Abstract
Adhesion to host cells is considered important for Lactobacillus plantarum as well as other lactic acid bacteria (LAB) to persist in human gut and thus exert probiotic effects. Here, we sequenced the genome of Lt. plantarum strain NL42 originating from a traditional Chinese dairy product, performed comparative genomic analysis and characterized a novel adhesion factor. The genome of NL42 was highly divergent from its closest neighbors, especially in six large genomic regions. NL42 harbors a total of 42 genes encoding adhesion-associated proteins; among them, cwaA encodes a protein containing multiple domains, including five cell wall surface anchor repeat domains and an LPxTG-like cell wall anchor motif. Expression of cwaA in Lactococcus lactis significantly increased its autoaggregation and hydrophobicity and conferred the new ability to adhere to human colonic epithelial HT-29 cells by targeting cellular surface proteins and not carbohydrate moieties, for CwaA adhesion. In addition, the recombinant Lc. lactis inhibited adhesion of Staphylococcus aureus and Escherichia coli to HT-29 cells, mainly by exclusion. We conclude that CwaA is a novel adhesion factor in Lt. plantarum and a potential candidate for improving the adhesion ability of probiotics or other bacteria of interest.
Similar content being viewed by others
Introduction
Lactobacillus plantarum is a highly flexible and versatile species which can be found in various environmental as well as human intestinal niches1. This species is one of the food-grade lactic acid bacteria (LAB) that offers health-promoting properties to humans, including potential treatment effects for irritable bowel syndrome2 and recurrent Clostridium difficile-associated diarrhea3, protection of the epithelial barrier4, reduction of gastrointestinal symptoms during antibiotic treatment5 and cholesterol-lowering6 and immunomodulatory effects7,8. Specific strains of Lt. plantarum, 299v8 for example, are now being added to commercially available probiotic products9,10. In view of the beneficial health effects of Lt. plantarum to humans, much effort has been invested in isolating and screening new strains, which might have improved or new probiotic traits, from various environmental niches, including natural fermented foods, plants and the human body11,12,13.
Adhesion of probiotic bacteria to human intestinal epithelial cells may favor their persistence in the gut, allowing them to exert beneficial effects on the host14. Bacterial adhesion to host mucosa is often mediated by the interaction of cell-surface components, including receptor-specific binding and charge and hydrophobic interactions15; mucus and epithelial adhesion represent the early and late stages of adhesion, respectively14. Autoaggregation and hydrophobicity are two indirect methods of evaluating the adhesion ability of bacteria16,17. Different adhesion mechanisms and molecules have been revealed in Lactobacillus, including surface-layer (S-layer) proteins in Lt. acidophilus, Lt. gasseri, Lt. johnsonii, Lt. crispatus and Lt. brevis15, cell wall-anchored mucus-binding protein in Lt. reuteri18, cell-surface collagen-binding protein in Lt. reuteri NCIB1195119, mannose-specific adhesin in Lt. plantarum20 and the mucus-binding pilin SpaC in Lt. rhamnosus GG21, among others. In comparison, there is less information on Lactococcus adhesion because these bacteria are not traditionally considered to be natural colonizers of humans22. In recent years, however, the presence of proteins containing a mucus-binding domain23, pili encoded by plasmids24 and surface physicochemical properties of charge or hydrophobicity25 has been predicted or verified to be correlated with the adhesive properties of Lc. lactis.
Genetic manipulation is a potent approach to designing new probiotic strains with improved or novel probiotic traits26. Various bacterial or even human targets of interest, such as enzymes27, cytokines and/or antigens28,29, adhesion proteins30 and so forth have been verified to be functional in existing probiotics. As for adhesion, Koo et al.30 demonstrated that recombinant probiotic Lt. paracasei expressing Listeria adhesion protein effectively blocks adhesion, invasion and translocation of Listeria monocytogenes, thereby aiding in the targeted clearance of Listeria infection. In addition, a newly identified Bifidobacterium bifidum-specific protein (BopA) involved in adhesion improved the adhesive properties of recombinant bifidobacteria31. Among the expression of different heterologous genes in LAB hosts, Lc. lactis has proven to be optimal for heterologous protein production and the delivery of therapeutic and prophylactic molecules32, mainly because Lc. lactis is considered to be a noninvasive and nonpathogenic organism which secretes relatively few proteins and does not produce extracellular proteases33.
We previously isolated 30 different LAB strains from traditional dairy products produced by herders in the western Tianshan Mountains of China. General features of these isolates, in particular their fermentative characteristics, were analyzed34. Among those isolates, a Lt. plantarum strain (NL42) displayed both high autolytic activity and high autoaggregation ability (reflecting potential high adhesive ability). To reveal the genome features of this isolate and to characterize its adhesion-associated factors, we sequenced the whole genome of Lt. plantarum NL42 and performed comparative genomic analysis. Based on this, a multidomain-containing, cell wall-anchored, adhesion-associated protein termed CwaA was predicted and features of Lc. lactis expressing this protein were characterized.
