Abstract
Calnexin (CANX) and calreticulin (CALR) chaperones mediate nascent glycoprotein folding in the endoplasmic reticulum. Here we report that these chaperones have distinct roles in male and female fertility. Canx null mice are growth retarded but fertile. Calr null mice die during embryonic development, rendering indeterminate any effect on reproduction. Therefore, we conditionally ablated Calr in male and female germ cells using Stra8 (mcKO) and Zp3 (fcKO) promoter-driven Cre recombinase, respectively. Calr mcKO male mice were fertile, but fcKO female mice were sterile despite normal mating behavior. Strikingly, we found that Calr fcKO female mice had impaired folliculogenesis and decreased ovulatory rates due to defective proliferation of cuboidal granulosa cells. Oocyte-derived, TGF-beta family proteins play a major role in follicular development and molecular analysis revealed that the normal processing of GDF9 and BMP15 was defective in Calr fcKO oocytes. These findings highlight the importance of CALR in female reproduction and demonstrate that compromised CALR function leads to ovarian insufficiency and female infertility.
Similar content being viewed by others
Introduction
Proper folding in the endoplasmic reticulum (ER) is a prerequisite for correct localization and function of most secreted and transmembrane proteins1. Failures in protein folding and quality control compromise cellular functions and cause disease including amyloidosis, cystic fibrosis and diabetes2,3. Failure in ER quality control also results in male infertility4,5,6. In the ER, soluble calreticulin (CALR) and membrane bound calnexin (CANX) were originally discovered as homologous calcium binding proteins and later shown to be lectin like chaperones that chiefly mediate nascent glycoprotein folding7,8,9. Despite their extensive homology, CALR and CANX have contrasting functions. For example, proteins in Calr−/− cells have accelerated folding with an accumulation of misfolded proteins whereas folding is significantly impaired in Canx−/− cells10. Differences in CALR and CANX function are further reflected in the distinct phenotypes of knock-out mice. Calr−/− mice are embryonic lethal due to defective heart development whereas Canx−/− mice are viable but growth retarded with neurological deficits11,12,13. However, the client specificity of calnexin/calreticulin in vivo has not been fully established.
We have previously demonstrated that calmegin (CLGN) and calsperin (CALR3) are testis specific homologues of CANX and CALR, respectively and are expressed in the ER of spermatogenic cells4,5,14. CLGN mediates the heterodimerization of ADAM1A/ADAM2 that is required for the maturation of ADAM3, a sperm membrane protein. In contrast to this indirect activity, CALR3 binds directly to ADAM3 and regulates its maturation. Although a pseudogene in humans15, mouse ADAM3 is essential for sperm migration from the uterus into the oviduct and Adam3 null male mice are sterile16,17. Both Clgn and Calr3 null mice lack ADAM3 on their sperm surface and null males are infertile. However, other membrane and secretory proteins are normally present in these mutant spermatozoa, indicating the importance of CLGN/CALR3 rather than CANX/CALR, for the maturation of ADAM3 related proteins4,5. The restricted client specificity of CLGN and CALR3 is further highlighted by the fact that the N-glycosylated sperm membrane fusion protein IZUMO1 is functionally presented on Clgn/Calr3 mutant spermatozoa18.
CLGN and CALR3 are not expressed in the ovary and thus female germ cells rely on the CANX/CALR system for quality control of nascent proteins in the ER. During oogenesis, oocytes secrete many factors that regulate the growth and differentiation of granulosa cells, including GDF9 and BMP15. These two proteins are structurally complex with extensive disulfide-bonds and N-linked glycans. Mutations that alter their ability to fold have been implicated as causes of premature ovarian failure19,20,21,22. Gdf9−/− and Bmp15−/− females are sterile or subfertile, respectively23,24 and a recent paper has reported the physiological importance of the GDF9:BMP15 heterodimer25.
In the present study, we have established mouse lines in which either Canx or Calr has been ablated to investigate their roles in male and female germ cell development and function. Although growth retardation was observed, both male and female Canx knockout mice were fertile. For CALR, we generated male and female germ cell specific knockout mice to circumvent the embryonic lethality. Whereas CALR was dispensable for spermatogenesis and sperm fertilizing ability, it was required in the oocyte for the maturation of the TGF-beta family proteins, GDF9 and BMP15, as well as the subsequent development of the cumulus oocyte complex (COC). Our results highlight the importance of CALR for female reproduction and suggest that compromised CALR function can lead to ovarian insufficiency and female sterility.
