culture methods, T-cell-based interferon-γ release and advanced imaging techniques8. However, the use of these methods in developing countries where majority of TB cases occur is limited by cost. In addition, these techniques are not suitable for large-scale surveillance and screening tests. Therefore, more accurate and economic diagnostic tools for TB are urgently required.
Rapid, point-of-care diagnostics that allow highly accurate diagnosis of active TB in the field are urgently required. Immunodiagnostic detection of TB antigens or their antibodies, which is simple, cheap, robust and easily implemented, represents an attractive option for this purpose although the sensitivities of the currently available options are quite variable ranging from 0.09% to 59.7%9. Recent efforts involving high-throughput screening of the entire M. tuberculosis proteome have identified some promising diagnostic antigens, including the 38-kDa antigen, which has been used in some commercial immunodiagnostic kits10,11. Moreover, the combination of several antigens significantly improved the diagnostic sensitivity12,13.
The availability of M. tuberculosis genome sequence information along with the corresponding proteomic datasets has facilitated the comprehensive, systematic and unbiased identification of novel antigenic proteins at a whole proteome scale14,15. Although a large number of antigens have been identified for the detection of circulating antibodies to M. tuberculosis and evaluated for their diagnostic value alone or in combination, their use is limited by low sensitivity and specificity16,17. Thus, further studies are needed to identify more effective diagnostic protein markers. Many previous screenings of diagnostic antigens either used denatured recombinant protein expressed by Escherichia coli18, thus, increasing the possibility that some conformational epitopes may be lost, or used native materials extracted from the bacteria or carrier proteins19,20,21, although folding, probably limited by the low level expression of the immunologically meaningful proteins, such as secreted proteins and transmembrane proteins22,23, therefore undetected. It was noteworthy that immunodominant antigens identified in vast literatures were enriched for secreted and transmembrane proteins24,25,26,27. Two recent proteome-wide approaches for identification of potential M. tuberculosis protein biomarkers revealed that the subset of the proteome targeted by a human immune response was predominated by secreted and transmembrane proteins18,28. These conclusions were consistent with much earlier diagnostic works which had identified some well-known immunodominant antigens of M. tuberculosis, including ESAT-6, the 38 kDa antigen, CFP-21 and MPT64 that were secreted by the bacteria during infection29,30 and LprA and HspX that were extracted from isolated plasma membrane31. Moreover, though it was widely recognized that protective immunity against intracellular bacteria such as M. tuberculosis was primarily elicited by T-cell-mediated response, antibodies were still produced during infection and secreted and transmembrane proteins had been identified to be targeted by B-cells in other intracellular bacterial species, including Chlamydia, Ehrlichia and Listeria32,33,34. As a consequence, we focused our search for serodiagnostic antigens on secreted and transmembrane proteins of M. tuberculosis. Recently, we developed a glutathione S-transferase (GST) fusion protein assay, in which each protein is refolded, arrayed in solution and at similar amounts. This technique could potentially identify antigens present at low levels, or antigens with short half-lives and has been successfully applied to identify diagnostic markers for other pathogen35. In the current study, we used this technology to generate over four hundred M. tuberculosis secreted and transmembrane proteins, which we analyzed using a large number of serum samples from TB patients. We discovered several novel diagnostic antigens and constructed a multiple-antigens combination set that significantly increased the sensitivity and specificity of immunodiagnostic testing of TB infection in humans.
Results
Production of recombinant GST-TB fusion proteins
A total of 239 putative secreted proteins and 358 transmembrane proteins were predicted from 3,924 M. tuberculosis open reading frames (ORFs) using bioinformatics tools. We excluded 118 transmembrane proteins, of which the protein sequence of the largest extracellular domain was less than 50 residues. In addition, we selected 91 latent-associated proteins and 129 RD (region of difference) proteins, which were absent from BCG but present in M. tuberculosis.
The full-length sequences of secreted proteins, RD proteins, latent-associated proteins and the largest extracellular domain of transmembrane proteins were amplified by PCR (Fig. 1a). A total of 441 genes were successfully cloned from the resulting PCR products into the pGEX-His vector (Supplementary Table S1). Western blot analysis showed that a total of 432 (97.9%) GST-TB fusion proteins were successfully expressed in E. coli (Fig. 1b and Supplementary Table S5) and of these, 389 (85.3%) were mainly expressed in inclusion bodies.
