Abstract
Molting is a critical developmental process for crustaceans, yet the underlying molecular mechanism is unknown. In this study, we used RNA-Seq to investigate transcriptomic profiles of the hepatopancreas and identified differentially expressed genes at four molting stages of Chinese mitten crab (Eriocheir sinensis). A total of 97,398 transcripts were assembled, with 31,900 transcripts annotated. Transcriptomic comparison revealed 1,189 genes differentially expressed amongst different molting stages. We observed a pattern associated with energy metabolism and physiological responses during a molting cycle. In specific, differentially expressed genes enriched in postmolt were linked to energy consumption whereas genes enriched in intermolt were related to carbohydrates, lipids metabolic and biosynthetic processes. In premolt, a preparation stage for upcoming molting and energy consumption, highly expressed genes were enriched in response to steroid hormone stimulus and immune system development. The expression profiles of twelve functional genes detected via RNA-Seq were corroborated through real-time RT-PCR assay. Together, our results, including assembled transcriptomes, annotated functional elements and enriched differentially expressed genes amongst different molting stages, provide novel insights into the functions of the hepatopancreas in energy metabolism and biological processes pertaining to molting in crustaceans.
Similar content being viewed by others
Introduction
Molting is an essential biological process occurring multiple times throughout the life history of crustaceans. Many behavioral and physiological activities such as growth, development and reproduction depend on successful molting1,2,3,4. Crustaceans experience rhythmic molting cycles, each cycle with four major stages, ecdysis (molting), postmolt, intermolt and premolt5. During ecdysis, individuals must loosen the connectives between living tissues and extracellular cuticles, absorb water to expand the new flexible exoskeletons and then quickly harden the skeletons for defense and locomotion6. During postmolt, the stage immediately following ecdysis, the exoskeleton is hardened via sclerotization and mineralization6. Intermolt is the longest period in a molting cycle during which individual crustaceans accumulate energy and gain weight. In premolt, the stage prior to ecdysis, the old exoskeleton is absorbed and the new exoskeleton will be formed.
Many regulatory hormones/genes and potential pathways that are pertaining to molting have been identified. These include ecdysteriods and crustacean farnesoate (MF), regulatory hormones that promote molting7. The primary site for ecdysteroid synthesis is the molting glands (Y-organs or YOs). The neuroendocrine hormones, molt-inhibiting hormone (MIH) and crustacean hyperglycaemic hormone (CHH), which are synthesized and stored in the sinus gland/X-organ complex, were found to inhibit crustacean molting8. A number of other hormones/genes, including vitellogenesis-inhibiting hormone (VIH), ecdysone receptor (EcR) and retinoid X receptor (RXR), have been connected to molting. A possible signaling pathway linking MIH to the regulation of ecdysteroidogenesis in decapod crustacean molting gland has been proposed, which include the triggering phase (MIH reduction) and the summation phase (ecdysone synthesis and secretion)6. Although much progress has been made in understanding the mechanism of crustacean molting, the studies conducted so far focus mainly on a few important hormones/genes or individual pathways3,6,7,9.
The Chinese mitten crab Eriocheir sinensis, native to East Asia, is the most important economic crab species throughout the northern and central coastal regions of China10. This species has also gained notoriety as an invasive species in Europe and North America11. The economic and biological significance of this species makes the Chinese mitten crab a unique system to explore the genes involved in the molting cycle. Periodic molting occurs approximately 18 times during its life cycle12. The duration of these molting stages ultimately determines the lifespan of E. sinensis11,13,14,15. Through each molting cycle, the crab shows saltatory changes or increments in growth and autotomized organ regeneration. Understanding the molecular mechanism pertaining to the molting of E. sinensis could promote aquaculture efforts in China and assist its biological control in Europe and North America.
The hepatopancreas plays important roles in carbohydrate and lipid metabolism, nutritional status, energy storage and breakdown in various crustaceans16. It is an important organ for the steroid hormone biosynthesis and catabolism related to reproduction system17,18. The hepatopancreas is also responsible for removing ecdysteroids from the hemolymph and adjusting ecdysteroid changes during the molting cycle19,20. In addition, large amounts of energy are stored in the hepatopancreas in preparation for molting, reproduction, limb regeneration and other life activities18. The hepatopancreas is thus an ideal organ to study transcriptomic changes during molting cycles.
The advent and advance of high-throughput next-generation sequencing (NGS) technology has revolutionized the way we conduct various biological research21,22,23. In this study, RNA-Seq was used to investigate hepatopancreas transcriptome profiles in E. sinensis. The specific objectives are (1) assemble and annotate a comprehensive de novo transcriptome of E. sinensis hepatopancreas, (2) identify differentially expressed genes enriched at different molting stages and (3) validate expression patterns of important functional genes via quantitative real-time PCR (qRT-PCR).
Materials and Methods
Animal Sampling
One-year-old crabs from a full-sib family were sampled from our research facility at the Shanghai Ocean University (China, Shanghai). These individuals were cultured in glass tanks with adequate aeration, temperature (20 °C) and food (twice daily). Based on our previous observations, one molting cycle takes approximately 30 days for a one-year-old E. sinensis under glass tank conditions, male crabs grow faster than female and intermolt (stage) is longer than postmolt or premolt24. In our experiment, crabs from four different time points after molting (Fig. 1) were sampled for analysis. Sampling started at the following days: Day 2 after molting (postmolt, PoM) - the exoskeleton of the crabs was soft and the crabs sheltered in the corner and did not ingest food; Day 10 after molting (intermolt-I, InM-I) - the exoskeleton was consolidated and hardened, the color of the carapace turned cyanish and the crabs started to ingest food; Day 20 after molting (intermolt-II, InM-II) - the external morphological pattern of the crabs looked similar to that at InM-I stage except that the color of the carapace turned brownish; Day 30 after molting (premolt, PrM) - pleural suture cracks between the carapace and the abdomen of the crabs were present, the shell color turned dark-brown and the crabs stopped eating. According to Phlippen’s description of molting stages, PoM should correspond to stage B, InM-I and InM-II both correspond to stage C and PrM corresponds to stage D3 25. Three individual crabs were sampled at each molting stage. The carapace length, carapace width and body weight of each crab were measured prior to tissue collection (Table S1). Fresh hepatopancreas tissue from each crab was quickly collected and immediately stored into liquid nitrogen for RNA isolation.