Results
Generation of conditional knockout (KO) mice for Canx and Calr genes
Mice with genetically disrupted Canx and Calr genes are growth retarded or die during embryonic development, respectively11,12,13. Thus, we generated male (mcKO) and female (fcKO) germ cell-specific conditional knockout mice to investigate the role of these genes in fertility. We first generated mice carrying floxed alleles (fl/+) using gene targeting vectors that floxed exons 3 and 4 for Canx and exons 4–7 for Calr (Supplementary Fig. S1A and Supplementary Fig. S2A, related to Fig. 1). The correct gene targeting event in embryonic stem cells and subsequent germ-line transmission was confirmed by PCR analysis (Supplementary Fig. S1B and Supplementary Fig. S2B). To remove the floxed exons in male and female germ cells, we used transgenic mouse lines expressing Cre recombinase under the Stra8 and Zp3 promoters, respectively26,27. Homozygous knockout (−/−) mice were generated by crossing heterozygous mutant (+/−) mice.
Abnormal ovarian follicular development in Calr fcKO mice.
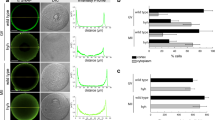
(A) Pregnancy rate (pregnancy/vaginal plug formation) obtained by mating control (Calrfl/+; Zp3-cre), Calr fcKO (Calrfl/−; Zp3-cre) and Canx fcKO (Canxfl/−; Zp3-cre) females with B6D2F1 wild-type male mice. The total number of plugs observed is indicated in parentheses. (B) Average litter sizes obtained by mating control, Calr fcKO and Canx fcKO females with B6D2F1 wild-type male mice. (C–F) Histological analysis of ovarian sections. Preovulatory follicles from Calr fcKO mice demonstrate immature follicles just under the surface of the ovary. The cumulus mass surrounding oocytes is composed of fewer cells than control. Scale bar = 300 μm (C,E) and 50 μm (D,F). Error bars represent standard deviation (B).
Fertility in Canx knockout mice
When we crossed Canx+/− females with Canx+/− males, Canx−/− mice were born in expected Mendelian ratios (+/+:+/−:−/−=14:27:16, n = 8 litters from 3 breeding pairs). However, 50% of the homozygous null pups died within 48 hours and very few survived to three months, as reported previously12. Similar postnatal lethality was not observed in Canx gene trapped mice despite the complete absence of CANX protein13. Paradoxically, truncated CANX protein was present in one of the two lines in the earlier report with the more severe phenotype. Using antibodies against the N- and C- terminal residues, CANX protein was not detected in our −/− mice (Supplementary Fig. S1D). Therefore the variance in postnatal viability reported earlier may reflect the combined effect of CANX deficiency and other factors, including genetic background and animal husbandry.
To examine Canx−/− mouse fertility, adult (8 week-old) Canx−/− females and males were mated with wild-type (+/+) males and females, respectively. Although the testis size was smaller in Canx−/− male mice, the ratio of testis weight to body weight was comparable to that of wild-type (0.37 ± 0.03% and 0.39 ± 0.06%, respectively) and normal spermatogenesis was observed (Supplementary Fig. S3, related to Fig. 1). Canx−/− males were fertile and average litter sizes were 7.1 ± 2.2 and 7.5 ± 0.8 (avg. ± s.d. pups, n = 6 litters from 3 males) in normal and null mice respectively.
Canx−/− females were also fertile, but litter sizes were smaller (3.3 ± 1.5 pups, n = 4 litters from 4 females) possibly due to their smaller body size. We produced oocyte specific Canx fcKO mice by introducing the ZP3-cre transgene (Canxfl/−; Zp3-cre). Mice were genotyped by PCR analysis and the lack of CANX protein was confirmed by immunoblot (Supplementary Fig. S1C, related to Fig. 1). Canx fcKO female mice had normal fertility when mated with wild type males (8.4 ± 2.6 pups, n = 9 litters from 3 females) (Fig. 1A,B). All 76 pups carried the knockout allele, which confirmed successful excision of the floxed exons by the Zp3 promoter driven Cre recombinase. Therefore, we conclude that CANX is not required for either male or female reproduction.
Female infertility in Calr conditional knockout mice
It was reported that the Stra8 promoter driven Cre recombinase was expressed at the postnatal day 3 in early-stage spermatogonia and the recombination efficiency was >95%26. In the present study, with our Stra8-cre transgenic line, Calr disruption was confirmed in most (84.2%, 219/260) of the testicular germ cells as determined by immunostaining (Supplementary Fig. S4A and B, related to Fig. 1). When mated with normal female mice, Calr mcKO males had comparable fertility (10.1 ± 1.5 pups, n = 26 litters from 6 males) with normal male mice (10.6 ± 1.3 pups, n = 8 litters from 3 males). Whereas some pups inherited the floxed allele, the majority (82.1%, 215/262) of the offspring inherited the knockout allele, which was consistent with the aforementioned observations in testicular germ cells.
We next used ZP3-cre transgenic lines to disrupt the Calr gene during oogenesis (Supplementary Fig. S2C and Supplementary Fig. S4C, related to Fig. 1). Whereas control female mice (Calrfl/+; Zp3-cre) had normal fertility, Calr fcKO female mice had a profound decrease in fecundity (Fig. 1A,B). Only 2 litters with 1 pup each were obtained from 16 copulations with 4 Calr fcKO females, whereas 10 litters from 10 copulations were obtained with 4 control females. The average litter sizes were 0.1 ± 0.3 and 8.4 ± 2.6 pups, respectively. When we superovulated Calr fcKO females with gonadotropins, successful copulation was observed. Thus, the female infertility was not caused by disrupted mating behavior. However, ovulation was severely impaired in the Calr fcKO female mice (Table 1).
