Abstract
Using ab initio evolutionary simulations, we predict the existence of five novel stable Li-N compounds at pressures from 0 to 100 GPa (Li13N, Li5N, Li3N2, LiN2 and LiN5). Structures of these compounds contain isolated N atoms, N2 dimers, polyacetylene-like N chains and N5 rings, respectively. The structure of Li13N consists of Li atoms and Li12N icosahedra (with N atom in the center of the Li12 icosahedron) – such icosahedra are not described by Wade-Jemmis electron counting rules and are unique. Electronic structure of Li-N compounds is found to dramatically depend on composition and pressure, making this system ideal for studying metal-insulator transitions. For example, the sequence of lowest-enthalpy structures of LiN3 shows peculiar electronic structure changes with increasing pressure: metal-insulator-metal-insulator. This work also resolves the previous controversies of theory and experiment on Li2N2.
Similar content being viewed by others
Introduction
Li-N system contains two well-known compounds: lithium nitride (Li3N) and lithium azide (LiN3). Li3N has potential use as an electrolyte in Li-batteries and a hydrogen storage medium1,2,3,4. Extensive experimental and theoretical investigations show that Li3N undergoes a sequence of phase transitions with increasing pressure. At ambient conditions, X-ray diffraction identified in Li3N a mixture of two phases: α-Li3N (P6/mmm) and metastable β-Li3N (P63/mmc); at about 0.5 GPa, α-Li3N fully transforms to β-Li3N; a new phase γ-Li3N ( ) occurs near 40 GPa5,6. Normal ionic materials usually become metallic with increasing pressure, but Li3N is abnormal since the increasing pressure makes it into a much more strongly ionic state7. LiN3 has been widely used in industry as a nitrogen source, initial explosives and photographic materials8. Before 2013, LiN3 was known in a single phase C2/m and seemed so simple and well understood. However, several other phases of LiN3 have been found using evolutionary crystal structure prediction methods coupled with first-principles calculations two years ago. At above 36 GPa, a hexagonal phase (P6/m) of LiN3 with pseudo-benzene N6 ring has been predicted by two research groups independently9,10. Some other phases appear as metastable:
) occurs near 40 GPa5,6. Normal ionic materials usually become metallic with increasing pressure, but Li3N is abnormal since the increasing pressure makes it into a much more strongly ionic state7. LiN3 has been widely used in industry as a nitrogen source, initial explosives and photographic materials8. Before 2013, LiN3 was known in a single phase C2/m and seemed so simple and well understood. However, several other phases of LiN3 have been found using evolutionary crystal structure prediction methods coupled with first-principles calculations two years ago. At above 36 GPa, a hexagonal phase (P6/m) of LiN3 with pseudo-benzene N6 ring has been predicted by two research groups independently9,10. Some other phases appear as metastable:  with a polyacetylene-like infinite linear nitrogen chain structure; C2/m and
with a polyacetylene-like infinite linear nitrogen chain structure; C2/m and  with puckered extended 2D decagonal and quasi-2D hexagonal nitrogen layers, respectively10. Above 375 GPa, Wang et al. identified the phase of P21 which consists of zigzag N polymeric chains with N5 ring sharing N-N pairs11. The band structures indicate that there are two metal-insulator transitions in LiN3: first from insulator to metal at 36 GPa and then from metal back to insulator at 375 GPa. Adding the new phases found in this work (see below), the sequence becomes metal-insulator-metal-insulator.
with puckered extended 2D decagonal and quasi-2D hexagonal nitrogen layers, respectively10. Above 375 GPa, Wang et al. identified the phase of P21 which consists of zigzag N polymeric chains with N5 ring sharing N-N pairs11. The band structures indicate that there are two metal-insulator transitions in LiN3: first from insulator to metal at 36 GPa and then from metal back to insulator at 375 GPa. Adding the new phases found in this work (see below), the sequence becomes metal-insulator-metal-insulator.
Besides nitrides (with N3− anion) and azides ([N3]−), in 2001, Kniep et al. proved the existence of diazenides [N2]2− by synthesizing SrN2 and BaN2 under high N2 pressure12,13. Since then, discovery of new diazenides has been of constant interest. In 2010, alkali diazenides Na2N2 and Li2N2 (Pmmm) were predicted14, but then, a different structure of Li2N2 (Immm) was obtained under HP/HT conditions (9 GPa, 750 K) by decomposition of LiN315. This discrepancy encourages us to study Li2N2 under high pressure in detail.