PCR amplification and expression of M. tuberculosis ORFs.
(a) A total of 699 unique pairs of primers based on bioinformatics analysis were designed for PCR amplification and 630 (90%) ORFs of the correct size were successfully obtained. The PCR products were separated by agarose gel electrophoresis. The corresponding ORF proteins are annotated at the top of the figure according to the M. tuberculosis H37Rv nomenclature (http://genolist.pasteur.fr/TubercuList/). (b) All samples were separated by 12% SDS-PAGE and Coomassie blue stained. The corresponding protein identities are annotated at the top of the figure. Bands corresponding to the correct size of ORF and proteins are indicated by red dots. The gels were cropped and run under the same experimental conditions.
Correct refolding of the GST-TBs fusion proteins expressed in inclusion bodies is essential for their binding to the GSH-immobilized microplates. When detected by ELISA using anti-GST-tag and anti-polyhistidine-tag mouse monoclonal antibodies, the relative light units (RLUs) of most of the refolded proteins were significantly higher (Fig. 2b) than those of the denatured proteins (Fig. 2a), indicating that the recombinant proteins were successfully refolded and most were full-length. Moreover, chemiluminescent ELISA indicated that 409 (94.7%) of the 432 GST-TB proteins were successfully bound to the GSH-immobilized microplates.
Screening of 409 GST-TBs with TB patient serum samples
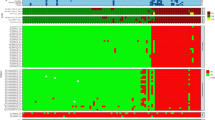
Initial screening of the interactions of the 409 GST-TB fusion proteins with 10 TB patient serum samples was assessed by chemiluminescent ELISA; three healthy serum samples were used as negative controls. Of 409 GST-TBs, 92 had seropositive reactions (Fig. 3, Supplementary Table S6). The number of reactive antigens varied with different serum samples. Of the 92 antigens, 24 (26.1%) and 43 (46.7%) interacted with the serum samples from Patients 7 and 8 respectively, while only 12 (13.0%) and 6 (6.2%) interacted with serum samples from Patients 5 and 9.
Screening of the GST-TBs with tuberculosis patient sera.
Ten TB patient sera and three healthy control sera were used for initial screening. The reactions of the serum samples to 409 putative M. tuberculosis proteins were determined by chemiluminescent ELISA. The RLUs were detected by the chemiluminescent reader SpectraMax M5. The ratio of RLUs of a human serum to a GST-TBs and the same serum to GST control was calculated using the formula: R = (RLUs of GST-TBs − RLUs of PBS)/(RLUs of GST − RLUs of PBS). GST-TBs with R ≥ 2 were considered to indicate seropositivity.
Of the 92 seropositive antigens, 55 reacted with only one TB patient serum sample, while 14 reacted strongly with at least two samples and showed no cross-reactivity with serum samples from healthy controls. Of these, eight were novel protein markers for TB serodiagnosis. The 14 proteins were selected as candidate protein markers for further analysis and the proteins without the GST-tag were re-produced in E. coli as shown in Supplementary Figure S1 and Supplementary Table S2.
Assessment of 14 candidate diagnostic antigens
The potential of the 14 most reactive antigens as protein markers for the serodiagnosis of active TB was evaluated using a panel of 64 randomly archived serum samples (42 infected samples and 22 uninfected samples). The specificities of all the recombinant proteins exceeded 80% (81.8–100%), whereas their sensitivities ranged from 9.5% to 33.33% (Fig. 4a and Supplementary Table S3). Clearly, no single antigen was representative of the antibody profiles of all TB patients; therefore, a combination of various antigens is required to improve the diagnostic potential.
Reactivity of the 14 selected TB proteins.
(a) Their responses to the panel of 42 archived infected serum samples and 22 healthy controls are displayed in a heat map. (b) The caret package in R statistical software was used for the analysis. The sensitivities and specificities of variable combinations of proteins (range, 2–14) were calculated. Each dot represents one combination (combinations with identical sensitivities and specificities are overlapping). Combinations with higher sensitivities and specificities (more than 0.7 and 0.8, respectively) are marked with red dots.