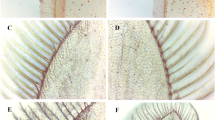
Energy metabolism and physiological responses in a typical molting cycle in Chinese mitten crab.
(A) Day 2 after molting (postmolt, PoM), soft exoskeleton; (B) Day 10 after molting (intermolt-I, InM-I), hardened exoskeleton and cyanish carapace; (C) Day 20 after molting (intermolt, InM-II), brownish carapace; (D) Day 30 after molting (Premolt, PrM), pleural suture cracks between carapace and abdomen and dark brown carapace. M - molting stage (ecdysis), yellow arrows - molting cycle, green bars - boundaries of different molting stages, black circle - physiological responses and red circle - energy metabolism pattern in different molting stages.
RNA isolation and RNA-Seq library preparation
Total RNA was extracted from approximately 80 mg of hepatopancreas tissue with TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. The RNA integrity and quantity were determined using an Agilent 2100 Bioanalyzer (Agilent, Shanghai, China). A total of 4 μg RNA with RNA integrity number above 7.0 was used for RNA-Seq library construction using the NEBNext® UltraTM RNA Library Prep Kit for Illumina (NEB, USA). Indexed libraries were then pooled and sequenced on Illumina HiseqTM2500, with 150 bp pair-end reads produced.
Cleaning and assembly of transcriptome sequencing reads
Raw reads from all four stages were first combined and quality-filtered using the Trimmomatic read trimming tool26. Reads containing 3′ or 5′ ends with an average quality score below 20 in a 4 bp sliding window were trimmed and reads with quality below 10 at the beginning or the end were also removed26. Any reads shorter than 120 bp were excluded from further assembly. All the cleaned reads were used for the reference transcriptome assembly based on Trinity version 2014-04-13 with the paired-end mode27. To filter out the misassembled transcripts, raw sequenced reads were mapped to the assembled reference transcriptome using Bowtie 1.0.028. Transcript abundance was estimated using RSEM software, the FPKM (fragments per kilobase per transcript per million mapped reads) value was calculated and transcripts with FPKM < 1 were filtered out29. Filtered transcripts were then used as E. sinensis reference transcriptome for downstream analysis.
To identify transcriptomic differences amongst the four molting stages of the molting cycle (PoM, InM-I, InM-II and PrM), four de novo assembled transcriptomes (one at each stage) were assessed using Trinity software. To investigate the accuracy of the assembled transcriptome, raw reads were mapped to the assembled transcriptome using Bwa-0.7.9a with BWA-MEM algorithm30 and the mapping statistics was calculated using SAMtools 0.1.19 with the samtools flagstat command31. To test the saturation level of our assembled transcriptome, 20, 40, 60, 80 and 100 million reads were randomly selected from the raw reads and then assembled using Trinity software and the number of transcripts with FPKM ≥ 1 was counted.
Transcriptome annotation
Assembled transcriptomes were annotated using BLASTX against NCBI-NR and UniProt protein databases, with a cutoff E-value smaller than 1e-6. The BLASTX results were imported into BLAST2GO software and Gene Ontology (GO) terms and EC numbers for the KEGG pathway were annotated32. The protein-coding DNA sequence region (CDS) was predicted using TransDecoder implemented in Trinity software. Sequences with a corresponding protein length greater than 100 were retained for further analysis. The predicted CDS was also subjected to BLASTP against the eggNOG V4.0 database to predict Clusters of Orthologous Groups (COGs)33. SignalP 4.1 software was used for predicting the presence and location of signal peptide cleavage sites in amino acid sequences34. To predict transcription factors (TFs), protein sequences were used as queries against the PFAM-A database using HMMER v3.1b1 software with hmmscan command35. The curated DNA-binding domain HMM list (PFAM 18) download from the DBD database was used to map the TF ID36. To identify novel noncoding RNAs, we removed the transcripts that could be annotated as functional genes based on the NCBI-NR database, as well as transcripts with the number of amino acids more than 100. The remaining transcripts were used as query sequences to BLAST against the Rfam database using rfam_scan.pl_v_1.04 software37.