Nitrogen can form many anionic species, e.g. [N2]−, [N2]3− and [N5]−, which have just been obtained in molecular complexes16,17,18,19. We wonder if solid-state compounds with these anions in Li-N system could be synthesized under high pressure. Evolutionary algorithm USPEX has been widely used to predict new ground state structures in various systems without any experimental information, such as B-H, Xe-O and Na-Cl20,21,22. The predicted counterintuitive compounds NaCl3 and Na3Cl in the Na-Cl system have been confirmed by experiment22. In this work, we have performed extensive structure searches on the Li-N system using variable-composition evolutionary algorithm USPEX and indeed found many new stable compounds with very diverse and unusual crystal structures.
Results and Discussion
We first studied the phase stability in the Li-N system by calculating the enthalpy of formation (ΔH) of Li-N compounds in the pressure range from 0 to 100 GPa. Stability of compounds is explored by the thermodynamic convex hull construction. If the enthalpy of decomposition of a compound into any other compounds is positive, then the compound is stable, which is depicted on the convex hull. The convex hulls are shown in Fig. 1 at selected pressures: 0, 20, 50 and 100 GPa. The various known phases of solid Li, N2, Li3N, LiN3 and Li2N2 are reproduced readily in our evolutionary structure searches. Interestingly, five previously unreported compositions of Li-N system: Li13N, Li5N, Li3N2, LiN2 and LiN5 are found to be on the convex hull under ambient or high pressure in our calculations. The calculated phonon spectra confirmed that all predicted structures are dynamically stable. In total, we have found three new N-rich compounds and two new Li-rich compounds.
Simultaneously with our work (in fact, with submission date after our paper appeared on arxiv.org) Peng et al.23 investigated the Li-N system and found two new stable compounds, LiN2 and LiN5. However, the phase diagram of the Li-N system published by Peng et al.23 missed a number of stable compounds (Li13N, Li5N, Li3N2). The enthalpies of reported phases in ref. 23 are recalculated and compared with our results. Detailed comparisons are shown in Figures S2 and S3 of the Supporting Information. Hence this paper presents a more complete and reliable picture, correcting omissions and presenting more stable crystal structures than those presented before.
We find that: (i) At ambient conditions (0 GPa), besides Li3N and Li2N2, LiN2 with space group P63/mmc is surprisingly stable. These three compositions are always stable in the pressure range from 0 to 100 GPa. (ii) However, the long-known LiN3 is metastable below 49 GPa, which is in agreement with the known fact that it decomposes into N2 and Li under external influences (heat, irradiation, etc) at 0 GPa. (iii) At 20 GPa, LiN5 becomes stable, meanwhile Li13N, Li3N2 and LiN3 lie very close to (or nearly on) the convex hull. At 50 GPa, Li13N, Li3N2 and LiN3 are all stable and Li5N lies very close to the convex hull. At 100 GPa, Li5N is stable, however, Li13N and Li3N2 are becoming metastable although they both lie nearly on the convex hull.
The pressure-composition phase diagram of the Li-N system is depicted in Fig. 2. For pure Li, with increasing pressure, the bcc phase ( ) transforms into fcc (
) transforms into fcc ( ), cI16 (
), cI16 ( ), Aba2–40 and Pbca phases in sequence, which is in accordance with previous experimental and theoretical data24,25,26. For pure N, the known
), Aba2–40 and Pbca phases in sequence, which is in accordance with previous experimental and theoretical data24,25,26. For pure N, the known  , P21/c, P41212 and I213 structures are reproduced in our searches and agree well with other theoretical predictions27,28.
, P21/c, P41212 and I213 structures are reproduced in our searches and agree well with other theoretical predictions27,28.