The sensitivities and specificities of variable combinations of proteins (2–14) were analyzed statistically. A total of 16,369 different combinations were shown (Fig. 4b). A total of 207 different combinations of sensitivity and specificity were identified, of which, a combination of six proteins (the 38kDa antigen, LprG, Rv1566c, Rv1623c, MPT64 and HspX) provided the best performance (71.4% sensitivity with 86.4% specificity) and was selected for comprehensive evaluation (Supplementary Table S3). Among the six proteins, Rv1566c and Rv1623c were novel proteins for TB serodiagnosis.
Diagnostic validation of the multiple-antigen combination set
The sensitivity and specificity of the multiple-antigen combination were further analyzed for TB serodiagnosis using serum samples from 96 patients infected with TB and 49 healthy individuals (Supplementary Figure S2 and Table 1). All antigens had a specificity of 95.9% or greater, while the sensitivities of the six individual antigens varied from 15.6% to 33.3%. Of the infected serum samples, 67.7% (65/96) recognized at least one antigen, while only 6.2% (4/65) recognized all the antigens and 55.4% (36/65) reacted only with the discrete antigens. Of these, seven serum samples interacted with the 38 kDa antigen alone, two interacted with LprG, three with Rv1566c, eleven with Rv1623c, seven with MPT64 and six with HspX. The combination of the six antigens significantly increased the sensitivity to 67.7% with a specificity of 91.8% (Table 1). Thus, the combined antigens were identified for further validation as serodiagnostic markers of TB.
Comparison of the combination test with a commercially available kit
To examine the potential diagnostic value of the multiple-antigen test, we collected an additional 384 serum samples, including 288 infected serum samples from the patients with clinically diagnosed TB during the period from September 2012 to October 2014. We compared the specificity and sensitivity of the combined antigens with a commercial diagnostic test (TAID-Kit, Tuberculosis Antibody (IgG) Detection Kit (ELISA), Shanghai). Of the 288 TB patients, the combined antigen test identified 191 as seropositive (66.3% sensitivity), whereas the TAID-Kit diagnosed only 91 as seropositive (31.6% sensitivity). The specificity of both methods was similar (89.6% and 90.6% respectively). Moreover, the combined antigen test provided higher accuracy compared with the TAID-Kit (72.1% vs. 46.4%) (Table 2).
Discussion
The WHO has developed a new six point Stop TB Strategy (the Stop TB Partnership's Global Plan to Stop TB 2006–2015), which aims to achieve the gradual elimination of TB and considerable effort has been directed towards preventing its high prevalence36. However, none of the current strategies are likely to achieve this level of disease control; consequently, the identification of new diagnostic biomarkers presents a priority in TB research. In this study, we used the recently developed high-throughput GST-fusion protein array technology to generate over 400 TB proteins. Fourteen highly reactive proteins were identified by screening with TB patient serum samples, eight of which were novel. These results provide experimental evidence of the immunogenicity of novel TB proteins that are suitable for the development of valuable serodiagnostic tools.
The availability of MTB genome sequences provides the opportunity for the systematic identification of diagnostic protein markers and vaccine candidates at a genome-wide scale. However, limited progress has been made, mainly due to difficulties in limiting the bias introduced by differences in the amounts of proteins present in such screening procedures. Many previous studies based on ‘omics’ approaches perform suboptimally and have respective drawbacks. For example, 2-D gel electrophoresis and liquid chromatography followed by mass spectrometry (MS) used native samples extracted from M. tuberculosis culture supernatant20,22,24. However, because of the complexity of protein extract preparation and separation, the success of these investigations were limited by the abundance of the expressed proteins, with immunologically relevant proteins, such as secreted proteins and transmembrane proteins, often present in low abundance and therefore undetected. On the other hand, one recent proteome-wide approach was developed to identify serodiagnostic antigens of M. tuberculosis at an unbiased way18. However, the expressed recombinant proteins dissolved in urea were at denatured state, thus increased the possibility that important conformational epitopes were lost. Other recently developed technologies involve a protein microarray method named Nucleic Acid Programmable Array (NAPPA), in which functional proteins are transcribed and translated in situ directly from printed full-length cDNAs just-in-time for assay37. The recently modified NAPPA method further shows multiple advantages over others such as highly consistent display levels, flexibility, high throughput and so on. Moreover, this method uses purified cDNA as the key substrate, which is much easier to prepare, print, quantify and store than proteins38. It exhibits the great potential and capacity to screen of candidate targets and has successfully applied to other human diseases39,40. In this study, we use an indirect