Differential expression analysis
The filtered transcriptome generated through de novo assembly was used as a reference transcriptome for RNA-Seq expression analysis. Raw reads generated from each stage were mapped to the reference transcriptome and FPKM values were calculated by RSEM software29. The resulting data matrix that contains the expression value (FPKM) for the samples of all four stages was generated by “rsem-generate-data-matrix” script. This data matrix was imported into edgeR 2.14 to identify the differentially expressed genes (DEGs) with P < 0.001 for FDR38. FPKM values of DEGs were normalized by log2 and median centered and cluster analysis was performed using the hierarchical cluster method based on Euclidean distance using Trinity Perl script27. We chose clusters of genes that showed different expression patterns following the instructions of Trinity software. For each defined cluster, expression patterns were plotted by R scripts. GO enrichment analysis of the DEGs detected in specific clusters was conducted by DAVID function annotation tools39.
qRT-PCR validation
To validate the results from RNA-Seq differential gene expression analysis, quantitative real-time PCR (qRT-PCR) was carried out amongst four molting stage. Twelve genes from different gene expression clusters were chosen for qRT-PCR assays. PCR primers were designed based on the assembled transcriptome sequences (Table S2). Internal reference genes were chosen based on the estimation of RefFinder (http://www.leonxie.com/referencegene.php). RefFinder integrates the currently available major computational programs (geNorm, Normfinder and BestKeeper) and the comparative ∆Ct method to compare and rank the tested candidate reference genes40,41,42,43. We used alpha-tubulin, ubiquitin conjugating enzyme and β-actin as internal reference genes to normalize the gene expression level (Figure S1). The qRT-PCR was conducted by using SYBR Green Premix Ex Taq (Takara, Japan) in a CFX96 real-time PCR system (Bio-Rad, USA). A standard curve was firstly generated to assess accuracy and primers with efficiency of amplification between 95% and 105% were chosen for following qRT-PCR. Four biological and three technical replicates were used for each gene. qRT-PCR was performed in a 25 μl reaction mixture including 12.5 μl SYBR Premix Ex TaqTM II (2×), 1 μl each primer (10 μM), 2 μl cDNA and 8.5 μl ddH2O. The PCR procedure used was: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s, a 0.5 °C/5 s incremental increase from 60 °C to 95 °C, 30 s elapse time for each cycles. The relative expression was estimated using the 2–∆∆Ct method with PoM stage samples as calibration control, therefore, ∆∆Ct = [(Ct, target – Ct, reference) molt stage] – [(Ct, target – Ct, reference) PoM stage]44. Relative expression results were presented as the fold-change relative to PoM stage. Statistical significance (P < 0.05) was determined using one-way ANOVA and Duncan’s multiple range tests under SPSS 17.0.
Results
Transcriptome assembly
A total of 125,852,483 paired-end reads with 150 bp each were obtained from 12 samples at the four molting stages. After trimming low quality reads, 100,979,293 clean reads were used for de novo transcriptome assembly, resulting in 256,361 transcripts (each with more than 200 bp). The N50 for the assembled transcripts were 766 bp and the average length was 585 bp. A large portion of transcripts were smaller than 400 bp (Figure S2A). After filtering transcripts with FPKM < 1, 97,398 transcripts remained for downstream analysis (Figure S3). These transcripts resulted in a filtered reference transcriptome with N50 of 1,432 bp and an average length of 746 bp (Figure S2B). Although the number of transcripts decreased after filtering, the proportion of reads mapped to the transcriptomes was comparable (87.7% vs 86.3%) (Table 1), indicating the transcripts with FPKM < 1 were likely to be misassembled.
The number of transcripts assembled from over 60 million reads varied from 113,599 to 151,932, depending on the molting stage. The number of transcripts with FPKM ≥ 1 ranged from 79,273 to 104,104 (Fig. 2A, Table S3). Sequencing reads from the specimens of each stage were mapped back to the filtered reference transcriptome and the corresponding stage-specific transcriptome. More than 80% of the reads were mapped to the filtered reference transcriptome and the corresponding transcriptome (Fig. 2B, Table S4). The number of transcripts (FPKM ≥ 1) assembled from 20, 40, 60, 80 and 100 million clean reads ranged from 82,379 to 102,527, indicating that 20 million clean reads were nearly sufficient for E. sinensis transcriptome assembly. These results demonstrated that our reference transcriptome assembled with more than 100 million clean reads was saturated and thus of very high quality (Figure S4).
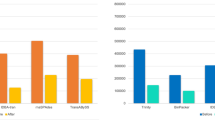
Assembly, mapping, annotation and comparisons of transcriptomes in different molting stages of Chinese mitten crab.
(A) Total number of de novo assembled transcripts and number of transcripts with FPKM ≥ 1; (B) Total number of reads, number of reads mapped to reference transcriptome and number of reads mapped to stage-specific transcriptome for each molting stage; (C) Number of transcripts annotated using NCBI-NR and UniProt protein database; (D) Venn diagram for the number of annotated genes. PoM: postmolt (Day 2), InM-I: intermolt-I (Day 10), InM-II: intermolt-II (Day 20) and PrM: premolt (Day 30).
Transcriptome annotation
BLASTX searches against two protein databases (NCBI-NR and UniProt) resulted in 31,900 (32.8% of total transcripts) and 23,582 transcripts annotated, respectively, with significant BLAST hits to the reference transcriptome (Fig. 2C, Table 1). Most top BLAST hits were matched to Daphnia pulex sequences (Figure S5). Further GO analysis showed that 24,402 transcripts were mapped to at least one GO category (Figure S6). In biological processes, most of the GO-mapped transcripts were related to cellular and metabolic processes. A high number of transcripts (2,280) were mapped to the GO category: response to stimulus (GO:0050896). In addition, we identified 91, 170 and 753 transcripts involved in growth (GO:0040007), reproduction (GO:0000003) and developmental processes (GO:0032502), respectively. A total of 14,760 transcripts were annotated with corresponding enzymes and 131 KEGG pathways were identified through functional analysis of the E. sinensis transcriptome (Table S5). Purine metabolism, pyrimidine metabolism, thiamine metabolism, pyruvate metabolism and oxidative phosphorylation were the top pathways with the highest number of genes represented.