For Li3N there is a peculiar situation: the experimentally known at ambient conditions P6/mmm structure is predicted to be stable only at pressures above 0.2 GPa – at lower pressures, at the GGA level of theory, the  structure is more stable (at 0 GPa, by 22 meV/formula unit). This small upward shift of phase transition pressures is typical of the GGA, but one wonders whether
structure is more stable (at 0 GPa, by 22 meV/formula unit). This small upward shift of phase transition pressures is typical of the GGA, but one wonders whether  structure could be stabilized by impurities, temperature etc. The subsequent phases of Li3N in our calculations are all in agreement with the previous works5,6,7.
structure could be stabilized by impurities, temperature etc. The subsequent phases of Li3N in our calculations are all in agreement with the previous works5,6,7.
LiN3 is a thermodynamically stable compound (on the convex hull) only above 49 GPa, but it is well known also at ambient conditions as a metastable material. We found  to have the lowest enthalpy in the pressure range 0–0.9 GPa, followed by C2/m and P6/m phases on increasing pressure. C2/m is the phase known experimentally at ambient conditions.
to have the lowest enthalpy in the pressure range 0–0.9 GPa, followed by C2/m and P6/m phases on increasing pressure. C2/m is the phase known experimentally at ambient conditions.
For LiN (actually Li2N2), the obtained structure at 0 GPa is Pmmm, which is consistent with the previous theoretical result14. At 8.2 GPa, Pmmm phase of LiN transforms into the Immm structure, indicating that the experimental result obtained at around 9 GPa is also perfectly correct15. At 8.9 GPa, the Immm structure will lose its stability and the Pnma phase becomes stable in the pressure range from 8.9 to 66.4 GPa. Then the Cmcm phase is stable up to 100 GPa.
Phase transformations of five new compositions of Li-N system are as follows: (i) For Li13N, the Immm structure is predicted to be stable from 43 to 76 GPa, following which the C2/m structure is stable up to 83 GPa. In fact, Immm and C2/m phases have nearly identical enthalpies (within 0.2 meV/atom), suggesting that Li13N can exist as a mixture of Immm and C2/m phases in the whole range of stability of this compound. (ii) Li5N has a single stable phase P6/mmm from 80 to at least 100 GPa. (iii) From 30 to 89 GPa, Li3N2 has two stable phases P4/mbm and C2/c. The pressure-induced structural transition from P4/mbm to C2/c occurs at about 39 GPa. (iv) Besides the P63/mmc structure, LiN2 has another phase  , stable above 56 GPa, (v) For LiN5, the P21/c structure becomes stable at 15 GPa and then transforms into the C2/c phase at the pressure of 65 GPa. The C2/c structure is stable at least up to 100 GPa.
, stable above 56 GPa, (v) For LiN5, the P21/c structure becomes stable at 15 GPa and then transforms into the C2/c phase at the pressure of 65 GPa. The C2/c structure is stable at least up to 100 GPa.
The representative structures of above-mentioned Li-N compounds under ambient conditions and high pressure are presented in Fig. 3. We first analyze the structures of Li3N and LiN3 and remind that the lengths of nitrogen-nitrogen bonds are 1.10 Å for the triple N–N bond, 1.25 Å for the double N = N bond and 1.45 Å for the single N-N bond. (i) For Li3N, the calculated lattice parameters of P6/mmm, P63/mmc and  are in agreement with experimental data within 0.5%. The
are in agreement with experimental data within 0.5%. The  structure is very simple, an anti-ReO3-type structure made of corner-sharing NLi6 octahedra (Fig. 3a). Interestingly, across the phase transitions, the number of Li atoms surrounding each N atom increases from 6 for
structure is very simple, an anti-ReO3-type structure made of corner-sharing NLi6 octahedra (Fig. 3a). Interestingly, across the phase transitions, the number of Li atoms surrounding each N atom increases from 6 for  to 8 for P6/mmm, 11 for P63/mmc and 14 for
to 8 for P6/mmm, 11 for P63/mmc and 14 for  . (ii) for LiN3 at ambient conditions, C2/m structure consists of Li+ cations and linear azide anions [N3]− 10. As illustrated in Fig. 3b, unlike the C2/m structure,
. (ii) for LiN3 at ambient conditions, C2/m structure consists of Li+ cations and linear azide anions [N3]− 10. As illustrated in Fig. 3b, unlike the C2/m structure,  phase does have [N3]− anions, but instead its unit cell contains two Li atoms and three N2 groups with the N-N distance of 1.151 Å at 0 GPa, smaller than that in the azide-ion [N3]− (1.184 Å), but larger than that in the gas-phase N2 molecule (1.10 Å) and indicating a bond order between 2 and 3.
phase does have [N3]− anions, but instead its unit cell contains two Li atoms and three N2 groups with the N-N distance of 1.151 Å at 0 GPa, smaller than that in the azide-ion [N3]− (1.184 Å), but larger than that in the gas-phase N2 molecule (1.10 Å) and indicating a bond order between 2 and 3.