ELISA-based GST-fusion protein array technique, which integrates multiple advantages as follows: (1) High throughput screening: this technology allows us to produce a large number of GST-TB fusion proteins that are bound to the 96 or 384 GSH-immobilized plates for screening, which provides a possibility of the systematic identification of novel protein markers for serodiagnostics at an ORFeome scale; (2) Faster and less labor-intensive: no purification procedure is needed for the GST fusion proteins in the bacterium extracts that are directly added to the GSH-immobilized plates for the binding through the interaction of GST with its substrate glutathione; (3) Potential identification of the protein markers at low expression at nature condition: the immunologically meaningful proteins such as secreted and transmembrane proteins normally presenting at a low level expression at nature condition could be produced at a large volume and high-quality of the products using this standard technology and potentially identified as diagnostic protein markers; (4) Correct refolding of the GST fusion proteins: binding of the GST-TB proteins to the GSH-immobilised plates means that all the bound fusion proteins could refold correctly. In addition, the recombinant proteins expressed in E. coli particularly those in inclusion bodies are essential for refolding before their binding to the GSH-immobilized microplates. We have identified a universal refolding buffer C7 from the iFOLD Protein Refolding System 1 for the refolding of the GST inclusion bodies35. We demonstrated the correct refolding of the GST-TB proteins by comparison of the interactive signal intensity with both anti-GST and anti-polyhistidine-tag antibodies with that of the denatured proteins (Fig. 2a). All of the refolded proteins but twenty-three bound successfully to the plate and were detected by the anti-GST monoclonal antibody (Fig. 2b), indicating correct folding. The remaining twenty-three proteins failed to bind to the plate probably due to incorrect folding. The ability to generate correctly refolded recombinant proteins is critical for the discovery of ideal diagnostic proteins. Using this system, we successfully identified fourteen highly immunoreactive proteins, eight of which were novel and had not been reported previously as diagnostic candidates. The detection of previously established antigens in addition to the relatively high rate of novel antigen detection confirms the efficacy of this approach. The ELISA-based GST-fusion protein array technique has also been successfully used in the identification of antigens for the serodiagnosis of Schistosoma japonicum infection35. Thus, the results of our study indicate the potential application of this approach for serodiagnosis of other bacterial and parasitic diseases.
A total of 409 GST-TB fusion proteins were produced and bound to the GSH-immobilized plates. Serum samples from active TB patients and healthy controls were used to screen of the putative GST-TB fusion proteins. The M. tuberculosis ORFeome reactivity of sera from different individuals was distributed among three distinct groups. The first and smallest group, which comprises roughly 3.4% (14/409) of the ORFeome, includes antigens that are most frequently recognized only during active TB. Of these antigens, Rv1488, Rv1566c, Rv1579c, Rv1623c, Rv1825, Rv3128, Rv3601c and Rv3921c were newly identified. A second, larger group includes additional, frequently recognized antigens as well as proteins that are rarely detected. Together, the first two groups of proteins define the immune-ORFeome of M. tuberculosis, which is approximately a quarter of the size of the total ORFeome established in this study. These targets are rich in secreted and transmembrane proteins. The remaining and largest, group of the ORFeome contains proteins that do not react with TB sera.
Previous evaluations of serological responses indicate a heterogeneous antibody response to TB antigens13,41,42, which is consistent with our results. While many TB patient sera reacted with different antigens, some sera were exceedingly restricted in their reactivity (Fig. 4a). Some sera reacted almost exclusively with discrete antigens, while other sera reacted with almost all antigens. However, most sera reacted with a variety of multiple antigens. Variations in specific antibody responses to TB antigens in different individuals may be linked to human leukocyte antigen (HLA) phenotype43,44, bacillary load and disease progression of the patient populations28. No single TB antigen has so far been identified as a useful serodiagnostic marker due to the complexity of the human immune response to TB antigens. To overcome this issue, a combination of several recombinant antigens is clearly required to improve the accuracy of such diagnostic tools. Our data revealed that the combination of six diagnostic proteins yielded a diagnostic sensitivity of 66.3% and specificity of 89.6%, which were significantly higher than that of the individual proteins. In addition, it can be speculated that our approach has the advantage of covering a greater extent of the M. tuberculosis proteome using correctly refolded proteins. This is important due to the heterogeneity of antibody responses to TB among individuals and different ethnic groups42.