Results
Genome features and phylogeny of Lactobacillus plantarum strain NL42
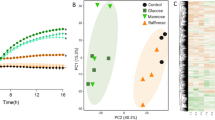
The whole genome of Lt. plantarum NL42 was sequenced using the Illumina HiSeq 2000 platform. A total of 4,241,606 paired-end reads with a read length of 100 bp were generated, in total 848 M of raw data corresponding to 250-fold coverage of the genome. After quality filtering and assembly, we obtained the draft genome of NL42 consisting of 3,353,072 bp (52 contigs) with a GC content of 44.3% (Fig. S1). Rapid Annotation Using Subsystem Technology (RAST) annotation of the genome revealed 3,297 coding sequences (CDSs), 349 SEED subsystems and 83 RNA genes. The 16S rRNA gene of NL42 was 100% identical to Lt. plantarum WCFS1, ATCC 14917, Lp90, LP91, AG30, NC8 and JCM 1149 and showed 99.6–99.9% similarity to the others (Fig. 1a and Fig. S2); however, whole-genome single nucleotide polymorphism (SNP)-based phylogenetic analysis grouped NL42 with AY01 and EGD-AQ4, forming a clade that was very distant from another two distinct clades. In addition, though NL42, AY01 and EGD-AQ4 were in the same clade, they were more divergent from each other than from members in the other two clades (Fig. 1b). These results suggested that even though its 16S rRNA genetic marker is closely related or even the same as those of other isolates, the NL42 genome is highly variable.
Phylogenetic analysis of different Lt. plantarum strains.
(a) Genetic marker 16S rRNA gene-based and (b) whole-genome SNP-based phylogenetic trees. The trees were constructed using MEGA 5.1 software with the neighbor-joining method. The confidence of the trees was assessed by 1000-replicate bootstrapping. The scale bar in panel (a) means sequence divergence; while the tree shown in panel (b) is a topological structure in which three distinct clades are colored in red, blue and green, respectively. Only strains having complete or draft genome sequences in GenBank were included. The 16S rRNA gene of strain 4_3 was not available and thus is not presented in the marker gene-based tree.
Comparative genomics of Lactobacillus plantarum
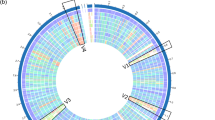
To reveal the genomic variations in NL42, we compared the CDSs of NL42 with six other available complete Lt. plantarum genomes, using WCFS1 as a reference. The results, shown in a heat map, revealed that the variations always occur in abnormal GC regions in the Lt. plantarum genomes (Fig. 2). Compared with WCFS1, NL42 displayed six large and highly varied genomic regions designated V1 to V6 (each covering more than 40 CDSs) (Data S1). According to their gene content, the functions of these six regions were predicted as follows: V1 (locus tags from lp_0373 to lp_0431 in WCFS1)—a bacteriocin biosynthesis gene cluster; V2 (lp_0624 to lp_0687)—prophage P1 locus; V3 (lp_1176 to lp_1233)—a polysaccharide biosynthesis gene cluster; V4 (lp_2399 to lp_2480)—prophage P2a and P2b loci; V5 (lp_3093 to lp_3164) and V6 (lp_3590 to lp_3650)—probably involved in sugar metabolism and transport, respectively. Notably, these variant regions were also present in the other Lt. plantarum genomes and thus may be major contributors to the genome plasticity of this species.
Comparison of protein sequence similarity among Lt. plantarum genomes.
Protein sequences in NL42 and the other six complete genomes were aligned using WCFS1 as the reference genome. The innermost track shows the GC content of the reference. Rings from inside to outside are WCFS1, 16, JDM1, P8, ST_III, ZJ316 and NL42, respectively. Red, yellow and blue indicate 90–100%, 60–89% and less than 59% protein sequence identities, respectively. Six large and highly varied genomic regions (V1 to V6) are labeled outside the outer ring.
We then compared the genes’ functional categories based on COG assignment among these Lt. plantarum genomes. The various Lt. plantarum genomes were found to harbor similar numbers of genes in each functional category (Fig. S3), with the highest number of genes assigned to the category ‘post-translational modification, protein turnover and chaperones’ (from 336 to 402 genes in the different genomes), followed by ‘cell wall/membrane/envelope biogenesis’ (270 to 305) and ‘replication, recombination and repair’ (251 to 299). Compared to the other six genomes, NL42 was slightly enriched in genes belonging to ‘transcription’ (263 genes), ‘lipid transport and metabolism’ (173 genes), ‘secondary metabolite biosynthesis, transport and catabolism’ (201 genes) and ‘cell motility’ (68 genes). We further sought and compared adhesion-associated proteins (adhesion-associated and cell wall anchor domain-containing proteins) in NL42 and the other six genomes. Strain ZJ316 harbored the highest number of these proteins (49 genes), whereas JDM1 had the lowest (40 genes) (Fig. S4). In general, proteins containing PepGly-associated (peptidoglycan-binding), cell wall anchor-associated and mucus-associated domains were the three most prevalent proteins in Lt. plantarum. Interestingly, NL42 was found to harbor a gene that encodes a protein containing five cell wall surface anchor repeat domains and an LPxTG-like cell wall anchor motif, termed cell wall-anchored protein A (CwaA). We then focused on characterizing this protein and its encoding gene, cwaA.
CwaA is a multidomain-containing, cell wall-anchored, adhesion-associated protein
The cwaA gene in the NL42 genome is probably the structural gene of an operon composed of five different open reading frames that encode three hypothetical proteins, a transcriptional regulator and the cell wall-anchored protein CwaA (Fig. 3). This putative operon structure was also found in the other six complete Lt. plantarum genomes. To further investigate the cwaA gene distribution and the sequence diversity, we searched and compared the homologues of cwaA in all 21 known Lt. plantarum genomes. Interestingly, cwaA homologues were found harbored by all the known Lt. plantarum genomes, with nucleotide identity ranging from 57.8% to 100% with cwaA in NL42. Phylogenetic analysis indicated that cwaA genes in the known Lt. plantarum genomes are clustered into two major groups, i.e., Group I and Group II. Most of the Lt. plantarum genomes (a total of 16) belong to Group I and only 6 genomes (NL42 included) are affiliated to Group II (Fig. 4a). The majority members among each group are similar to each other, showing more than 90% nucleotide identity, while members between the two groups are relative more divergent, usually less than 75% identity (Fig. 4b). Taken together, though the sequence of cwaA in different Lt. plantarum isolates are diverse and separately clustered, none of these genomes are devoid of cwaA homologues, suggesting that cwaA may play essential roles for this species.