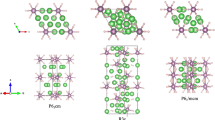
Crystal structures of Li-N compounds.
(a)  -Li3N at 0 GPa, (b)
-Li3N at 0 GPa, (b)  -LiN3 at 0 GPa, (c) Pmmm-Li2N2 at 0 GPa, (d) Immm-Li2N2 at 10 GPa, (e) Pnma-Li2N2 at 10 GPa, (f) Immm-Li13N at 50 GPa, (g) P6/mmm-Li5N at 90 GPa, (h1) P4/mbm-Li3N2 at 30 GPa, (h2) ELF isosurfaces (ELF = 0.85) of (h1), (i) C2/c-Li3N2 at 40 GPa, (j) P63/mmc-LiN2 at 0 GPa, (k)
-LiN3 at 0 GPa, (c) Pmmm-Li2N2 at 0 GPa, (d) Immm-Li2N2 at 10 GPa, (e) Pnma-Li2N2 at 10 GPa, (f) Immm-Li13N at 50 GPa, (g) P6/mmm-Li5N at 90 GPa, (h1) P4/mbm-Li3N2 at 30 GPa, (h2) ELF isosurfaces (ELF = 0.85) of (h1), (i) C2/c-Li3N2 at 40 GPa, (j) P63/mmc-LiN2 at 0 GPa, (k)  -LiN2 at 60 GPa, (l) P21/c-LiN5 at at 50 GPa, (m) C2/c-LiN5 at 80 GPa.
-LiN2 at 60 GPa, (l) P21/c-LiN5 at at 50 GPa, (m) C2/c-LiN5 at 80 GPa.
The Pmmm structure of Li2N2 consists of face-sharing Li8 parallelepipeds (Fig. 3c). The N2 groups sit in the center of parallelepipeds, which can be viewed that there are six Li atoms connecting to each N atom of N2 molecule and each of four Li atoms connects to both N atoms. The N-N bond length is 1.263 Å at 0 GPa, slightly larger than that of Na2N2 (1.24 Å)14 and indicating a double N = N bond and ideal charge of the N2 group equal to −2, which matches perfectly the formula Li2N2. Our calculated lattice constants of Immm structure (Fig. 3d) are in good agreement with experimental results15. The predicted N-N bond length is 1.271 Å, slightly smaller than the experiment. Figure 3e presents the Pnma structure of Li2N2 at 10 GPa. Its unit cell contains four N22− groups and eight Li+ ions. The N-N bond length is 1.269 Å.
For Li13N, Immm and C2/m phases have similar structures. The Immm structure of Li13N at 50 GPa is shown in Fig. 3f. This structure is an interesting example of Li-N compounds which can be viewed as a combination of a single Li atom and a slightly distorted Li12N icosahedral group (with N atom inside the Li12 icosahedron). A similar Li12Cs icosahedron is present in the Pnna structure of Li3Cs compound29, where neighboring icosahedra share Li-Li edges. However, the Li12N icosahedra are isolated and do not share Li atoms with each other in our Li13N compound. The Li-N bond lengths in the Li12N icosahedron are 1.934, 1.951 and 2.011 Å, i.e. nearly identical and Li-Li distances are also nearly identical, ranging from 2.026 to 2.113 Å (maximum difference 4.3%, to compare with 22.3% in Li3Cs29).
The P6/mmm phase of Li5N has a layered structure, made of alternating layers of stoichiometry Li4N (here, N atoms are sandwiched between two Li-graphene sheets) and Li, see Fig. 3g. Such unusual layered structures with alternation of “metallic” and “non-metallic” layers have been previously reported by some of us for the Na-Cl system (e.g., Na3Cl, also confirmed experimentally22) and for the K-Cl system30. Bader analysis shows that Li5N at 90 GPa has charge configuration [Li4N]–0.68 Li+0.68, indicating that most of the valence electrons of Li layer transfer to the Li4N sandwich layer31. Interestingly, the Bader charge of Li atom in upper Li-graphene sheet of Li4N sandwich layer is nearly neutral (+0.1 e) and the charge of Li atom in bottom Li-graphene sheet is +0.74 e.