Over the recent decades, TB immunodiagnostics including cell-immune-based diagnostic tests and serological tests were developed for diagnosing infected individuals with M. tuberculosis. However, majority of them had poor performances in terms of their sensitivities and specificities17,45,46. The cell-immune-based diagnostic tests have several irreversible drawbacks. For example, the century old TST is limited to use by its low specificity because of cross-reactivity with previous BCG vaccination and other environmental mycobacterium infection while the recently developed interferon-γ release assays (IGRAs), despite of high specificity compared with TST, is expensive and need particular expertise which presents an additional barrier in resource-limited settings. Moreover, both of the tests have the poor positive predictive value (PPV) for progression of latent tuberculosis infection (LTBI) to active TB46. In addition, none of two immune-based tests performs optimal sensitivity in individuals who are immunocompromised and is able to discriminate active or past disease from latent infection in areas with a high burden of TB. Serological tests represent an attractive option for diagnosing active TB. However, antibody-based diagnosis is controversial and is widely regarded as useless. WHO currently recommends against using these TB serological tests due to the suboptimal sensitivity and specificity47. Therefore, further identification and screening of novel serodiagnostic antigens is still highly recommended by WHO. In this study, after a stepwise selection process (represented schematically in Fig. 5), a multiple-antigen combination set was identified that consisted the six most immunoreactive diagnostic proteins (i.e. the 38 kDa antigen, LprG, Rv1566c, Rv1623c, MPT64 and HspX) for active TB. This combination set was selected through analysis of over 16,000 different combinations, which revealed higher accurate diagnosis compared to any of the other combinations and the individual antigens. Of the combined antigens, four proteins, the 38 kDa antigen, LprG, MPT64 and the LTBI-associated antigen HspX, have been reported previously as candidate diagnostic biomarkers27,28,48. In contrast, Rv1566c and Rv1623c were identified as novel antigens with one or more transmembrane helixes as predicted by TMHHM. A comparative study showed that the multiple-antigen combination set conferred a significant increase in diagnostic accuracy compared to a commercially available kit. Although the multiple-antigen combination set could be potentially developed as a novel serodiagnostic tool for active TB, the sensitivity and specificity of the multiple-antigen combination set still need to be improved. The diagnostic value of the combination set would be expected to be even lower in clinical practice, where TB contacts and healthy, latently-infected individuals would cloud the diagnosis. Potentially, this approach could be developed for the detection of latent and preclinical TB, which represents a reservoir for transmission. The increased sensitivity of such a diagnostic tool will provide support for the TB control program in achieving a reduction in the transmission of this disease.
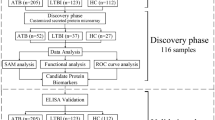
Overview of the experimental design.
The putative proteins were predicted from the whole genome of M. tuberculosis. The selected genes were amplified by PCR and cloned into a GST-fusion protein expression vector. The fusion genes (GST-TBs) were expressed in E. coli. The refolded proteins were arrayed on GSH-immobilized plates and the GST-TB protein library was constructed. Serum samples from TB patients were used to probe the GST-TB arrayed microplates. Seropositive antigens were identified by ELISA. Proteins strongly associated with TB were further assessed with a panel of TB patient serum samples. A multiple-antigen combination set was predicted by R statistical software and validated.
In conclusion, we used an indirect ELISA-based GST-fusion protein array technique, in which each protein was represented in solution and correctly refolded, to identify fourteen highly reactive proteins with the TB patient serum samples including eight novel proteins as diagnostic candidates. Further, the six diagnostic proteins were identified from them through analysis of various combinations and constructed as a multiple-antigen combination set for diagnosis of active TB. A comparative study demonstrated that the set of novel protein markers had higher sensitivity and equivalent specificity compared with the current commercially available kit for diagnosis of active TB. The increased sensitivity of the diagnostic tool based on the combination set will provide support for the TB control program in achieving a reduction in the transmission of this disease.