After TransDecoder prediction, the number of predicted protein coding sequences (CDS) with a minimum amino acid length of 100 was 36,948. The predicted protein sequences were used for BLASTP against the eggNOG database to find COGs and the results are presented in Figure S7. A total of 28,904 transcripts were found in one COG group. Most proteins were annotated as “S: functional unknown” due to the lack of annotated protein sequences of closely related species. Using predicted amino acid sequences, 2,149 signal peptides with cleavage sites were predicted (Table S6). With the HMMER software and PFAM-A database, 1,167 protein sequences were predicted to have 106 TFs (Table S7) compared with 112 TFs in D. pulex36. The top 20 TFs in E. sinensis were compared with the top 20 TFs in D. pulex (Figure S8A,B) and both had around 60% C2H2-type zinc finger (PF00096) TFs. In addition, we were able to identify 135 transcripts corresponding to 27 noncoding RNAs that include micro-RNAs, small RNAs, 5S_rRNAs and tRNAs (Table S8).
The number of transcripts annotated through BLAST search against NCBI-NR database ranged from 21,405 to 23,666, depending on the molting stage and the number of transcripts annotated with UniProt ranged from 16,881 to 18,715 (Fig. 2C). We identified 5,860 annotated genes shared among stages (Fig. 2D) corresponding to 18,014, 16,509, 17,782 and 18,032 transcripts in PoM, InM-I, InM-II and PrM, respectively. The numbers of transcripts in the PrM and PoM stages were significantly higher than those in the InM-I and InM-II stages (P < 0.01, t-test) (Table S9).
Differentially expressed genes (DEGs) in different molting stages
A total of 1,189 differentially expressed genes (DEGs) were identified across the four molting stages. No DEGs were observed between the two intermolt stages (InM-I and InM-II); more DEGs were found in PoM compared with other stages (Fig. 3). We identified 740, 491 and 550 genes differentially expressed respectively between PoM and each of other three stages, InM-I, InM-II and PrM. A total of 195 DEGs were identified between PrM and InM-I whereas 24 DEGs were found between PrM and InM-II. Eight clusters were defined based on the hierarchical clustering results revealing different expression patterns in the four stages (Fig. 3 and S9). DEGs in clusters 1, 3, 5 and 6 were highly expressed in PoM compared to other stages. DEGs in cluster 2 showed that the expression of genes increased with the days following molting (from PoM to PrM). Clusters 4, 7 and 8 showed that the genes had low expression levels in the PoM and PrM stages but relatively high expression levels in the InM-I and InM-II stages (Fig. 3 and S9).
Heat map of differentially expressed genes clustered in two ways - molting stages and GO categories.
PoM: postmolt (Day 2), InM-I: intermolt-I (Day 10), InM-II: intermolt-II (Day 20) and PrM: premolt (Day 30). Numbers - replicate samples, C1–C8 - functional clusters of differentially expressed genes and Color key value - FPKM fold change.
GO enrichment analysis of differentially expressed genes showed that genes in clusters 1, 3, 5 and 6 (highly expressed in PoM; Fig. 3) were mainly associated with “energy consumption process”, “homeostasis process” and “response to stimulus”. Genes such as sterol o-acyltransferase 1(soat1), niemann-pick C1(npc1), apoptosis 2 inhibitor (iap2), G-protein coupled receptor moody (moody) and nocturnin (ccrn4l) in cluster 1 were enriched in biological processes such as lipid and sterol homeostasis and metabolism, programmed cell death and endothelial cell differentiation, response to stimulus (smell, chemicals and cocaine) and rhythmic processes (Fig. 3, Table S10). Genes such as glucose-6-phosphate translocase (g6pt1), carnitine O-palmitoyltransferase 1 (cpt1a), phosphoenolpyruvate carboxykinase (pck), fumarylacetoacetase (fah), long chain acyl-CoA synthetase 8 (lacs8), probable proline dehydrogenase 2 (prodh2) and glutamate dehydrogenase (gdh) in clusters 3, 5 and 6 were associated with glucose, monosaccharide and hexose metabolism; neutral lipid metabolism and fatty-acid metabolism; and amino acid catabolic and metabolic processes (Fig. 3, Table S10).
DEGs in clusters 4, 7 and 8 (highly expressed in InM-I and InM-II) were related to energy storage and specific developmental processes. Genes such as probable C-5 sterol desaturase (erg3), aldehyde dehydrogenase family 8 member A1 (aldh8a1), fatty acid synthase (fas), ATP-citrate synthase (acly), mitotic checkpoint serine (bub1), centromere protein F (cenpf), actin-binding protein anillin (anln) and cyclin-A2 (ccna2) in cluster 4 were strongly enriched in lipids, fat-soluble vitamins, cholesterol and sterol biosynthetic process and mitosis; they were mostly related to the accumulation of nutrient substances and cell proliferation (Fig. 3, Table S10). Genes such as aldehyde dehydrogenase (aldh2), superoxide dismutase (sod2) and acetylcholinesterase (ache) in cluster 7 were enriched in liver development, neurotransmitter receptor biosynthetic process, receptor biosynthetic process and neurotransmitter catabolic and metabolic processes. Genes such as glucose-6-phosphate isomerase (pgi), triosephosphate isomerase B (tpi1b), phosphoglycerate mutase 2 (pgam2), ATP-dependent 6-phosphofructokinase (pfk), phosphoglycerate kinase (pgk), ADP-dependent glucokinase (adpgk), elongation of very long chain fatty acids protein 6 (elovl6) and acyl-coA desaturase (scd) in cluster 8 were strongly enriched in carbohydrate metabolic, biosynthesis, fatty acid synthesis and metabolism (Fig. 3, Table S10).