Phylogenetic relationship, nucleotide sequence diversity and conserved functional domains of CwaA.
(a) Phylogenetic tree of cwaA gene and its homologues in 21 Lt. plantarum genomes. The tree was constructed using MEGA 5.1 software with the neighbor-joining method (1000-replicate bootstrapping). Bootstrap values are shown beside each node and the values less than 50% are not shown; (b) Heat-plot of the similarity matrices of cwaA gene in different genomes based on pairwise sequence alignments; and (c) Conserved functional domains in CwaA annotated by CDD. Different confidence levels are represented by specific hits and nonspecific hits and the domain model scope includes superfamilies and multidomains. Specific hits indicate the top-ranking RPS-BLAST hits, meaning a high-confidence association between a query protein and a conserved domain; nonspecific hits meet or exceed the RPS-BLAST threshold for statistical significance; superfamilies are the domain clusters to which the specific and/or nonspecific hits belong; multidomains are domain models likely to contain multiple single domains.
The cwaA gene in NL42 is 2.772 kb long; it encodes 923 amino acids with a predicted molecular weight of 93.7 kD—47 strongly basic (+), 81 strongly acidic (−), 275 hydrophobic and 398 polar amino acids—with a secondary structure consisting mostly of β-sheets and turns (Fig. S5). The N terminus of CwaA is a KxYKxGKxW-type signal peptide (Fig. 4c), which tends to occur on long, low-complexity proteins of the phylum Firmicutes. The SignalP4.1 tool predicted a cleavage site between amino acid positions 48 and 49. The C terminus of CwaA contains an LPQTDE (LPxTG-like cell wall anchoring) motif belonging to the gram-positive LPxTG anchor superfamily. Interestingly, aside from the hexapeptide motif at the C terminus, CwaA possesses five cell wall surface anchor repeat domains (repeats 1 to 5, each 57 amino acids in length) (Fig. 4c and Fig. S6) which were first found in L. monocytogenes35. The LPxTG-like motif and three of the five cell wall surface anchor repeat domains (repeats 3 to 5) were all ranked as specific hit levels by the Conserved Domain Database (CDD) CD-Search tool, which represents a very high confidence level for the inferred function of the query protein36; we therefore concluded that CwaA is a cell wall-anchored protein. The specific hit domains of CwaA also included epiglycanin (tandem-repeating region of mucin, pfam05647), OmpC (outer membrane protein, COG3203), PT (the tetrapeptide XPTX repeat, pfam04886) and BF2867_like_N (N-terminal domain found in Bacteroides fragilis Nctc 9343 BF2867 and related proteins, cd13120), probably with a role in cell adhesion. Moreover, these specific hit domains also overlapped with other nonspecific hits; for example, the cell wall surface anchor repeats 3, 4 and 5 overlapped with MucBP (mucin binding protein) domains (pfam06458). Interestingly, when single domains were considered together (multidomain hit results), CwaA was more related to Hia (COG5295) and FhaB (COG3210) multidomains with e-values of 1.28e-20 and 1.30e-19, respectively. Hia and FhaB are, respectively, annotated as autotransporter adhesion and large exoproteins involved in heme utilization or adhesion. Taken together, these results strongly support CwaA as a multidomain-containing cell wall-anchored protein that is very likely involved in cell adhesion.
Cell wall-anchored domains in CwaA are relatively conserved
Multiple sequence alignment of CwaA with its homologues in another five complete Lt. plantarum genomes indicated that the whole protein sequence of CwaA is most similar to hypothetical protein LBP_cg2016 in Lt. plantarum P8 (90.9% identity). However, the N-terminal signal peptide, the C-terminal LPxTG-like cell wall-anchoring motif and the cell wall surface anchor repeats 3, 4 and 5 of CwaA were nearly identical in these strains (Fig. S6). We further used the ConSurf server to analyze the conservation of amino acids in CwaA and all of its homologous sequences in the database. The results again showed that the amino acids in the regions mentioned above are more conserved (Fig. S7), suggesting that these amino acids per se and the cell wall-anchored domains containing them are critical to CwaA-like proteins.
Overexpression of CwaA in Lactococcus lactis
The cwaA gene in NL42 was cloned, 6×His-tagged and expressed in L. lactis NZ9000 using lactococcal expression vector pNZ401, resulting in the recombinant strain NZ9000-pNZ401-cwaA. Western blotting assay using an anti-His-tagged antibody revealed the expected 93-kD protein product in both the total protein extract and the cell wall-associated protein extract of this recombinant strain (Fig. 5). No corresponding products were found in the parent strain (NZ9000) harboring the empty vector. These results indicated that cwaA is efficiently expressed in Lc. lactis and the presence of its expression product CwaA in the cell wall protein extracts further proved that the protein is anchored to the cell wall.