As observed in Fig. 3h1, the P4/mbm structure of Li3N2 consists of a three-dimensional network of Li atoms, which has open channels along z direction. This structure is very similar to the structure of the new compound Mg3O2 predicted by some of us recently32, except that in Li3N2 there is pairing of N atoms with the N-N distance of 1.353 Å at 30 GPa, indicating bond order between 1 and 2. Just like in P4/mbm-Mg3O2, we can clearly see columns of face-sharing body-centered cubes of metal atoms. The electron localization function (ELF) of Li3N2 (Fig. 3h2) shows strong charge transfer from Li to N. However, unlike Mg3O2 which is an electride, there is no strong interstitial electron location in Li3N2. Bader analysis also confirms the above result. The charges of P4/mbm-Li3N2 are +0.794 e for one Li atom, +0.809 e for the other two Li atoms and −1.146e and −1.266 e for two N atoms, respectively. The C2/c structure has a more complex three-dimensional network of lithium atoms with N2 groups also sitting in its channels (Fig. 3i), with the N-N distance of 1.391 Å at 40 GPa.
The P63/mmc structure of LiN2 can be described as a NiAs-type structure, where anionic positions are occupied by the N2 groups (Fig. 3j). At zero pressure, the N-N distance is 1.173 Å, indicating a bond order between 2 and 3. The  phase contains an infinite polyacetylene-like nitrogen chain (Fig. 3k), similar to the metastable phase of LiN310. The N-N distances are 1.316, 1.320 and 1.333 Å at 60 GPa, suggesting bond order between 1 and 2. We can clearly see how pressure destroys molecular groups, favoring extended structures.
phase contains an infinite polyacetylene-like nitrogen chain (Fig. 3k), similar to the metastable phase of LiN310. The N-N distances are 1.316, 1.320 and 1.333 Å at 60 GPa, suggesting bond order between 1 and 2. We can clearly see how pressure destroys molecular groups, favoring extended structures.
As observed in Fig. 3l, the P21/c structure of LiN5 consists of isolated Li atoms and N5 rings, which up to now were only detected in molecular complexes19. At 50 GPa, the N-N distances are 1.286, 1.291, 1.299, 1.303 and 1.305 Å, respectively. The higher-pressure C2/c phase also consists of isolated Li atoms and N5 rings (Fig. 3m). Unlike in P21/c, the N5 ring here is a nearly isosceles pentagon, with N-N distances of 1.277, 1.277, 1.301, 1.301 and 1.281 Å, respectively, at 80 GPa.
To obtain deeper insight into these new Li-N compounds, we calculated their band structures and density of states (DOS) at selected pressures. We found that all stable phases of Li13N, Li5N and Li3N2 are metallic. The  phase of Li3N is a semiconductor with the DFT band gap of 0.84 eV. All three stable phases of Li2N2 are also metallic, in agreement with experiment15. Interestingly, LiN2 has a metal-insulator transition: P63/mmc is metallic at low pressure, but semiconducting in the high-pressure
phase of Li3N is a semiconductor with the DFT band gap of 0.84 eV. All three stable phases of Li2N2 are also metallic, in agreement with experiment15. Interestingly, LiN2 has a metal-insulator transition: P63/mmc is metallic at low pressure, but semiconducting in the high-pressure  phase, with the band gap of 0.13 eV at 60 GPa. Since the newly predicted
phase, with the band gap of 0.13 eV at 60 GPa. Since the newly predicted  phase of LiN3 is also metallic, combining with previously known phases of LiN3, we find that the sequence of transitions of LiN3 under pressure is extremely unusual: from metallic to insulating to metallic to insulating. The P21/c and C2/c phases of LiN5 are wide-gap insulators: e.g., the DFT band gap of the C2/c phase at 80 GPa is 2.19 eV.
phase of LiN3 is also metallic, combining with previously known phases of LiN3, we find that the sequence of transitions of LiN3 under pressure is extremely unusual: from metallic to insulating to metallic to insulating. The P21/c and C2/c phases of LiN5 are wide-gap insulators: e.g., the DFT band gap of the C2/c phase at 80 GPa is 2.19 eV.