Methods
Cloning and expression of GST-TB fusion proteins
All methods were carried out in accordance with the approved guidelines. The putative secreted and transmembrane proteins were predicted by bioinformatics tools, i.e. SignalP (http://www.cbs.dtu.dk/services/SignalP/)49 and TMHMM software (http://www.cbs.dtu.dk/services/TMHMM/)50, respectively. The RD proteins and the latent-associated proteins were selected from the relevant published data51,52,53. All the selected genes were amplified by PCR and expressed in E. coli as GST fusion proteins (the details were included in supplementary materials).
Serum sample collection
Serum samples from TB patients were collected from Shanghai Pulmonary Hospital during the period from October 2011 to October 2014. Patients were included in the study if they fulfilled all the following criteria: all patients were diagnosed as newly treated active TB and defined according to the fourth edition of Guidelines for treatment of tuberculosis54. Specifically, the active TB case definition was a patient with a positive sputum culture for the M. tuberculosis complex or a patient who has been diagnosed as having active TB by a clinician and has been decided to treat with a full course of TB treatment according to clinical diagnostic criteria. Diagnostic criteria included bacteriological and pathological results, typical radiological manifestation and clinical response to anti-TB treatment consistent with active TB. All negative control serum samples were collected from healthy individuals with no history of TB and who tested negative by T-SPOT.TB or QFT-G. Individuals with HIV, hepatitis infections or autoimmune disorders were excluded. The serum samples used for every round of screening were independent and not recycled. Patients were tested by TB-DOT (Diagnostic Kit for Antibody to Mycobacterium tuberculosis (Colloidal Gold), Shanghai) and more details regarding age, gender and sputum smear were listed in Supplementary Table S4. The serum samples were pre-adsorbed with E. coli lysate and the GST protein to block antibodies against E. coli antigens and GST proteins. A mixture of 0.1 ml human serum, 3 ml bacterial lysate, 0.4 ml GST bound Glutathione Sepharose 4B beads and 1.5 ml PBS (pH 7.4) was rocked for 5 h at room temperature. After centrifugation, the adsorbed serum was stored at −20 °C for later use.
GST-TB protein screening using TB patient sera
The ratio of RLUs of a human serum sample to GST-TBs and the GST control was calculated using the formula: R = (RLUs of GST-TB − RLUs of PBS)/(RLUs of GST − RLUs of PBS). GST-TBs with R ≥ 2 were considered to indicate seropositive reactions.
Indirect ELISA
The 14 identified antigens were expressed in E. coli and purified by Ni-NTA affinity chromatography. The optimal conditions for ELISA (antigen concentration, dilution of human serum sample dilution and HRP-conjugated anti-human IgG secondary antibody dilution) were determined according to the phalanx titration principle.
Selection of antigens for a multiple-antigen system
To overcome the poor diagnostic sensitivity of a single antigen, variable combinations of proteins (range, 2–14) were calculated using the caret package in R statistical software (http://www.r-project.org/).
Other Materials and Methods
A detailed description of methodologies (Cloning and expression of GST-TB fusion proteins, GST-TB arrayed plates, GST-TB protein screening using TB patient sera, Indirect ELISA, etc.) used in this study can be found in the Supplementary materials.
Statistical analysis
Heat maps and analysis of multiple-antigen combinations were performed with R statistical software. Sensitivity and specificity were calculated according to the following formulas: sensitivity = number of true positives/(number of true positive + number of false negatives) and specificity = number of true negatives/(number of false positives + number of true negatives).
Ethics statement
All study procedures were conducted with Internal Review Board approval (Tongji University School of Medicine, China). All participants received information on the aim and procedures of the study and written informed consent was obtained from all subjects.
Additional Information
How to cite this article: Zhou, F. et al. Protein array identification of protein markers for serodiagnosis of Mycobacterium tuberculosis infection. Sci. Rep. 5, 15349; doi: 10.1038/srep15349 (2015).
References
World Health Organization. Global tubeculosis report 2014. (2014) Available at: http://www.who.int/tb/publications/global_report/en/. (Accessed: 28 March 2015).
Dye, C., Scheele, S., Dolin, P., Pathania, V. & Raviglione, M. C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 282, 677–86 (1999).
Zignol, M. et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 194, 479–85 (2006).
Gandhi, N. R. et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 375, 1830–43 (2010).