CwaA increases adhesion of Lactococcus lactis to HT-29 cells
To evaluate whether CwaA is involved in adhesion, we first performed autoaggregation and hydrophobicity assays. Compared with the negative control strain NZ9000-pNZ401, the autoaggregation and hydrophobicity rates of NZ9000-pNZ401-cwaA were 1.8-fold and 5.4-fold higher, respectively (Fig. 6a,b), reaching 33.9% and 85.8%, which was comparable to the levels of Lt. plantarum NL42 (38.7% and 75.4%, respectively). Interestingly, CwaA seemed to be more proficient at improving Lc. lactis hydrophobicity (P < 0.001); the hydrophobicity rate of NZ9000-pNZ401-cwaA was even higher than that of NL42 (Fig. 6b). We then performed adhesion assays using the human colonic epithelial cell line HT-29 as a model. Similar to the trends in the autoaggregation and hydrophobicity assays, CwaA significantly improved the adhesive ability of Lc. lactis NZ9000, with 40-fold increase in the number of adherent bacterial cells (P < 0.01), representing a binding efficiency approaching that of NL42 (Fig. 6c). Taken together, these results confirmed that autoaggregation and hydrophobicity of Lc. lactis are closely correlated with its adhesive ability; CwaA played a critical role in autoaggregation and hydrophobicity improvement and thus increased the adhesion of Lc. lactis to HT-29 cells.
Lactococcus lactis expressing CwaA blocks adhesion of pathogens
To determine whether the increased adhesion of Lc. lactis inhibits adhesion of pathogenic bacteria and to elucidate the mode of action, we used Staphylococcus aureus and enterotoxigenic Escherichia coli (ETEC) as indicators in displacement, competition and exclusion blockage assays, performed by incubating Lc. lactis or Lt. plantarum before (displacement), simultaneously with (competition) or after (exclusion) the pathogens. In general, Lt. plantarum and Lc. lactis both inhibited the adhesion of the two pathogens to HT-29 cells; the greatest blockage effects were observed under conditions of exclusion. Compared with Lc. lactis NZ9000-pNZ401, recombinant strain NZ9000-pNZ401-cwaA further reduced the number of both pathogens adhered to HT-29 cells by approximately 40%, 20% and 20% under conditions of displacement, competition and exclusion, respectively. In the case of S. aureus, NZ9000-pNZ401-cwaA significantly reduced the number of adherent cells under conditions of competition and exclusion compared with NZ9000-pNZ401 (P < 0.05) (Fig. 7a), whereas for ETEC, this trend was only observed under condition of exclusion (Fig. 7b). The results suggested that the improved adhesion of Lc. lactis enhances the blocking effects on S. aureus and ETEC adhesion to HT-29 cells and that exclusion, i.e. occupation of the adhesion sites by Lc. lactis expressing CwaA prior to the pathogens, was the most effective blocking mode.
Inhibition effects of Lc. lactis expressing CwaA on the adhesion of (a) S. aureus and (b) enterotoxigenic E. coli (ETEC).
Adhesion of pathogen to HT-29 cells without added Lc. lactis or Lt. plantarum served as the control. Displacement, competition and exclusion indicate that Lc. lactis or Lt. plantarum was incubated before, simultaneously with and after the pathogens, respectively. Data are presented as means ± SEM of three independent repeats. *P ≤ 0.05, **P ≤ 0.01.
Cell-surface protein serves as a major receptor for CwaA adherence
To reveal which surface components of the HT-29 cell are targets for CwaA adhesion, we treated the cells prior to NZ9000-pNZ401-cwaA adhesion with periodate or protease (trypsin) to investigate the contributions of carbohydrate and protein factors, respectively. Periodate treatment of HT-29 cells seemed to have a concentration-dependent but not statistically significant effect on both Lt. plantarum NL42 and Lc. lactis NZ9000-pNZ401-cwaA adhesion (Fig. 8a). Significantly reduced adhesion to HT-29 cells was only observed for NZ9000-pNZ401-cwaA under treatment with a high periodate concentration (60 mg/ml) (P < 0.05). These results suggested that the surface carbohydrate moieties of HT-29 cells are probably not the major receptor for CwaA adhesion. This was further supported by the results of sugar-inhibition tests in which glucose, lactose, sucrose and mannose had no obvious competitive inhibitory effects on either NL42 or NZ9000-pNZ401-cwaA adhesion (P > 0.05 for each) (Fig. 8b). In contrast, trypsin treatment of HT-29 cells significantly reduced adhesion of both NL42 and NZ9000-pNZ401-cwaA and a significant reduction was even observed at the low trypsin concentration of 10 mg/ml (P < 0.01 and 0.001 for NL42 and NZ9000-pNZ401-cwaA, respectively) (Fig. 8c). In addition, bacterial cell binding to the HT-29 cells decreased gradually with increasing trypsin concentration, showing a strong concentration-dependent trend. Taken together, we suggest that a certain kind of protein, but not carbohydrate moieties, on the HT-29 cell surface is the major receptor for CwaA adhesion.
Discussion
Lt. plantarum is considered a flexible and versatile LAB1. This is reflected, to some extent, by the analysis provided herein, showing that although the 16S rRNA gene of strain NL42 is the same as that of strain WCFS1 and others, the genomes are highly divergent. The NL42 genome was similar in size to that of WCFS1 (the first Lt. plantarum genome), but was highly varied in six large genomic abnormal GC regions; two of these were prophage loci, suggesting the large contribution of horizontal gene transfer mediated by mobile genetic elements to the genome plasticity of this species. In addition, the variations in gene clusters related to polysaccharide biosynthesis (V3) and sugar metabolism and transport (V6) and the gene enrichment in transcription and lipid transport and metabolism might reflect NL42’s adaption to or fitness in the dairy environment.
A mannose-specific adhesion mechanism has been reported in Lt. plantarum strains 299 and 299v and a mannose-specific adhesin (Msa) has been identified in WCFS1 (gene locus, lp_1229)20. Here we found that lp_1229 is located in the V3 region and is lost in strain NL42 (Data S1), suggesting that adhesion of NL42 does not occur in a mannose-specific manner and that Msa is not the major adhesin in this strain. This assumption is also supported by our sugar-inhibition test results in which mannose did not inhibit the adhesion of NL42.