The DEGs in cluster 2 (increase form PoM to PrM) were enriched for genes broad-complex core protein isoforms 1(br), ecdysone receptor (ecr), niemann-pick C1 (npc1), arylsulfatase B (arsb) and forkhead box protein P1 (foxp1) associated with the response to steroid hormone stimulus, nutrient levels and acid transport, developmental cell growth, RNA transcription and immune system development (Fig. 3, Table S10).
qRT-PCR validation
Twelve genes were selected from the clusters of differentially expressed genes and then verified by qRT-PCR. One gene (casein kinase isoform epsilon) from cluster 1, four genes (cuticle protein cbm, myosin isoform e, beta-lactamase and phosphoenolpyruvate carboxykinase) from cluster 3 and two genes (methyltransferase and juvenile hormone-inducible protein) from cluster 5 had the highest expression levels during postmolt. Three genes (metalloreductase steap4, loc100186072 and loc100880818) from cluster 4, one gene (long chain fatty acid ligase acsbg 2) from cluster 8 presented the highest expression levels in intermolt. One gene (ecdysone receptor) from cluster 2 showed the highest expression level in premolt. qRT-PCR efficiency of amplification was above 95% and the correlation value (R2) was more than 0.98 for each gene (Table S2). The qRT-PCR results confirmed the findings of RNA-Seq (Fig. 4 and S10), with a correlation coefficient (r) from 0.798 to 0.997 depending on specific genes and reference genes.
Discussion
Transcriptomic repertoire for Chinese mitten crab
This study established a comprehensive repertoire of annotated genes, differentially expressed genes, non-coding RNA and transcription factors for the Chinese mitten crab from different molting stages throughout one molting cycle. In order to produce a comprehensive reference transcriptome, all raw reads from the four different molting stages were combined (more than 120 million paired-end reads) and assembled. The saturation analysis and the comparison of transcription factors between E. sinensis and Daphnia pulex (water flea) asserted a high-quality hepatopancreas transcriptome for E. sinensis.
Gene Ontology functional annotation analysis revealed a relatively large number of transcripts in “response to stimulus,” which suggest these genes may be involved in the adjustment of permeation pressure during the transition from saltwater to freshwater during the crab’s lifecycle and presumably prevent automutilation45. KEGG analysis demonstrated the top enriched pathways are energy related ones, including oxidative phosphorylation and glycolysis/gluconeogenesis. This is consistent with the assumption that the molting process requires a significant amount of energy to uptake water and swell the exoskeleton and to produce chitin.
Crustaceans are the second largest group of arthropods, the most speciose and morphologically diverse animal group on the planet46. To date, there is only one crustacean species - Daphnia pulex (water flea) with its genome sequence publically available, although a number of transcriptomic studies have been reported to address issues such as salinity stress, immune responses to microbial pathogens and the development of sexual organs in E. sinensis47,48,49,50. As of May 28, 2015, there are 9 Bio-Samples with over 60 Gb sequence data available in E. sinensis in the NCBI SRA database. One future direction for this project is to sequence transcriptomes of other tissues to corroborate the preliminary findings in this study and to integrate publically available transcriptomic data to enhance our transcriptomic repertoire for the Chinese mitten crab. Nevertheless, our transcriptomic data and analysis results will facilitate genomic sequencing and annotation of other crustaceans (including the Chinese mitten crab) in the future.
Transcriptomic variation among different molting stages
A comparable number of annotated transcripts were attained for each of the four transcriptomes assembled from different molting stages (Fig. 2C), despite the difference in the number of total assembled transcripts in different stages (Fig. 2A). The largest numbers of assembled transcripts were found in stages postmolt (PoM) and premolt (PrM), which are the two critical stages that may have more developmental and immune response genes involved as discussed below. With the mapping of raw reads from each molting stage to the reference transcriptome, we successfully identified differentially expressed genes and gene clusters associated with energy metabolism and physiological responses during one molting cycle in the E. sinensis hepatopancreas.
Postmolt (PoM) is a period when the Chinese mitten crab is recovering from molting. During postmolt the exoskeleton of E. sinensis is quickly hardened as a mechanism to avoid predation, which requires sufficient energy input. Our clustering analysis revealed several clusters of highly expressed genes during the postmolt stage. The genes in cluster 1 are involved in homeostasis (soat1, npc1), response to stimulus and programmed cell death and differentiation, which may suggest the crabs under postmolt are very sensitive to the external and internal environments. Clusters 3, 5 and 6 contain highly expressed genes enriched in carbohydrate, neutral lipid and amino acid metabolic process (pck, g6pt1, Cpt1a, Fah and prodh2), which may provide more energy to maintain homeostasis when dietary carbohydrate is unavailable in the PoM stage (Table S10, cluster 3, 5, 6)51,52,53,54. The pck gene has been considered as a vital marker for liver gluconeogenesis - a metabolic pathway to produce glucose when starvation occurs for extended periods51,52. Furthermore, we found the cuticle protein cbm gene (likely involved in chitin metabolic process) enriched in cluster 3. The over expression of this gene is likely to promote the synthesis of chitin to quickly harden their shell for defense.
Intermolt (InM-I and InM-II) is an important period in growth and development and thus it is an essential stage for energy storage55,56. We found a number of highly expressed genes associated with the glycolysis pathway. These genes including adpgk, pgi, pfk, tpi1b, pgk and pgam2, are involved in the conversion of glucose into pyruvic acid to produce energy. Note that the two genes, adpgk and pfk, are associated with the irreversible steps in this glycolysis process compared to gluconeogenesis52. The product of glycolysis, i.e., pyruvic acid, could supply sufficient energy to cells through the citric acid cycle under aerobic condition and consequently facilitate the growth and development for E. sinensis in InM stage. Our clustering analysis revealed several highly expressed genes such as bub1, cenpf, anln and ccna2 and these genes are likely pertaining to mitosis (Table S10, cluster 4). Two genes, aldh2 and sod2, were linked to liver development and one gene, ache, was presumably related to eye development (Table S10, cluster 7).