Zumla, A. et al. Drug-resistant tuberculosis–current dilemmas, unanswered questions, challenges and priority needs. J Infect Dis. 205 Suppl 2, S228–40 (2012).
Siddiqi, K., Lambert, M. L. & Walley, J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 3, 288–96 (2003).
Harboe, M. Antigens of PPD, old tuberculin and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am Rev Respir Dis. 124, 80–7 (1981).
Lawn, S. D. & Zumla, A. I. Tuberculosis. Lancet. 378, 57–72 (2011).
Steingart, K. R. et al. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax. 62, 911–8 (2007).
Pottumarthy, S., Wells, V. C. & Morris, A. J. A comparison of seven tests for serological diagnosis of tuberculosis. J Clin Microbiol. 38, 2227–31 (2000).
Ben Selma, W. et al. Rapid detection of immunoglobulin G against Mycobacterium tuberculosis antigens by two commercial ELISA kits. Int J Tuberc Lung Dis. 14, 841–6 (2010).
Zhang, S. L. et al. Development and evaluation of a novel multiple-antigen ELISA for serodiagnosis of tuberculosis. Tuberculosis (Edinb). 89, 278–84 (2009).
Feng, X. et al. Enhanced serodiagnostic utility of novel Mycobacterium tuberculosis polyproteins. J Infect. 66, 366–75 (2013).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 393, 537–44 (1998).
Camus, J. C., Pryor, M. J., Medigue, C. & Cole, S. T. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 148, 2967–73 (2002).
Steingart, K. R. et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 16, 260–76 (2009).
Steingart, K. R. et al. Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med. 8, e1001062 (2011).
Li, Y. et al. A proteome-scale identification of novel antigenic proteins in Mycobacterium tuberculosis toward diagnostic and vaccine development. J Proteome Res. 9, 4812–22 (2010).
Samanich, K. M. et al. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J Infect Dis. 178, 1534–8 (1998).
Jungblut, P. R. et al. Comparative proteome analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG strains: towards functional genomics of microbial pathogens. Mol Microbiol. 33, 1103–17 (1999).
Samanich, K. M. et al. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 7, 662–8 (2000).
Sonnenberg, M. G. & Belisle, J. T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing and electrospray mass spectrometry. Infect Immun. 65, 4515–24 (1997).
Malen, H., Pathak, S., Softeland, T., de Souza, G. A. & Wiker, H. G. Definition of novel cell envelope associated proteins in Triton X-114 extracts of Mycobacterium tuberculosis H37Rv. BMC Microbiol. 10, 132 (2010).
Bahk, Y. Y. et al. Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics. 4, 3299–307 (2004).
Ben Amor, Y. et al. Immunological characterization of novel secreted antigens of Mycobacterium tuberculosis. Scand J Immunol. 61, 139–46 (2005).
Kunnath-Velayudhan, S. & Porcelli, S. A. Recent Advances in Defining the Immunoproteome of Mycobacterium tuberculosis. Front Immunol. 4, 335 (2013).
Bekmurzayeva, A., Sypabekova, M. & Kanayeva, D. Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis (Edinb). 93, 381–8 (2013).
Kunnath-Velayudhan, S. et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci USA 107, 14703–8 (2010).
Silva, V. M., Kanaujia, G., Gennaro, M. L. & Menzies, D. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int J Tuberc Lung Dis. 7, 478–84 (2003).
Wang, B. L. et al. Antibody response to four secretory proteins from Mycobacterium tuberculosis and their complex antigen in TB patients. Int J Tuberc Lung Dis. 9, 1327–34 (2005).
Sinha, S. et al. Proteome analysis of the plasma membrane of Mycobacterium tuberculosis. Comp Funct Genomics. 3, 470–83 (2002).
Bannantine, J. P., Griffiths, R. S., Viratyosin, W., Brown, W. J. & Rockey, D. D. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell Microbiol. 2, 35–47 (2000).
Li, J. S. & Winslow, G. M. Survival, replication and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect Immun. 71, 4229–37 (2003).
Grenningloh, R., Darji, A., Wehland, J., Chakraborty, T. & Weiss, S. Listeriolysin and IrpA are major protein targets of the human humoral response against Listeria monocytogenes. Infect Immun. 65, 3976–80 (1997).