To elucidate the cause of infertility in the Calr fcKO females, we superovulated 3 (Fig. 1C–F) or 12 (Supplementary Fig. S5C–H) week-old female mice and prepared ovarian sections 2 hours before the anticipated time of ovulation. In Calr fcKO mice, the ovary was smaller, possibly due to defective folliculogenesis (Table 2). A few preovulatory follicles appeared, but most of the follicles at the surface of the ovary were immature. Follicular development was arrested at the early antral stage, the number of cumulus cells that surrounded an oocyte after germinal vesicle breakdown in cross sections was reduced (33.1 ± 13.6 in fcKO and 141.6 ± 30.5 in control) and cumulus expansion was impaired (Fig. 1C–F and Supplementary Fig. S5A–H). Corpora lutea were rarely observed in Calr fcKO ovaries. Because antral follicles were present, we assayed in vitro maturation and in vitro fertilization. Comparable numbers of oocytes were collected from control and Calr fcKO mice 46–48 hours after PMSG injection. Both control and mutant oocytes underwent germinal vesicle breakdown (GVBD) and matured to the metaphase II (MII) stage in vitro. The oocytes collected from the Calr fcKO had slightly larger diameters than those collected from control mice (77.6 ± 0.3 μm in fcKO and 73.5 ± 0.4 μm in control, P < 0.01), as reported in Gdf9 KO mice23. Although the efficiency was lower than in controls, these MII eggs could be fertilized and developed to term after transfer into pseudopregnant females (Table 3).
CALR mediated quality control of GDF9 and BMP15
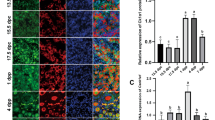
Calr fcKO had impaired ovulation with defects in cumulus expansion. GDF9 and BMP15 are two growth factors that are secreted from oocytes and stimulate cumulus cell expansion23,28. To examine the effect of CALR disruption on the production of these proteins, primary mouse embryonic fibroblast (MEF) cells were prepared from Calr+/+ and Calr−/− mice and transfected with mouse Gdf9 or Bmp15 expression vectors. Using co-immunoprecipitation, we confirmed that CALR associates with GDF9 and BMP15 in wild-type cells (Fig. 2A). In Calr+/+ MEFs, GDF9 and BMP15 were secreted into the extracellular fluid for 4–5 days, whereas in Calr−/− MEFs, secretion stopped within 1–2 days (Fig. 2B). When carefully observed, GDF9 proproteins appeared as a doublet on SDS-PAGE29, but the upper band was not present in Calr−/− cell supernatants (Fig. 2C). Recovery of the doublet was observed after co-transfection of a Calr expression vector. Similar results were observed for BMP15. To examine whether GDF9 is expressed and/or secreted in Calr fcKO oocytes, we collected 300 oocytes and examined their lysates by immunoblot (Fig. 2D). Whereas comparable amounts of GDF9 proproteins (57 kDa) were observed in control and fcKO oocytes, mature type GDF9 (17 kDa) was detected in control, but not in CALR deficient oocytes. There were comparable amounts of other fertilization related N-glycoproteins, including ZP3 and CD9.
CALR is essential for extracellular secretion of GDF9 and BMP15.
(A) Calr+/+ or Calr−/− MEFs expressing Flag/His tagged GDF9 or BMP15 were immunoprecipitated (IP) with anti-CALR antibody from the cell lysates (50 μg) and the immunoprecipitates were probed with anti-His antibody on immunoblots. (B) Secreted GDF9 and BMP15 from Calr+/+ or Calr−/− MEFs were examined every 24 hours after transfection. Calr−/− MEFs stopped secreting these proteins after 1–2 days. (C) Secreted GDF9 and BMP15 were both observed as a single band in Calr−/− MEFs. The upper band was recovered by co-transfection with a plasmid expressing Calr. (D) The 57 kDa GDF9 proprotein was present, but not the 17.5 kDa mature GDF9 in CALR deficient oocytes. Other glycoproteins, CD9 and ZP3, were normally expressed. 300 oocytes (GDF9) or 20 oocytes (ZP3 and CD9) were loaded per lane.
Complementation of defective GOC (granulosa-oocyte complex) development by recombinant GDF9 and BMP15
External addition of recombinant BMP15 or GDF9 improves cumulus cell proliferation, differentiation and steroidogenesis in Bmp15/Gdf9 null mice30,31. In the present study, we examined whether GDF9 and BMP15 supplements could restore the defective GOC development in Calr fcKO mice using an in vitro follicle culture system. Without supplementation, granulosa cells proliferated and the size of the follicles gradually increased when CALR was present. In Calr fcKO GOC, the follicle size did not increase compared to controls (Fig. 3). When the GOC growth medium was supplemented with either recombinant GDF9 or BMP15, both factors significantly enhanced Calr fcKO GOC growth. Conditioned medium from cells expressing both GDF9 and BMP15 did not show a synergistic effect.