During intermolt (InM-I and InM-II) fatty acid synthesis occurs and it is primarily controlled by the fas and acly genes, which catalyze the lipids synthesis pathway through converting the carbohydrates into fatty acids18,57,58. We found these two genes highly expressed in InM stage in addition to the genes involved in lipid synthesis such as erg3, aldh8a1, elovl6 and scd. These genes are likely to convert excessive carbohydrates into fatty acids that could be eventually stored in the hepatopancreas52. The storing of lipids shall play an important role in energy supply when E. sinensis is fasting in other molting stages12. Several studies have reported that crustaceans synthesize a large amount of carbohydrates, lipids, fatty acids and fat-soluble vitamins during the intermolt stage for subsequent molting and limb regeneration59. Reduction in food supply has been linked to a longer intermolt period59.
During premolt, we found the ecdysone receptor gene differentially expressed. This gene is an essential regulatory element for ecdysone hormone and has been shown to regulate the molting of crustaceans6,60. Other enriched genes included those associated with steroid hormone stimulus, immune system, response to nutrient levels and amino acid transport. Gene expression analysis revealed several other genes highly expressed during the premolt (PrM) stage, including EcR, br and foxp1. EcR is a heterodimer composed of the products of the EcR and RXR/USP nuclear hormone genes and was found to regulate the ecdysone hormone, one vital hormone for successful molting in crustaceans9,61,62,63,64. Up-regulation of EcR has been observed in other crustacean species such as the freshwater prawn Macrobrachium nipponense, Pacific white shrimp, Litopenaeus vannamei and grapsid crab, Metopograpsus messor during the premolt stage65,66,67. The br and foxp1 genes are assumed to be associated with responses to steroid hormone stimuli as shown in the fruit fly, Drosophila melanogaster, and honeybee, Apis mellifera68,69.
Understanding the dynamic process of molting is an essential goal for both aquaculture and invasive species management. For aquaculture, the intermolt stage is an ideal period to enhance nutrition to shorten the intermolt period interval and foster growth of crabs. During the postmolt stage it is essential to increase protection for these crabs in the form of additional habitats to ensure they are not victims of predation. For invasive species management, limiting the amount of nutrients available to the crabs is essential to extend the intermolt period in order to limit the number of sexually mature individuals. Furthermore, additional research needs to be done to determine if the postmolt stage might be extended which would cause invasive crabs to be more susceptible to predation as potential management technique.
In conclusion, we performed a comprehensive transcriptomic study on the hepatopancreas of E. sinensis amongst four molting stages. We established the first reference transcriptome of the hepatopancreas of E. sinensis, characterized genes and functional categories at each molting stage and identified regular patterns of differentially expressed genes amongst different molting stages. Our study thus provides unique insight into the functions of the hepatopancreas in energy metabolism and biological processes for molting in crustaceans. Future research directions include transcriptomic studies of more tissues and samples from different molting cycles and genomic sequencing of the Chinese mitten crab.
Data Archiving
Sequencing reads are available at NCBI SRA database (SRX824594, SRX845724 and SRX845725).
Additional Information
How to cite this article: Huang, S. et al. Transcriptomic variation of hepatopancreas reveals the energy metabolism and biological processes associated with molting in Chinese mitten crab, Eriocheir sinensis. Sci. Rep. 5, 14015; doi: 10.1038/srep14015 (2015).
References
Panganiban, G., Sebring, A., Nagy, L. & Carroll, S. The development of crustacean limbs and the evolution of arthropods. Science. 270, 1363–1366 (1995).
Hopkins, P. M. Limb Regeneration in the Fiddler Crab, Uca pugilator: Hormonal and Growth Factor Control. Am Zool. 41, 389–398 (2001).
Morris, S. et al. The adaptive significance of crustacean hyperglycaemic hormone (CHH) in daily and seasonal migratory activities of the Christmas Island red crab Gecarcoidea natalis. J Exp Biol. 213, 3062–3073 (2010).
Jung, H., Lyons, R. E., Hurwood, D. A. & Mather, P. B. Genes and growth performance in crustacean species: a review of relevant genomic studies in crustaceans and other taxa. Rev Aquacult. 5, 77–110 (2013).
Kurup, N. The intermolt cycle of an anomuran, Petrolisthes cinctipes Randall (Crustacea-Decapoda). Biol Bull. 127, 96–107 (1964).
Chang, E. S. & Mykles, D. L. Regulation of crustacean molting: A review and our perspectives. Gen Comp Endocr. 172, 323–330 (2011).
Hopkins, P. Crustacean Ecdysteriods and Their Receptors in Ecdysone: Structures and Functions (ed Guy Smagghe ) Ch. 3, 73–97 (Springer Netherlands, 2009).
Chen, H.-Y., Dillaman, R. M., Roer, R. D. & Watson, R. D. Stage-specific changes in calcium concentration in crustacean (Callinectes sapidus) Y-organs during a natural molting cycle and their relation to the hemolymphatic ecdysteroid titer. Comp Biochem Phys A. 163, 170–173 (2012).
Nakagawa, Y. & Henrich, V. C. Arthropod nuclear receptors and their role in molting. FEBS J. 276, 6128–6157 (2009).