Some electronic structures are shown in Fig. 4. As is seen from Fig. 4(a–c), the PDOSs of two different phases of Li3N (or Li2N2 or LiN3) at 0 GPa are obviously different. The newly found phases  -Li3N, Pmmm-Li2N2 and
-Li3N, Pmmm-Li2N2 and  -LiN3 have one character in common: the states near the Fermi level come mostly from Li-s and N-p orbitals. Figure 4(d–e) show the band structures of
-LiN3 have one character in common: the states near the Fermi level come mostly from Li-s and N-p orbitals. Figure 4(d–e) show the band structures of  -LiN2 and P63/mmc-LiN2 at different pressures, respectively. The band structures at different pressures for the same phase are similar. When pressure increases, the band structure is more dispersive and the bandwidths also increase: both the conduction and valence bands broaden and conduction band tends to shift upwards in energy. These changes can lead to both metallization and demetallization: for example,
-LiN2 and P63/mmc-LiN2 at different pressures, respectively. The band structures at different pressures for the same phase are similar. When pressure increases, the band structure is more dispersive and the bandwidths also increase: both the conduction and valence bands broaden and conduction band tends to shift upwards in energy. These changes can lead to both metallization and demetallization: for example,  -LiN2 is metallic at 0 GPa, whereas it becomes semiconductor with the gap of 0.13 eV at 60 GPa.
-LiN2 is metallic at 0 GPa, whereas it becomes semiconductor with the gap of 0.13 eV at 60 GPa.
Conclusions
A number of new Li-N compounds have been predicted using ab initio evolutionary structure search. Other than the well-known compositions Li3N, Li2N2 and LiN3, we found five novel compositions which should be experimentally synthesizable under pressure, including Li13N, Li5N, Li3N2, LiN2 and LiN5. Notably, the N-N bonding patterns evolve from isolated N ions to N2 dumbbells, to linear N3 groups, infinite nitrogen chains, N5 rings with increasing N content. Interestingly, for the experimentally known compounds Li3N and LiN3 at ambient conditions we find new lowest-energy structures ( and
and  , respectively): these are stable (at the GGA level of theory) in very narrow pressure ranges near 0 GPa. While this is most likely an artefact of the GGA (known to slightly overstabilize open structures and shift phase transition pressures upwards), these phases may be stabilized by doping, temperature, etc. We also resolve previous discrepancy on stable phases of Li2N2. In conclusion, this paper presents a more complete and reliable picture, correcting omissions and presenting more stable crystal structures than those presented before. Our work provides the basis for the future experimental investigations of the Li-N system.
, respectively): these are stable (at the GGA level of theory) in very narrow pressure ranges near 0 GPa. While this is most likely an artefact of the GGA (known to slightly overstabilize open structures and shift phase transition pressures upwards), these phases may be stabilized by doping, temperature, etc. We also resolve previous discrepancy on stable phases of Li2N2. In conclusion, this paper presents a more complete and reliable picture, correcting omissions and presenting more stable crystal structures than those presented before. Our work provides the basis for the future experimental investigations of the Li-N system.
Methods
To search for stable compounds, the Li-N system was first explored using the variable-composition evolutionary technique, as implemented in the USPEX code33,34,35. Evolutionary crystal structure predictions were performed in the pressure range from 0 to 100 GPa. Initial structures included up to 16 atoms in the unit cell. The first generation of structures was produced randomly. The child structures were obtained applying heredity, transmutation, softmutation and random symmetric generator, with probabilities of 40, 20, 20 and 20%, respectively. Then we performed detailed fixed-composition evolutionary calculations to explore the most promising compositions.
All structure relaxations and electronic structure calculations were done using the Vienna Ab Initio Simulation Package (VASP) in the framework of density functional theory36. The Perdew-Burke-Ernzerhof generalized gradient approximation (PBE-GGA) was employed to treat the exchange-correlation energy37 and the all-electron projector augmented wave (PAW) potentials were used to describe the core-valence interactions38. The cut-off energy of 650 eV and Monkhorst-Pack k-point meshes for sampling the Brillouin zone with resolution 2π × 0.04 Å−1 ensured that all the enthalpy calculations were well converged to better than 1 meV/atom. To ensure that the structures of predicted compounds in Li-N system are dynamically stable, phonon calculations were carried out using the Phonopy code39. Our tests showed that the effect of van der Waals interactions40,41 on stability of lithium nitrides is negligible, which is consistent with other works23.