Recombinant GDF9 and BMP15 partially restored follicular development of Calr cKO.
(A) Control and Calr fcKO follicles were collected from 12 day-old mice and cultured in vitro for 9 days. In Calr fcKO mice, the addition of GDF9 or BMP15 partially promoted granulosa cell proliferation and increased the follicular volume of Calr fcKO. (B) The average follicular volumes from three independent experiments were determined. Error bars represent standard error of the mean. Columns with the same letter are not significantly different (P > 0.05). (n = 43, 39, 43, 38 and 12 for Control (Calrfl/+; Zp3-cre), Calr fcKO, Calr fcKO+GDF9, Calr fcKO+BMP15 and Calr cKO+GDF9/BMP15, respectively).
Discussion
Although CANX is not essential for mouse development in vivo12,13, it has been difficult to investigate its role in the reproductive system since few Canx KO mice survived to adulthood and they were smaller than their littermates. In the present study, we showed that Canx KO males were able to copulate and successfully impregnate females. Canx KO females showed reduced litter size, but oocyte specific disrupted cKO females were fully fertile. Therefore we conclude that CANX is dispensable for both male and female reproductive systems.
We next examined the role of CALR, the soluble homologue of CANX, in the reproductive system. Calr KO mice are embryonic lethal but ectopic expression of calcineurin in the heart enabled Calr KO mice to survive until adulthood32. However, the surviving mice exhibited growth retardation, hampering any study of their reproduction. Here, we generated male germ cell specific cKO mice and showed that CALR is dispensable for spermatogenesis and sperm fertilizing ability. Although CANX and CALR have contrasting functions in other tissues12,32, their major roles may be redundant and complementary to each other, at least in developing male germ cells. In contrast, the homologues of CANX and CALR, CLGN and CALR3, respectively, have different substrate specificity and both, albeit by different mechanisms, are required for fertilization by controlling ADAM3 presentation on the sperm surface4,5. Thus, the present study reinforces the uniqueness and importance of the CLGN/CALR3 ER chaperone system in the male reproductive system.
In the female reproductive system, we have shown that CALR has a novel and indispensable role in COC development and female fertility. Since GDF9 and BMP15 are secreted proteins, soluble CALR might be more accessible to these molecules than membrane-tethered CANX. However it is also reported that soluble CANX and CALR have different substrate specificity33 and an in-depth analysis of the molecular interactions of these chaperones with these two growth factors requires future investigation. Our data indicated CALR plays an important role in regulating the folding of GDF9 and BMP15 in the ER. The folding defects could cause various effects, such as protein instability, secretion defects, abnormal cleavage or aberrant post-translational modifications of GDF9 and BMP15 at later stages of the secretory pathway.
In the present study we could not detect mature GDF9 and BMP15 proteins in MEF cells. The defect in the processing machinery may cause different behaviors in unfolded GDF9 and BMP15 in MEF and oocytes. It has been reported that the mature recombinant mouse BMP15 is not processed in HEK 293T cells and CHO cells34. Further, the post-translational processing of BMP15 is precisely regulated during meiosis and the mature BMP15 can be detected 9 hours after the hCG injection in mice28. These studies imply that oocytes may have a unique post-translational processing mechanism for GDF9 and BMP15.
The follicular development in the Gdf9 KO is arrested at the primary stage and the Bmp15 KO showed normal folliculogenesis despite decreased ovulation and rates of fertilization23,24. However, follicular development in Calr fcKO mice arrested at the early antral stage. In Calr fcKO mice, GDF9 proprotein is expressed in oocytes, but not cleaved. In rat, macaque and human, GDF9 proprotein or BMP15 is detected in the follicular fluid35,36. In addition, mutation in the protease cleavage site of GDF9 causes female infertility in ewes37. The hypoplastic ovaries of homozygous mutated ewe lambs contain large numbers of primordial follicles and developing follicles up to the early antral stage. These data suggest that the proprotein of GDF9 accounts for the different phenotypes in Gdf9 KO and Calr fcKO mice.
The mechanism of COC development has been well investigated and many key factors have been identified in both oocyte and surrounding granulosa cells38,39. Among these factors, GDF9 and BMP15 are secreted from developing oocytes and stimulate granulosa cell proliferation and expansion24,31,40. GDF9 and BMP15 have a unique disulfide bond arrangement among TGF-beta family proteins41,42,43 and showed different mobility in SDS electrophoresis under reducing or non-reducing conditions31,44, indicating the importance of disulphide bond formation during their proper folding, targeting and function. Here we showed that CALR interacts with GDF9 as well as BMP15 and the lack of CALR compromised their secretion. This is reminiscent of the misfolding and disappearance of ADAM3 in Calr3 knockout male mice. In testis, CALR3 recruits PDILT (protein disulfide isomerase like in testis) and assists in the quality control of ADAM36. From studies of other nascent glycoproteins folding in somatic cells45,46,47, CALR may cooperate with PDIA3 (ERP57) to regulate the quality control of GDF9/BMP15.