Cell wall-anchored surface proteins, especially those with an N-terminal LPxTG-like motif, have frequently been identified to be involved in adhesion in LAB as well as bacterial pathogens37,38. CwaA was identified as a cell wall-anchored adhesion-associated protein based on the facts that: 1) it contains an LPxTG-like motif, 2) it harbors five cell wall surface anchor repeat domains, three of them overlapping with MucBP domains, 3) its multidomain is similar to those of other adhesion-associated proteins and most importantly 4) it enhances autoaggregation and hydrophobicity of Lc. lactis, thereby providing it with the ability to adhere. CwaA was similar (90.9% amino acid identity) to a hypothetical protein of another isolate originating from a traditional Chinese dairy product—Lt. plantarum P839—and showed 67.3% identity with WCFS1 lp_2486 which has been annotated as a “mucus-binding protein, LPxTG-motif cell wall anchor”, probably due to overlap of the putative MucBP domains with the cell wall surface anchor repeat domains in CwaA. However, our CCD CD-Search results suggested that the cell wall surface anchor repeat domains are ranked as specific hits in the database, i.e., top-ranking RPS-BLAST hits compared to other hits in overlapping intervals, whereas the MucBP domains were ranked as nonspecific hits. We therefore named this protein cell wall-anchored protein A to reflect its features according to the specific domains it contains.
The LPxTG-like motif-containing proteins are sorted and covalently coupled to the cell wall by sortase in gram-positive bacteria40. A previous study indicated that sortase A (SrtA) of Lc. lactis has different LPxTG-like motif-containing substrates, such as LPKTGE, LPFTGG, LPETGD and LPSTGD41. The successful expression of CwaA (LPQTDE) in Lc. lactis prompts us to suggest that LPQTxE, an LPxTG-like sorting motif in cell wall-bound proteins of Lt. plantarum42, is another potential substrate of Lc. lactis sortase. Actually, Lt. plantarum NL42 SrtA and Lc. lactis NZ9000 SrtA shared more than 60% amino acid similarity (data not shown).
Different adhesion-associated proteins of either probiotic or pathogen origin, including the collagen-binding S-layer protein CbsA of Lt. crispatus43, the N-terminal region of the S-layer protein SlpA of Lt. brevis44, cell wall-associated polypeptides SspA and SspB of Streptococcus gordonii45, the lipoprotein BopA of Bifidobacterium bifidum31 and the pneumococcal surface protein PspC of Streptococcus pneumoniae46 have been characterized and demonstrated to confer adhesive properties to Lc. lactis or others with varying degrees. For examples, Lc. lactis MG1363 expressing cell surface Streptococcus gordonii SspA and SspB exhibited 10-fold- and 5-fold-increased binding, respectively45, to immobilized salivary agglutinin glycoprotein compared with controls; and the adhesion abilities of B. longum/infantis E18 to T84, Caco-2 and HT-29 cells were improved by 511%, 180% and 209%, respectively32. Here, CwaA increased the number of Lc. lactis NZ9000 adhering to HT-29 cells by about 40-fold, leading to the recombinant strain nearly reaches the level of the adhesive ability of CwaA’s original host, Lt. plantarum NL42, suggesting that it is a favorable candidate for improving the adhesion of Lc. lactis. However, compared with the degree of the improvement of adhesion, the pathogen blocking effects of Lc. lactis expressing CwaA is relative minor (Fig. 6C). These results prompt us to suggest that the adhesion sites of CwaA and the pathogens tested are not completely overlapped. For example, the E. coli ETEC H10407 we used has been demonstrated to use different strategies to adhere to human epithelial cells, such as using adhesins Tia and Tib and through the interaction of exoprotein EtpA and flagella47,48.
In summary, we sequenced the whole genome of a Lt. plantarum strain NL42 originally isolated from a traditional Chinese dairy product. Comparative genomic analysis of this genome with other available Lt. plantarum genomes was performed, predicting a candidate cell wall-anchored adhesion-associated protein, CwaA, in Lt. plantarum. CwaA conferred Lc. lactis strain NZ9000 with significantly improved adhesive ability to HT-29 cells, probably via adhesion to a surface protein on these cells. In addition, The Lc. lactis expressing CwaA not only acquired improved adhesion capability, but also blocked the adhesion of bacterial pathogens. We therefore expect that a recombinant Lc. lactis with these properties would be most valuable for future efficient delivery of interesting molecules. Furthermore, we should stress that future efforts are still needed to address the issues including the specific target of CwaA and the attachment mechanism, the core functional domains of CwaA, the relationship between cawA gene diversity and its adhesive characteristics and the efficiency of CwaA to improve the probiotic traits in vivo.
Methods
Bacterial strains and growth conditions
The strains and plasmids used in this study are listed in Table S1. Lt. plantarum strain NL42 was grown anaerobically at 37 °C in MRS broth (Difco Laboratories, Detroit, MI) for 16 h. Lactococcus strains were cultured in M17 (Difco) containing 0.5% (w/v) glucose at 30 °C without shaking. When required, erythromycin was added at 5 μg/ml. The S. aureus and E. coli were incubated in LB medium containing 1% (w/v) tryptone, 0.5% (w/v) yeast extract and 1% (w/v) NaCl at 37 °C with shaking at 220 rpm for 16 h.