Additional Information
How to cite this article: Shen, Y. et al. Novel lithium-nitrogen compounds at ambient and high pressures. Sci. Rep. 5, 14204; doi: 10.1038/srep14204 (2015).
References
Boukamp, B. A. & Huggins, R. A. Lithium ion conductivity in lithium nitride. Phys. Lett. A 58, 231–233 (1976).
Rabenau, A. Lithium nitride and related materials case study of the use of modern solid state research techniques. Solid State Ionics 6, 277–293 (1982).
Chen, P., Xiong, Z., Luo, J., Lin, J. & Tan, K. L. Interaction of hydrogen with metal nitrides and imides. Nature 420, 302–304 (2002).
Hu, Y. H. & Ruckenstein, E. Ultrafast reaction between Li3N and LiNH2 to prepare the effective hydrogen storage material Li2NH. Ind. Eng. Chem. Res. 45, 4993–4998 (2006).
Lazicki, A. et al. New cubic phase of Li3N: Stability of the N3−ion to 200 GPa. Phys. Rev. Lett. 95, 165503 (2005).
Lazicki, A. et al. Pressure-induced loss of electronic interlayer state and metallization in the ionic solid Li3N: Experiment and theory. Phys. Rev. Lett. 78, 155133 (2008).
Cui, S., Feng, W., Hu, H., Feng, Z. & Wang, Y. Structural transition of Li3N under high pressure: A first-principles study. Solid State Commun. 149, 612–615 (2009).
Evans, B. L., Yoffe, A. D. & Gray, P. Physics and chemistry of the inorganic azides. Chem. Rev. 59, 515–568 (1959).
Zhang, M., Yan, H., Wei, Q., Wang, H. & Wu, Z. Novel high-pressure phase with pseudo-benzene “N6” molecule of LiN3 . EPL-Europhys Lett. 101, 26004 (2013).
Prasad, D. L. V. K., Ashcroft, N. W. & Hoffmann, R. Evolving structural diversity and metallicity in compressed lithium azide. J. Phys. Chem. C 117, 20838–20846 (2013).
Wang, X. et al. Polymerization of nitrogen in lithium azide. J. Chem. Phys. 139, 164710 (2013).
Auffermann, G., Prots, Y. & Kniep, R. SrN and SrN2: Diazenides by synthesis under high N2 pressure. Angew. Chem. Int. Ed. 40, 547–549 (2001).
Vajenine, G. et al. Preparation, crystal structure and properties of barium pernitride, BaN2 . Inorg. Chem. 40, 4866–4870 (2001).
Zhang, X., Zunger, A. & Trimarchi, G. Structure prediction and targeted synthesis: A new NanN2 diazenide crystalline structure. J. Chem. Phys. 133, 194504 (2010).
Schneider, S. B., Frankovsky, R. & Schnick, W. High-pressure synthesis and characterization of the alkali diazenide Li2N2 . Angew. Chem. Int. Ed. 51, 1873–1875 (2012).
Chiesa, M. et al. Reductive activation of the nitrogen molecule at the surface of “electron-rich” MgO and CaO. The N2− surface adsorbed radical ion. J. Phys. Chem. B 105, 497–505 (2001).
Kaim, W. & Sarkar, B. N23−: Filling a gap in the N2n− Series. Angew. Chem. Int. Ed. 48, 9409–9411 (2009).
Chirik, P. J. One electron at a time. Nature Chem. 1, 520–522 (2009).
Vij, A., Pavlovich, J. G., Wilson, W. W., Vij, V. & Christe, K. O. Experimental detection of the pentaazacyclopentadienide (pentazolate) anion, cyclo-N5−. Angew. Chem. Int. Ed. 41, 3051–3054 (2002).
Hu, C.-H. et al. Pressure-induced stabilization and insulator-superconductor transition of BH. Phys. Rev. Lett. 110, 165504 (2013).