GDF9 and BMP15 play important roles in energy metabolism and/or cholesterol biosynthesis in granulosa cells25,48,49. Because TGF-beta/Smad3 signaling regulates glucose and energy homeostasis and Smad3 KO mice exhibit improved glucose tolerance and enhanced glucose-stimulated insulin secretion from pancreatic islet beta cells50,51, it will be interesting to investigate the role of CALR in the secretion of TGF-beta family proteins in other tissues. That might also explain why Calr KO mice, transgenically rescued from embryonic lethality, still die mainly due to metabolic failure32. Of interest, it was also reported that TGF-beta stimulates cells and induces extracellular matrix secretion that depends on CALR mediated Ca2+ signaling52. Thus the ability of CALR to regulate calcium availability might also be important downstream of the TGF-beta signaling pathway. In the present study, we could not fully recover GOC development with recombinant GDF9/BMP15 (Fig. 3), implying that there are other CALR dependent factors required for complete oocyte development.
In conclusion, we document that CALR plays an indispensable role in the COC development by controlling the maturation of the TGF-beta proteins GDF9 and BMP15. Because GDF9 and BMP15 are known to be involved in premature ovarian failure and polycystic ovarian syndrome22,53,54,55, targeting CALR and the ER quality control system in follicular development should be considered as a novel avenue for female infertility treatment.
Methods
Animal experimentation
All animal experiments were carried out in accordance with the protocols approved by the Animal Care and Use Committee of the Research Institute for Microbial Diseases, Osaka University. The Stra8-cre mouse line was established in our laboratory by injecting a transgene that was constructed by replacing CAG promoter in the pCX-cre vector with the Stra8 promoter56. The Zp3-cre mouse line was obtained from the Jackson laboratory27.
Generation of KO and cKO mice
Targeting vectors were constructed by placing Canx genomic fragments (1.7 kbp, 2.0 kbp and 4.0 kbp) and Calr genomic fragments (2.0 kbp, 1.4 kbp and 3.5 kbp) into pNT1.157. D3 embryonic stem cells were electroporated with the NotI-linearized targeting vector. After G418/GANC selection, drug resistant clones (3/96 for Canx and 5/672 for Calr) with homologous recombinants (tm1a) were identified by PCR analysis. Three targeted clones were injected into C57BL/6 blastocysts, resulting in the birth of coat-color chimeric male mice. The neomycin-resistant cassette was removed by crossing with transgenic mice expressing FLPe under the CAG promoter and this allowed the generation of mice with the conditional floxed (fl or tm1b) allele (Canxfl/+ or Calrfl/+). These mice were crossed with transgenic mice expressing Cre under the CAG promoter to generate Canx+/− or Calr+/− mice (- or tm1c). Canx−/− or Calr−/− mice were generated by mating with each heterozygous mutated mouse that did not contain CAG-FLPe and CAG-Cre. To generate cKO mice, Canxfl/+ or Calrfl/+ mice were crossed with transgenic mice expressing Cre under the control of Stra8 or Zp3 promoters. For Canx fcKO, Canxfl/+; Zp3-cre mice were crossed with Canxfl/− to produce mice with the following genotype: Canxfl/+; Zp3-cre (for control) and Canxfl/−; Zp3-cre (for fcKO). Calr cKO were generated through the same breeding strategy as Canx cKO using Stra8-cre or Zp3-cre transgenic mice. Pr-FlpeF; 5′- ccacctaaggtcctggttcgtcagtttgtg -3′ and pr-FlpeR; 5- atacaagtggatcgatcctaccccttgcgc -3′ primers were used for the Flpe transgene in addition to the primers indicated in Figure S1 and Figure S2.
Histology of the ovary
Female mice were injected with pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) at 48 hour intervals. Ovaries were collected at 10 hours after hCG injection and fixed with 4% paraformaldehyde/PBS overnight. Then the ovaries were embedded into glycol methacrylate (Technobit 8100: Heraeus Kulzer, Germany) after treatment with a graded ethanol series. Plastic thin sections (5 μm) were stained with hematoxylin and eosin. For Periodic acid Schiff (PAS) staining, sections were rehydrated and treated with 2% metaperiodic acid for 15 minutes, followed by treatment with Schiff’s reagent (Wako, Japan) for 20 minutes. The sections were stained with hematoxylin prior to imaging.