Genome sequencing and comparative genomics
The whole genome of Lt. plantarum NL42 was sequenced on an Illumina HiSeq 2000 platform according to a standard protocol. Genome assembly and annotation were performed with SOAPdenovo (http://soap.genomics.org.cn) and RAST programs (Rapid Annotation using Subsystem Technology)49, respectively. Available Lt. plantarum genomes (6 complete and 15 draft sequences) were retrieved from NCBI GenBank and whole-genome alignment and SNP calling were performed using Mugsy50. Protein sequence similarity among genomes was determined by BLASP against the reference genome WCFS1 and visualized as a circle heat map using Circos51. Multiple sequence alignment of CwaA with its homologues in other genomes was performed by Clustal X version 2.052. Gene functional categories were analyzed using the COG database53. Adhesion- and cell wall anchor-associated domain-containing proteins were searched by BLAST against the Pfam protein families database54.
Other bioinformatics tools
MEGA software (version 5)55 was used to construct the phylogenetic trees. The CD-Search tool in the CDD (Conserved Domain Database)36 was used to search for conserved domains and functional annotations in CwaA. SignalP4.1 server (http://www.cbs.dtu.dk/services/SignalP/) was used to predict the CwaA signal peptide sequence and cleavage site. The evolutionary conservation of amino acids in CwaA was estimated with the help of ConSurf server (http://consurf.tau.ac.il/). The secondary structure of CwaA was predicted using Protean (Lasergene package, DNASTAR, Madison, WI).
Cloning and inducible expression
The cwaA gene was amplified from Lt. plantarum NL42 chromosomal DNA using primers of cwaAF (5′-GCTCTAGAATGTCAAAAGATAATCAAAAA-3′, XbaI site underlined) and cwaAR (5′-CCGCTCGAGTTAGTGGTGGTGGTGGTGGTGTGCTTCATGCTTCCGACGAGA-3′, XhoI site underlined) and sequence coding for a 6 × His tag (italics) was incorporated into the reverse primer. The PCR product was then cloned into plasmid pNZ401 between XbaI and XhoI sites, resulting pNZ401-cwaA. Plasmids pNZ401-cwaA and empty control pNZ401 extracted from E. coli DH5α were both transformed into Lc. lactis NZ9000. For inducible expression, the recombinant Lc. lactis NZ9000-401-cwaA and control (NZ9000-401) were grown overnight in glucose-M17 (GM17) medium containing 5 μg/ml erythromycin. A 2% (v/v) inoculum was transferred to fresh GM17 broth and grown at 30 °C without shaking to an optical density at 600 nm (OD600) of 0.4 to 0.6 and then 10 ng/ml nisin was added and the culture was incubated for 3 h before harvesting.
Western blot analysis
The induced recombinant Lc. lactis was harvested by centrifugation at 8000 g for 10 min at 4 °C. Pelleted cells were washed three times in PBS, resuspended in PBS and disrupted by ultrasonicator. The supernatant was mixed with 5 × SDS loading buffer (250 mM Tris-HCl pH 6.8, 10% w/v SDS, 0.5% w/v bromophenol blue, 50% v/v glycerol, 5% w/v β-mercaptoethanol) and separated by 12% SDS–PAGE and then transferred to a nitrocellulose membrane using a semi-dry transmembrane system. Protein bands were detected using a His-Tag XP@ Rabbit monoclonal antibody. The L. lactis NZ9000 harboring empty vector pNZ401 was used as a control. Cell wall proteins were extracted as described previously56.
Autoaggregation and hydrophobicity assays
Autoaggregation assays were carried out according to Collado et al.57 with some modifications. The bacterial cells were harvested by centrifugation at 8000 g for 10 min, washed twice in PBS and resuspended in PBS to an OD600 of around 0.5. The bacterial suspensions were then mixed by vortexing and incubated at 30 °C for 4 h. Autoaggregation percentage was calculated using the formula: 1−(A4/A0) × 100%, where A4 represents OD600 at 4 h and A0 the absorbance at 0 h. In the hydrophobicity assay, 1 ml xylene was added to 3 ml cell suspension; the mixture was shaken by vortexing for 90 s and incubated at room temperature for 20 min and then the OD600 of the aqueous phase was measured. The percentage of hydrophobicity was expressed as [(A0−A)/A0] × 100%, where A0 and A are the absorbance before and after extraction with xylene, respectively.
HT-29 cell culture and adhesion assay
Human colonic epithelial HT-29 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% (v/v) fetal calf serum and 100 U/ml of penicillin/streptomycin. For adhesion assays, 105 HT-29 cells were seeded in 24-well plates with glass cover slips and maintained at 37 °C under 5% CO2 for 3 days. Prior to the experiments, all bacterial cultures were harvested until the stationary phase or after induction and washed twice in PBS. Bacterial cells of 108 or 109 CFU/ml dissolved in 1 ml DMEM were inoculated into each well containing HT-29 cells. After co-incubation for 4 h at 37 °C, 5% CO2, the HT-29 cells were washed five times in PBS to remove the free bacterial cells and then lysed with 1 ml Triton X-100 (1% v/v) in PBS. The cell lysates were serially diluted and plated on agar plates.
Treatment of cells with sodium periodate and trypsin
Washed HT-29 cells were disposed with different concentrations of sodium periodate or trypsin (37 °C, 30 min) as previously described58 and then adhesion experiments were performed. Acetate buffer (0.2 M, pH 4.6) was used to dilute sodium periodate and trypsin and alone as a control. The sodium periodate was used at 0, 20, 40 and 60 mg/ml and the trypsin was used at 0, 10, 20 and 30 mg/ml.
Sugar-inhibition tests
Four types of sugar—glucose, mannose, sucrose and lactose—were tested for their ability to competitively inhibit adhesion. The adhesion assay was carried out in the presence of a sugar at 20 mg/ml and no sugar was added to the corresponding control.