Xu, X. et al. Serodiagnosis of Schistosoma japonicum infection: genome-wide identification of a protein marker and assessment of its diagnostic validity in a field study in China. Lancet Infect Dis. 14, 489–97 (2014).
Zumla, A., George, A., Sharma, V., Herbert, N. & Baroness Masham of, I. WHO's 2013 global report on tuberculosis: successes, threats and opportunities. Lancet. 382, 1765–7 (2013).
Ramachandran, N. et al. Self-assembling protein microarrays. Science. 305, 86–90 (2004).
Ramachandran, N. et al. Next-generation high-density self-assembling functional protein arrays. Nat Methods. 5, 535–8 (2008).
Anderson, K. S. et al. Application of protein microarrays for multiplexed detection of antibodies to tumor antigens in breast cancer. J Proteome Res. 7, 1490–9 (2008).
Ceroni, A. et al. Systematic analysis of the IgG antibody immune response against varicella zoster virus (VZV) using a self-assembled protein microarray. Mol Biosyst. 6, 1604–10 (2010).
Lyashchenko, K. et al. Heterogeneous antibody responses in tuberculosis. Infect Immun. 66, 3936–40 (1998).
Weldingh, K., Rosenkrands, I., Okkels, L. M., Doherty, T. M. & Andersen, P. Assessing the serodiagnostic potential of 35 Mycobacterium tuberculosis proteins and identification of four novel serological antigens. J Clin Microbiol. 43, 57–65 (2005).
Bothamley, G. H. et al. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J Infect Dis. 159, 549–55 (1989).
Bothamley, G. H., Schreuder, G. M., de Vries, R. R. & Ivanyi, J. Association of antibody responses to the 19-kDa antigen of Mycobacterium tuberculosis and the HLA-DQ locus. J Infect Dis. 167, 992–3 (1993).
Kunnath-Velayudhan, S. & Gennaro, M. L. Immunodiagnosis of tuberculosis: a dynamic view of biomarker discovery. Clin Microbiol Rev. 24, 792–805 (2011).
Rangaka, M. X. et al. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 12, 45–55 (2012).
Morris, K. WHO recommends against inaccurate tuberculosis tests. Lancet. 377, 113–4 (2011).
Yin, X. et al. Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: a meta-analysis. J Infect. 67, 369–77 (2013).
Bendtsen, J. D., Nielsen, H., von Heijne, G. & Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 340, 783–95 (2004).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 305, 567–80 (2001).
Behr, M. A. et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 284, 1520–3 (1999).
Muttucumaru, D. G., Roberts, G., Hinds, J., Stabler, R. A. & Parish, T. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb). 84, 239–46 (2004).
Albrethsen, J. et al. Proteomic profiling of Mycobacterium tuberculosis identifies nutrient-starvation-responsive toxin-antitoxin systems. Mol Cell Proteomics. 12, 1180–91 (2013).
World Health Organization. Guidelines for treatment of tuberculosis, fourth edition. (2009) Available at: http://www.who.int/tb/publications/2010/9789241547833/en/. (Accessed: 28 March 2014).
Acknowledgements
This work was supported by National S & T Program (2013ZX10003007) in China. We thank Prof. Zhongyi Hu from Shanghai Key Lab of Tuberculosis, Shanghai Pulmonary Hospital for invaluable help and advice; we also thank Mr. Zhonghua Liu for serum samples.
Author information
Authors and Affiliations
Contributions
F.Z., X.X. and W.P. conceived and designed the experiments. F.Z., S.W. and X.C. performed the experiments. F.Z., X.X., L.F. and W.P. analyzed the data. L.F. collected the samples. F.Z., X.X. and W.P. drafted the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhou, F., Xu, X., Wu, S. et al. Protein array identification of protein markers for serodiagnosis of Mycobacterium tuberculosis infection. Sci Rep 5, 15349 (2015). https://doi.org/10.1038/srep15349
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep15349
This article is cited by
-
Evaluation of immunodominant peptides of in vivo expressed mycobacterial antigens in an ELISA-based diagnostic assay for pulmonary tuberculosis
Brazilian Journal of Microbiology (2023)
-
Ribokinase screened from T7 phage displayed Mycobacterium tuberculosis genomic DNA library had good potential for the serodiagnosis of tuberculosis
Applied Microbiology and Biotechnology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.