Immunohistochemistry
Ovaries were collected from adult mice and fixed in 4% (wt/vol) paraformaldehyde/PBS overnight at 4 °C, cryopreserved in graded 10–30% (wt/vol) sucrose and embedded in TissueTek OCT compound (Sakura Finetechnical). Frozen sections (5 μm) were mounted on APS (aminosilane)-coated glass slides. After washing with PBS, slides were blocked with 10% (vol/vol) newborn calf serum (NBCS)/PBS for 1 hour and incubated with anti CALR antibody in 10% (vol/vol) NBCS/PBS at 4 °C overnight. After washing with PBS, the slides were incubated with secondary antibodies with Alexa Fluor 488 (Invitrogen) in 10% (vol/vol) NBCS/PBS for 2 hours. After washing with PBS, the slides were imaged with an Olympus IX-70 fluorescence microscope.
In vitro maturation and fertilization
For in vitro maturation58, immature GV (germinal vesicle) oocytes were collected from ovaries 46 hours after injection with PMSG. Antral follicles were punctured with 26G needles in FHM59 with 100 μM dibutyryl-cyclic AMP (Sigma). After dissociating the cumulus cells by pipetting, oocytes were washed 3 times and cultured in Minimum Essential Medium Alpha (GIBCO) with 3 mg BSA (A3311, Sigma). After 14 hours, partial zona dissection of MII oocytes was performed using a piezo-micromanipulator with a glass capillary needle (diameter of 5–10 μm)60 and incubated in TYH medium61 with 2 × 105/ml B6D2F1 capacitated sperm. Fertilization was determined by formation of two-cell embryos, which were then transferred into the oviduct of pseudopregnant mice.
Immunoblot and immunoprecipitation
Oocytes were collected from ovaries 46 hours after PMSG injection. Cumulus or granulosa cells were dissociated using glass pipettes and collected oocytes were boiled in SDS-PAGE sample buffer. Proteins were separated by SDS-PAGE and run under reducing conditions. Immunoprecipitation was performed as described5. Antibodies used included rabbit antisera against CAXN and CALR5, rabbit antibody against the N-terminus of CAXN (sc-11397, Santa Cruz Biotechnology), penta-His mouse monoclonal antibody (Qiagen), horseradish peroxidase conjugated goat anti-rabbit IgG and goat anti-mouse IgG antibodies (Jackson Immuno Research Laboratories); and a GDF9 monoclonal antibody62, kindly provided by Dr. Martin M. Matzuk.
Preparation of Calr deficient mouse embryonic fibroblasts (MEF)
MEFs were isolated from 13.5–14.5 day-old embryos. The head and all internal organs were removed from the embryo. The remaining tissue was minced with scissors and incubated in 0.25% trypsin containing penicillin (100 U/ml, Nacalai) and streptomycin (100 μg/ml, Nacalai) for 10–20 min. The cells were pipetted and plated onto a 10 cm tissue culture dish in culture medium [Dulbecco’s Modified Eagle Medium (DMEM, GIBCO) with 10% FCS, penicillin (100 U/ml) and streptomycin (100 μg/ml)]. The next day, the medium was replaced and cells were cultured at 37 °C until confluent, when the cells were harvested and frozen.
Production of recombinant GDF9 and BMP15
The HIV-1-based self-inactivating-type lentiviral vector plasmid pLV-EGFP was constructed by replacing the EGFP cDNA with mouse GDF9 or BMP15 cDNA, respectively and isolated for infection of HEK293T cells63.
In vitro follicle culture
Ovaries were collected from 12 day-old mice from which follicles were mechanically isolated prior to in vitro culture64. Each follicle was placed in a 30 μl droplet of DMEM (GIBCO) with 10% FCS, penicillin (100 U/ml) and streptomycin (100 μg/ml). The droplets were placed in 60 mm uncoated culture dishes (Iwaki) and covered with paraffin oil (Nacalai). After overnight culture, half of the culture medium was replaced by HEK293T cell-conditioned medium containing recombinant GDF9 or BMP15. Culture medium from non-transfected HEK293T cells was used as a negative control. Half of the medium was replaced every other day. Follicle volumes were calculated as described65.
Purification of His tagged GDF9 or BMP15 secreted into MEF cell media
Mouse Gdf9 or Bmp15 cDNA tagged with Flag/Hisx6 at the C-terminus was inserted into a pNCAG vector consisting of the CAG-promoter and rabbit β globin poly-adenylation signal, respectively56. Calr+/+ or Calr−/− MEF cells were cultured in 6-well plates and transfected with pNCAG-Gdf9 Flag/His or pNCAG-Bmp15 Flag/His using lipofectamine LTX (Invitrogen). After 18 hours, cell debris was removed by two washes in PBS (GIBCO) and the medium was replaced [DMEM with 3% FCS, penicillin (100 U/ml) and streptomycin (100 μg/ml)]. The culture medium was collected every 24 hours for 4 days. Purification of His tagged recombinant protein in the culture medium was performed using μMACS anti-His Isolation Kit (Miltenyi Biotec). Two milliliters of culture medium were used for purification and the proteins were eluted into 70 μl.