Wang, C., Li, C. & Li, S. Mitochondrial DNA-inferred population structure and demographic history of the mitten crab (Eriocheir sensu stricto) found along the coast of mainland China. Mol Ecol. 17, 3515–3527 (2008).
Dittel, A. I. & Epifanio, C. E. Invasion biology of the Chinese mitten crab Eriochier sinensis: A brief review. J Exp Mar Biol Ecol. 374, 79–92 (2009).
Wang, W., Wang, C. & Ma, X. Ecological Aquaculture of Chinese mitten crab (In Chinese). 2 edn, (China Agriculture Press, 2013).
Leersnyder, M. d., Dhainaut, A. & Porcheron, P. La vitellogenese chez le crabe Eriocheir sinensis. Bull. Soc. zool. Fr. 105, 413–419 (1980).
Tian, Z., Kang, X. & Mu, S. The molt stages and the hepatopancreas contents of lipids, glycogen and selected inorganic elements during the molt cycle of the Chinese mitten crab Eriocheir sinensis. Fish Sci. 78, 67–74 (2012).
Guo, Y.-R., Gu, S.-Q., Wang, X.-C., Zhao, L.-M. & Zheng, J.-Y. Comparison of fatty acid and amino acid profiles of steamed Chinese mitten crab. Fish Sci. 80, 621–633 (2014).
Wang, L., Yan, B., Liu, N., Li, Y. & Wang, Q. Effects of cadmium on glutathione synthesis in hepatopancreas of freshwater crab, Sinopotamon yangtsekiense. Chemosphere. 74, 51–56 (2008).
Uawisetwathana, U. et al. Insights into eyestalk ablation mechanism to induce ovarian maturation in the black tiger shrimp. PloS one. 6, e24427 (2011).
Wang, W., Wu, X., Liu, Z., Zheng, H. & Cheng, Y. Insights into Hepatopancreatic Functions for Nutrition Metabolism and Ovarian Development in the Crab Portunus trituberculatus: Gene Discovery in the Comparative Transcriptome of Different Hepatopancreas Stages. PLoS One. 9, e84921 (2014).
Jiang, H., Yin, Y., Zhang, X., Hu, S. & Wang, Q. Chasing relationships between nutrition and reproduction: A comparative transcriptome analysis of hepatopancreas and testis from Eriocheir sinensis. Comp Biochem Phys D. 4, 227–234 (2009).
Mykles, D. L. Ecdysteroid metabolism in crustaceans. J Steroid Biochem. 127, 196–203 (2011).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 10, 57–63 (2009).
Wachholtz, M. et al. Transcriptome analysis of two buffalograss cultivars. BMC Genomics. 14, 613 (2013).
Wang, C., Wachholtz, M., Wang, J., Liao, X. & Lu, G. Analysis of the skin transcriptome in two oujiang color varieties of common carp. PloS one. 9, e90074 (2014).
He, J. Research on the growth of the cultured population of Chinese mitten crab (In Chinese). Reser Fisher. 25, 10–11 (2005).
Phlippen, M. K., Webster, S. G., Chung, J. S. & Dircksen, A. H. Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph. J Exp Biol. 203, 521–536 (2000).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics. 1–7 (2014).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech. 29, 644–652 (2011).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, 1–6 (2009).
Li, B. & Dewey, C. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 12, 323 (2011).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 26, 589–595 (2010).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 25, 2078–2079 (2009).
Conesa, A. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 21, 3674–3676 (2005).
Powell, S. et al. eggNOG v4.0: nested orthology inference across 3686 organisms. Nucleic Acids Res. 42, 231–239 (2013).
Petersen, T. N., Brunak, S., von Heijne, G. & Nielsen, H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth. 8, 785–786 (2011).
Finn, R. D., Clements, J. & Eddy, S. R. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39, 29–37 (2011).
Wilson, D., Charoensawan, V., Kummerfeld, S. K. & Teichmann, S. A. DBD—taxonomically broad transcription factor predictions: new content and functionality. Nucleic Acids Res. 36, 88–92 (2008).
Burge, S. W. et al. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res. 41, 226–232 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26, 139–140 (2010).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols. 4, 44–57 (2008).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1–12 (2002).
Andersen, C. L., Jensen, J. L. & Ørntoft, T. F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Research. 64, 5245–5250 (2004).
Pfaffl, M., Tichopad, A., Prgomet, C. & Neuvians, T. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol Lett. 26, 509–515 (2004).
Silver, N., Best, S., Jiang, J. & Thein, S. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 7, 33 (2006).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25, 402–408 (2001).
Mao, H., Huang, S., Wang, Z., Zhou, L. & Wang, C. Molecular Cloning and Expression Analysis of Na+/K+-ATPase α1 Gene in Chinese Mitten Crab (Eriocheir sinensis) (In Chinese). J Agricult Biotech. 22, 343–350 (2014).
Brusca, R. & Brusca, G. Invertebrates. 2 edn, (Sinauer Associates, Sunderland, MA, 2003).
Colbourne, J. K. et al. The Ecoresponsive Genome of Daphnia pulex. Science. 331, 555–561 (2011).
He, L. et al. Comparative transcriptome analysis of the accessory sex gland and testis from the Chinese mitten crab (Eriocheir sinensis). PLoS One. 8, e53915 (2013).
Li, E. et al. Transcriptome sequencing revealed the genes and pathways involved in salinity stress of Chinese mitten crab. Eriocheir sinensis. Physiol Genomics. 46, 177–190 (2014).
Cui, Z. et al. High-density linkage mapping aided by transcriptomics documents ZW sex determination system in the Chinese mitten crab Eriocheir sinensis. Heredity. 115, 206–215 (2015).