Blocking pathogen adhesion
To investigate the ability of the recombinant Lc. lactis to block the adhesion of pathogens to HT-29 cells, S. aureus ATCC 25923 and E. coli ETEC H10407 (ATCC 35401) were used for displacement, competition and exclusion assays as described previously59. The S. aureus ATCC 25923 is a widely used indicator for both antimicrobial activity and adhesion assays and the E. coli ETEC H10407 (serotype O78:H11) is an enterotoxin producer, which was originally isolated from human feces60. Briefly, in the displacement assay, 500 μl pathogens (108 CFU/ml) and HT-29 cells (106) were incubated together (37 °C, 2 h) and then 500 μl Lc. lactis (108 CFU/ml) was added later and incubated for another 2 h; in the competition assay, Lc. lactis, pathogens and HT-29 cells were incubated together (37 °C, 4 h); in the exclusion assay, Lc. lactis and HT-29 cells were incubated together (37 °C, 2 h) and then 500 μl pathogens was added and the mixture incubated for another 2 h. After the adhesion incubation, HT-29 cells were washed five times with PBS and lysed with 1 ml Triton X-100 (1% in PBS). The cell lysates were serially diluted and plated on a LB agar plate.
Statistics
Statistical analyses were performed using unpaired two-tailed Student’s t-tests. P values less than 0.05 were considered to be statistically significant. Data are presented as means ± SEM of three independent repeats in each experiment.
Nucleotide sequence accession numbers
The genome sequence of Lt. plantarum NL42 and the nucleotide sequence of the cwaA gene have been deposited in the GenBank database under accession numbersJZSB00000000 and KP893285, respectively.
Additional Information
How to cite this article: Zhang, B. et al. Comparative genome-based identification of a cell wall-anchored protein from Lactobacillus plantarum increases adhesion of Lactococcus lactis to human epithelial cells. Sci. Rep. 5, 14109; doi: 10.1038/srep14109 (2015).
References
Kleerebezem, M. et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100, 1990–1995 (2003).
Niedzielin, K., Kordecki, H. & ena Birkenfeld, B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 13, 1143–1147 (2001).
Wullt, M., Hagslätt, M. L. & Odenholt, I. Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: a double-blind, placebo-controlled trial. Scand. J. Infect. Dis. 35, 365–367 (2003).
Karczewski, J. et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G851–G859 (2010).
Lönnermark, E. et al. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J. Clin. Gastroenterol. 44, 106–112 (2010).
Nguyen, T., Kang, J. & Lee, M. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 113, 358–361 (2007).
Herias, M. et al. Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin. Exp. Immunol. 116, 283–290 (1999).
Molin, G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 73, 380s–385s (2001).
De Vries, M. C., Vaughan, E. E., Kleerebezem, M. & de Vos, W. M. Lactobacillus plantarum-survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 16, 1018–1028 (2006).
Nagpal, R. et al. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol. Lett. 334, 1–15 (2012).
Ennahar, S. et al. Production of pediocin AcH by Lactobacillus plantarum WHE 92 isolated from cheese. Appl. Environ. Microb. 62, 4381–4387 (1996).
Giraud, E., Brauman, A., Keleke, S., Lelong, B. & Raimbault, M. Isolation and physiological study of an amylolytic strain of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 36, 379–383 (1991).
Siezen, R. J. et al. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ. Microbiol. 12, 758–773 (2010).
Ouwehand, A. C., Salminen, S. & Isolauri, E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82, 279–289 (2002).
Ljungh, A. & Wadstrom, T. Lactic acid bacteria as probiotics. Curr. Issues Intest. Microbiol. 7, 73–90 (2006).
Rahman, M. M., Kim, W.-S., Kumura, H. & Shimazaki, K.-I. Autoaggregation and surface hydrophobicity of bifidobacteria. World J. Microb. Biot. 24, 1593–1598 (2008).
Pan, W.-H., Li, P.-L. & Liu, Z. The correlation between surface hydrophobicity and adherence of Bifidobacterium strains from centenarians’ faeces. Anaerobe 12, 148–152 (2006).
Roos, S. & Jonsson, H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148, 433–442 (2002).
Aleljung, P. et al. Purification of collagen-binding proteins of Lactobacillus reuteri NCIB 11951. Curr. Microbiol. 28, 231–236 (1994).
Pretzer, G. et al. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 187, 6128–6136 (2005).
Kankainen, M. et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. USA 106, 17193–17198 (2009).
Le, D. T. L. et al. Unraveling the Role of Surface Mucus-Binding Protein and Pili in Muco-Adhesion of Lactococcus lactis. PloS One 8, e79850 (2013).
Boekhorst, J., Helmer, Q., Kleerebezem, M. & Siezen, R. J. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology 152, 273–280 (2006).
Meyrand, M. et al. Surface proteome analysis of a natural isolate of Lactococcus lactis reveals the presence of pili able to bind human intestinal epithelial cells. Mol. Cell. Proteomics 12, 3935–3947 (2013).
Giaouris, E., Chapot-Chartier, M.-P. & Briandet, R. Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int. J. Food Microbiol. 131, 2–9 (2009).
Steidler, L. Genetically engineered probiotics. Best Pract. Res. Cl. Ga. 17, 861–876 (2003).
Drouault, S., Juste, C., Marteau, P., Renault, P. & Corthier, G. Oral treatment with Lactococcus lactis expressing Staphylococcus hyicus lipase enhances lipid digestion in pigs with induced pancreatic insufficiency. Appl. Environ. Microbiol. 68, 3166–3168 (2002).
Steidler, L. et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289, 1352–1355 (2000).
Steidler, L. et al. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66, 3183–3189 (1998).
Koo, O. K., Amalaradjou, M. A. R. & Bhunia, A. K. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PloS One 7, e29277 (2012).