Statistical analysis
The values were the means ± standard deviation or standard error of the mean from at least three independent experiments. Statistical analyses were performed using Student’s t-test. Differences were considered significant at P < 0.05.
Additional Information
How to cite this article: Tokuhiro, K. et al. Calreticulin is required for development of the cumulus oocyte complex and female fertility. Sci. Rep. 5, 14254; doi: 10.1038/srep14254 (2015).
References
Braakman, I. & Bulleid, N. J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 80, 71–99 (2011).
Cohen, F. E. & Kelly, J. W. Therapeutic approaches to protein-misfolding diseases. Nature 426, 905–909 (2003).
Gregersen, N., Bross, P., Vang, S. & Christensen, J. H. Protein misfolding and human disease. Annu Rev Genomics Hum Genet 7, 103–124 (2006).
Ikawa, M. et al. The putative chaperone calmegin is required for sperm fertility. Nature 387, 607–611 (1997).
Ikawa, M. et al. Calsperin is a testis-specific chaperone required for sperm fertility. J Biol Chem 286, 5639–5646 (2011).
Tokuhiro, K., Ikawa, M., Benham, A. M. & Okabe, M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]. Proc Natl Acad Sci USA 109, 3850–3855 (2012).
Helenius, A. & Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73, 1019–1049 (2004).
Molinari, M. N-glycan structure dictates extension of protein folding or onset of disposal. Nat Chem Biol 3, 313–320 (2007).
Aebi, M., Bernasconi, R., Clerc, S. & Molinari, M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci 35, 74–82 (2010).
Molinari, M. et al. Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control. Mol Cell 13, 125–135 (2004).
Coppolino, M. G. et al. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature 386, 843–847 (1997).
Denzel, A. et al. Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol Cell Biol 22, 7398–7404 (2002).
Kraus, A. et al. Calnexin deficiency leads to dysmyelination. J Biol Chem 285, 18928–18938 (2010).
Watanabe, D. et al. Molecular cloning of a novel Ca(2+)-binding protein (calmegin) specifically expressed during male meiotic germ cell development. J Biol Chem 269, 7744–7749 (1994).
Frayne, J. & Hall, L. The gene for the human tMDC I sperm surface protein is non-functional: implications for its proposed role in mammalian sperm-egg recognition. Biochem J 334, (Pt 1), 171–176 (1998).
Shamsadin, R. et al. Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod 61, 1445–1451 (1999).
Yamaguchi, R. et al. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod 81, 142–146 (2009).
Inoue, N., Ikawa, M. & Okabe, M. Putative sperm fusion protein IZUMO and the role of N-glycosylation. Biochem Biophys Res Commun 377, 910–914 (2008).
Zhao, H. et al. Analyses of GDF9 mutation in 100 Chinese women with premature ovarian failure. Fertil Steril 88, 1474–1476 (2007).
Dixit, H. et al. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause 12, 749–754 (2005).
Di Pasquale, E., Beck-Peccoz, P. & Persani, L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 75, 106–111 (2004).
Otsuka, F., McTavish, K. J. & Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev 78, 9–21 (2011).
Dong, J. et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383, 531–535 (1996).
Yan, C. et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15, 854–866 (2001).
Peng, J. et al. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA 110, E776–785 (2013).
Sadate-Ngatchou, P. I., Payne, C. J., Dearth, A. T. & Braun, R. E. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 46, 738–742 (2008).
de Vries, W. N. et al. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis 26, 110–112 (2000).
Yoshino, O., McMahon, H. E., Sharma, S. & Shimasaki, S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci USA 103, 10678–10683 (2006).
Gilchrist, R. B. et al. Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biol Reprod 71, 732–739 (2004).
Sugiura, K. et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 134, 2593–2603 (2007).
Hayashi, M. et al. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology 140, 1236–1244 (1999).
Guo, L. et al. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem 277, 50776–50779 (2002).
Wada, I., Imai, S., Kai, M., Sakane, F. & Kanoh, H. Chaperone function of calreticulin when expressed in the endoplasmic reticulum as the membrane-anchored and soluble forms. J Biol Chem 270, 20298–20304 (1995).
Hashimoto, O., Moore, R. K. & Shimasaki, S. Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci USA 102, 5426–5431 (2005).
Gode, F. et al. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil Steril 95, 2274–2278 (2011).
Duffy, D. M. Growth differentiation factor-9 is expressed by the primate follicle throughout the periovulatory interval. Biol Reprod 69, 725–732 (2003).
Souza, C. J., McNeilly, A. S., Benavides, M. V., Melo, E. O. & Moraes, J. C. Mutation in the protease cleavage site of GDF9 increases ovulation rate and litter size in heterozygous ewes and causes infertility in homozygous ewes. Anim Genet 45, 732–739 (2014).
Juengel, J. L. & McNatty, K. P. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 11, 143–160 (2005).