Zhu, Q. et al. Stability of xenon oxides at high pressures. Nature Chem. 5, 61–65 (2013).
Zhang, W. et al. Unexpected stoichiometries of stable sodium chlorides. Science 342, 1502–1505 (2013).
Peng, F., Yao, Y., Liu, H. & Ma, Y. Crystalline LiN5 predicted from first-principles as a possible high-energy material. J. Phys. Chem. Lett. 6, 2363–2366 (2015).
Hanfland, M., Syassen, K., Christensen, N. E. & Novikov, D. L. New high-pressure phases of lithium. Nature 408, 174–178 (2000).
Pickard, C. J. & Needs, R. J. Dense low-coordination phases of lithium. Phys. Rev. Lett. 102, 146401 (2009).
Lv, J., Wang, Y., Zhu, L. & Ma, Y. Predicted novel high-pressure phases of lithium. Phys. Rev. Lett. 106, 015503 (2011).
Pickard, C. J. & Needs, R. J. High-pressure phases of nitrogen. Phys. Rev. Lett. 102, 125702 (2009).
Wang, X. et al. Cagelike diamondoid nitrogen at high pressures. Phys. Rev. Lett. 109, 175502 (2012).
Botana, J. & Miao, M.-S. Pressure-stabilized lithium caesides with caesium anions beyond the -1 state. Nat. Commun. 5, 4861 (2014).
Zhang, W. & Oganov, A. R. Stability of numerous novel potassium chlorides at high pressure. arXiv:1405.3007 (2014).
Bader, R. F. W. Atoms in Molecules-A Quantum Theory (Oxford Univ. Press, Oxford, 1990).
Zhu, Q., Oganov, A. R. & Lyakhov, A. O. Novel stable compounds in the Mg-O system under high pressure. Phys. Chem. Chem. Phys. 15, 7696–7700 (2013).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: principles and applications. J. Chem. Phys. 124, 244704 (2006).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works - and why. Acc. Chem. Res. 44, 227–237 (2011).
Lyakhov, A. O., Oganov, A. R., Stokes, H. T. & Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 184, 1172–1182 (2013).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Togo, A., Oba, F. & Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 78, 134106 (2008).
Bučko, T., Hafner, J., Lebègue, S. & Ángyán, J. G. Improved description of the structure of molecular and layered crystals: Ab Initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 114, 11814 (2010).
Bučko, T., Lebègue, S., Hafner, J. & Ángyán, J. G. Tkatchenko-Scheffler van der Waals correction method with and without self-consistent screening applied to solids. Phys. Rev. B 87, 064110 (2013).
Acknowledgements
Y. Shen thanks Dongxu Li, Xiao Dong, Xiangfeng Zhou, Qianku Hu, Shengnan Wang and Haiyang Niu for valuable discussions. The research is supported by National Natural Science Foundation of China (No. 11204053 and No. 11074059) and the China Postdoctoral Science Foundation (No. 2013M531028). A.R.O. thanks DARPA (No. W31P4Q1210008 and No. W31P4Q1310005) and the Government of Russian Federation (No. 14.A12.31.0003) for financial support.
Author information
Authors and Affiliations
Contributions
A.R.O. designed the project and Y.-Q.S. carried out structure prediction and electronic structure calculations. G.-R.Q. and J.Z. performed phonon calculations. H.-F.D. and Q.Z. computed the electron localization function and Bader charges. Y.-Q.S., A.R.O. and Z.-X.Z. analysed the data and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shen, Y., Oganov, A., Qian, G. et al. Novel lithium-nitrogen compounds at ambient and high pressures. Sci Rep 5, 14204 (2015). https://doi.org/10.1038/srep14204
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14204
This article is cited by
-
Stabilization of hexazine rings in potassium polynitride at high pressure
Nature Chemistry (2022)
-
Immunizing lithium metal anodes against dendrite growth using protein molecules to achieve high energy batteries
Nature Communications (2020)
-
Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains
Nature Communications (2018)
-
The polymerization of nitrogen in Li2N2 at high pressures
Scientific Reports (2018)
-
Materials discovery at high pressures
Nature Reviews Materials (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.






 -LiN2 and P63/mmc-LiN2 at two different pressures.
-LiN2 and P63/mmc-LiN2 at two different pressures.