Gleinser, M., Grimm, V., Zhurina, D., Yuan, J. & Riedel, C. U. Improved adhesive properties of recombinant bifidobacteria expressing the Bifidobacterium bifidum-specific lipoprotein BopA. Microb. Cell Fact. 11, 80–94 (2012).
Nouaille, S. et al. Heterologous protein production and delivery systems for Lactococcus lactis. Genet. Mol. Res. 2, 102–111 (2003).
Bermúdez-Humarán, L. G., Kharrat, P., Chatel, J.-M. & Langella, P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell. Fact. 10, S4 (2011).
Zuo, F., Feng, X., Chen, L. & Chen, S. Identification and partial characterization of lactic acid bacteria isolated from traditional dairy products produced by herders in the western Tianshan Mountains of China. Lett. Appl. Microbiol. 59, 549–556 (2014).
Bierne, H. & Cossart, P. Listeria monocytogenes surface proteins: from genome predictions to function. Microbiol. Mol. Biol. Rev. 71, 377–397 (2007).
Marchler-Bauer, A. et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39, D225–D229 (2011).
von Ossowski, I. et al. Functional characterization of a mucus-specific LPXTG surface adhesin from probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 77, 4465–4472 (2011).
Foster, T. J., Geoghegan, J. A., Ganesh, V. K. & Höök, M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12, 49–62 (2014).
Bao, Y. et al. Effect of Lactobacillus plantarum P-8 on lipid metabolism in hyperlipidemic rat model. Eur. J. Lipid Sci. Tech. 114, 1230–1236 (2012).
Call, E. K. & Klaenhammer, T. R. Relevance and application of sortase and sortase-dependent proteins in lactic acid bacteria. Front. Microbiol. 4 (2013).
Dieye, Y. et al. Functionality of sortase A in Lactococcus lactis. Appl. Environ. Microbiol. 76, 7332–7337 (2010).
Boekhorst, J., de Been, M. W., Kleerebezem, M. & Siezen, R. J. Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J. Bacteriol. 187, 4928–4934 (2005).
Sillanpää, J. et al. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182, 6440–6450 (2000).
Åvall-Jääskeläinen, S., Lindholm, A. & Palva, A. Surface display of the receptor-binding region of the Lactobacillus brevis S-layer protein in Lactococcus lactis provides nonadhesive lactococci with the ability to adhere to intestinal epithelial cells. Appl. Environ. Microbiol. 69, 2230–2236 (2003).
Holmes, A. R., Gilbert, C., Wells, J. M. & Jenkinson, H. F. Binding properties of Streptococcus gordonii SspA and SspB (antigen I/II family) polypeptides expressed on the cell surface of Lactococcus lactis MG1363. Infect. Immun. 66, 4633–4639 (1998).
Asmat, T. M., Klingbeil, K., Jensch, I., Burchhardt, G. & Hammerschmidt, S. Heterologous expression of pneumococcal virulence factor PspC on the surface of Lactococcus lactis confers adhesive properties. Microbiology 158, 771–780 (2012).
Fleckenstein, J. M., Kopecko, D. J., Warren, R. L. & Elsinghorst, E. A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 64, 2256–2265 (1996).
Roy, K. et al. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 457, 594–598 (2009).
Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9, 75 (2008).
Angiuoli, S. V. & Salzberg, S. L. Mugsy: fast multiple alignment of closely related whole genomes. Bioinformatics 27, 334–342 (2011).
Krzywinski, M. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Tatusov, R. L. et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41 (2003).
Punta, M. et al. The Pfam protein families database. Nucleic Acids Res. 40, D290–301 (2012).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
De Angelis, M. et al. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic and cell wall protein analyses. Appl. Environ. Microbiol. 67, 2011–2020 (2001).
Collado, M. C., Meriluoto, J. & Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 226, 1065–1073 (2008).
Lin, X., Wang, Z., Niu, Z., Peng, J. & Wang, Y. The nature of adhesion factors which lie on the surfaces of Lactobacillus adhering to cells. Adv. Biosci. Biotechnol. 3, 153–157 (2012).
Zárate, G. & Nader-Macias, M. E. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 43, 174–180 (2006).
Skerman, F. J. et al. Plasmid-associated enterotoxin production in a strain of Escherichia coli isolated from humans. Infect. Immun. 5, 622–624 (1972).
Acknowledgements
This work was supported by the ‘Five-twelfth’ National Science and Technology Support Program of China (2011AA100903).
Author information
Authors and Affiliations
Contributions
B.Z. and S.C. designed and conceived the experiments. F.Z. isolated strain NL42. B.Z., R.Y. and Z.Z. performed the experiments. B.Z. analyzed the data and wrote the manuscript. H.M. and S.C. reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, B., Zuo, F., Yu, R. et al. Comparative genome-based identification of a cell wall-anchored protein from Lactobacillus plantarum increases adhesion of Lactococcus lactis to human epithelial cells. Sci Rep 5, 14109 (2015). https://doi.org/10.1038/srep14109
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14109
This article is cited by
-
Genome Sequencing Unveils Nomadic Traits of Lactiplantibacillus plantarum in Japanese Post-Fermented Tea
Current Microbiology (2024)
-
Draft genome sequence and probiotic functional property analysis of Lactobacillus gasseri LM1065 for food industry applications
Scientific Reports (2023)
-
Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi
Food Science and Biotechnology (2021)
-
Lactiplantibacillus plantarum strains isolated from spontaneously fermented cocoa exhibit potential probiotic properties against Gardnerella vaginalis and Neisseria gonorrhoeae
BMC Microbiology (2021)
-
Metabolic engineering of Lactococcus lactis for high level accumulation of glutathione and S-adenosyl-l-methionine
World Journal of Microbiology and Biotechnology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.