Scaramuzzi, R. J. et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev 23, 444–467 (2011).
Otsuka, F. et al. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem 275, 39523–39528 (2000).
McPherron, A. C. & Lee, S. J. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem 268, 3444–3449 (1993).
Laitinen, M. et al. A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech Dev 78, 135–140 (1998).
Dube, J. L. et al. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 12, 1809–1817 (1998).
Li, Q., Rajanahally, S., Edson, M. A. & Matzuk, M. M. Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15. Mol Hum Reprod 15, 779–788 (2009).
Oliver, J. D., van der Wal, F. J., Bulleid, N. J. & High, S. Interaction of the thiol-dependent reductase ERp57 with nascent glycoproteins. Science 275, 86–88 (1997).
Hughes, E. A. & Cresswell, P. The thiol oxidoreductase ERp57 is a component of the MHC class I peptide-loading complex. Curr Biol 8, 709–712 (1998).
Morrice, N. A. & Powis, S. J. A role for the thiol-dependent reductase ERp57 in the assembly of MHC class I molecules. Curr Biol 8, 713–716 (1998).
Sugiura, K., Pendola, F. L. & Eppig, J. J. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 279, 20–30 (2005).
Su, Y. Q. et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135, 111–121 (2008).
Lin, H. M. et al. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem 284, 12246–12257 (2009).
Yadav, H. et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab 14, 67–79 (2011).
Zimmerman, K. A., Graham, L. V., Pallero, M. A. & Murphy-Ullrich, J. E. Calreticulin regulates transforming growth factor-beta-stimulated extracellular matrix production. J Biol Chem 288, 14584–14598 (2013).
Simpson, J. L. Genetic and phenotypic heterogeneity in ovarian failure: overview of selected candidate genes. Ann N Y Acad Sci 1135, 146–154 (2008).
Rossetti, R. et al. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat 30, 804–810 (2009).
Tiotiu, D. et al. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod 25, 1581–1587 (2010).
Matsumura, H., Hasuwa, H., Inoue, N., Ikawa, M. & Okabe, M. Lineage-specific cell disruption in living mice by Cre-mediated expression of diphtheria toxin A chain. Biochem Biophys Res Commun 321, 275–279 (2004).
Fujihara, Y. et al. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J Cell Sci 123, 1531–1536 (2010).
Miki, H., Ogonuki, N., Inoue, K., Baba, T. & Ogura, A. Improvement of cumulus-free oocyte maturation in vitro and its application to microinsemination with primary spermatocytes in mice. J Reprod Dev 52, 239–248 (2006).
Lawitts, J. A. & Biggers, J. D. Culture of preimplantation embryos. Methods Enzymol 225, 153–164 (1993).
Yamagata, K. et al. Sperm from the calmegin-deficient mouse have normal abilities for binding and fusion to the egg plasma membrane. Dev Biol 250, 348–357 (2002).
Toyoda, Y., Yokoyama, M. & Hoshi, T. Studies on the fertilization of mouse eggs in vitro I: In vitro fertilization of eggs by fresh epididymal sperm. Jpn J Anim Reprod 16, 147–151 (1971).
Elvin, J. A., Clark, A. T., Wang, P., Wolfman, N. M. & Matzuk, M. M. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 13, 1035–1048 (1999).
Okada, Y. et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol 25, 233–237 (2007).
Cortvrindt, R., Smitz, J. & Van Steirteghem, A. C. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod 11, 2656–2666 (1996).
Janik, P., Briand, P. & Hartmann, N. R. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res 35, 3698–3704 (1975).
Acknowledgements
We thank Yumiko Maruyama and Akiko Kawai for generating mutant mice and Dr. Jurrien Dean and Samantha A.M. Young (B. Sc. Hons) for review of the manuscript. This work was partly supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Contributions
K.Tokuhiro, A.M.B. and M.I. designed research; K.Tokuhiro, Y.S., K.N., A.I., Y.F., Y.H., H.M., K.Takumi, T.M. and M.I. performed research; K.Tokuhiro, M.O., A.M.B. and M.I. analyzed data; and K.Tokuhiro, A.M.B. and M.I. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tokuhiro, K., Satouh, Y., Nozawa, K. et al. Calreticulin is required for development of the cumulus oocyte complex and female fertility. Sci Rep 5, 14254 (2015). https://doi.org/10.1038/srep14254
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14254
This article is cited by
-
FIGNL1 AAA+ ATPase remodels RAD51 and DMC1 filaments in pre-meiotic DNA replication and meiotic recombination
Nature Communications (2023)
-
Spatiotemporal profiling of the bovine oviduct fluid proteome around the time of ovulation
Scientific Reports (2022)
-
Immunogenic cell stress and death
Nature Immunology (2022)
-
The ATF6β-calreticulin axis promotes neuronal survival under endoplasmic reticulum stress and excitotoxicity
Scientific Reports (2021)
-
Calreticulin and cancer
Cell Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.