Burgess, S. C. et al. Cytosolic Phosphoenolpyruvate Carboxykinase Does Not Solely Control the Rate of Hepatic Gluconeogenesis in the Intact Mouse Liver. Cell Metab. 5, 313–320 (2007).
Fromm, H. J. & Hargrove, M. Essentials of biochemistry. (Springer Science & Business Media, 2012).
Charkoudian, L. K., Farrell, B. P. & Khosla, C. Natural product inhibitors of glucose-6-phosphate translocase. MedChemComm. 3, 926–931 (2012).
Jogl, G. & Tong, L. Crystal Structure of Carnitine Acetyltransferase and Implications for the Catalytic Mechanism and Fatty Acid Transport. Cell. 112, 113–122 (2003).
Devaraj, H. & Natarajan, A. Molecular mechanisms regulating molting in a crustacean. FEBS J. 273, 839–846 (2006).
Kuballa, A. & Elizur, A. Novel molecular approach to study moulting in crustaceans. Bulletin Fisheries Research Agency Japan. 20, 53 (2007).
Chirala, S. & Wakil, S. Structure and function of animal fatty acid synthase. Lipids. 39, 1045–1053 (2004).
Mashima, T., Seimiya, H. & Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Brit J cancer. 100, 1369–1372 (2009).
Hartnoll, R. Growth in Crustacea – twenty years on. Hydrobiologia. 449, 111–122 (2001).
Chang, E. S. & Bruce, M. J. Ecdysteroid titers of juvenile lobsters following molt induction. J Exp Zool Part A. 214, 157–160 (1980).
Chang, E. S. & O’Connor, J. D. Secretion of alpha-ecdysone by crab Y-organs in vitro. P Natl Acad Sci USA. 74, 615–618 (1977).
Keller, R. & Schmid, E. In vitro secretion of ecdysteroids by Y-organs and lack of secretion by mandibular organs of the crayfish following molt induction. J Comp Physiol A. 130, 347–353 (1979).
Schoettker, P. J. & Gist, D. H. In vitro ecdysteroid production by Y-organs of the blue crab Callinectes sapidus. J Crustacean Biol. 10, 487–491 (1990).
Aranda, A. & Pascual, A. Nuclear Hormone Receptors and Gene Expression. Physiol Rev. 81, 1269–1304 (2001).
Shen, H., Zhou, X., Bai, A., Ren, X. & Zhang, Y. Ecdysone receptor gene from the freshwater prawn Macrobrachium nipponense: identification of different splice variants and sexually dimorphic expression, fluctuation of expression in the molt cycle and effect of eyestalk ablation. Gen Comp Endocr. 193, 86–94 (2013).
Qian, Z. et al. Identification of ecdysteroid signaling late-response genes from different tissues of the Pacific white shrimp, Litopenaeus vannamei. Comp Biochem Phys A. 172, 10–30 (2014).
Shyamal, S., Anilkumar, G., Bhaskaran, R., Doss, G. P. & Durica, D. S. Significant fluctuations in ecdysteroid receptor gene (EcR) expression in relation to seasons of molt and reproduction in the grapsid crab, Metopograpsus messor (Brachyura: Decapoda). Gen Comp Endocr. 211, 39–51 (2015).
Uhlirova, M. et al. Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis. P Natl Acad Sci USA. 100, 15607–15612 (2003).
Paul, R. K., Takeuchi, H. & Kubo, T. Expression of Two Ecdysteroid-Regulated Genes, Broad-Complex and E75, in the Brain and Ovary of the Honeybee (Apis mellifera L.). Zool Sci. 23, 1085–1092 (2006).
Acknowledgements
We would like to thank Yuanji Ci and Lian Wu for their assistance in RNA isolation and Professor Sheng Li for proof-reading the manuscript. We particularly thank the Holland Computing Center (HCC) at the University of Nebraska for computational support and technical assistance. This work was funded by Shanghai Technology System for Chinese Mitten Crab Industry (No. 2014-4), Shanghai Science and Technology Committee Programs (No. 13391912102; No. 03DZ2251800) and Innovation project of Shanghai Ocean University Graduate Education.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: C.W., S.H., J.W., G.L., W.L. Conducted the analysis: J.W., S.H., W.Y., J.C., C.W. Wrote the paper: J.W., G.L., S.G., S.H., C.W., W.L.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Huang, S., Wang, J., Yue, W. et al. Transcriptomic variation of hepatopancreas reveals the energy metabolism and biological processes associated with molting in Chinese mitten crab, Eriocheir sinensis. Sci Rep 5, 14015 (2015). https://doi.org/10.1038/srep14015
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep14015
This article is cited by
-
Histological, Physiological and Transcriptomic Analysis Reveal the Acute Alkalinity Stress of the Gill and Hepatopancreas of Litopenaeus vannamei
Marine Biotechnology (2023)
-
Chronic toxicity of broflanilide in Daphnia magna: changes in molting, behavior, and gene expression
Environmental Science and Pollution Research (2023)
-
Whole-body transcriptome analysis provides insights into the cascade of sequential expression events involved in growth, immunity, and metabolism during the molting cycle in Scylla paramamosain
Scientific Reports (2022)
-
Changes in the growth performance, antioxidant enzymes and stress resistance caused by dietary administration of synbiotic (fructooligosaccharide and probiotics) in juvenile Chinese Mitten Crab, Eriocheir sinensis
Aquaculture International (2022)
-
Identification of Genes Involved in Digestion from Transcriptome of Parasesarma pictum and Parasesarma affine Hepatopancreas
Thalassas: An International Journal of Marine Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.