Abstract
Eight phenolic compounds were isolated using bio-guided isolation and purified from the roots of Astragalus taipaishanensis Y. C. Ho et S. B. Ho (A. taipaishanensis) for the first time. Their structures were elucidated by ESI-MS, HR-ESI-MS, 1D-NMR and 2D-NMR as 7,2′-dihydroxy-3′,4′-dimethoxy isoflavan (1), formononetin (2), isoliquiritigenin (3), quercetin (4), kaempferol (5), ononin (6), p-hydroxybenzoic acid (7) and vanillic acid (8). Six flavonoids (compounds 1-6) exhibited stronger antioxidant activities (determined by DPPH, ABTS, FRAP and lipid peroxidation inhibition assays) than those of BHA and TBHQ and also demonstrated noticeable protective effects (particularly quercetin and kaempferol) on Escherichia coli under oxidative stress. Additionally, the chemical constituents compared with those of Astragalus membranaceus and the structure-activity relationship of the isolated compounds were both analyzed. The results clearly demonstrated that A. taipaishanensis has the potential to be selected as an alternative medicinal and food plant that can be utilized in health food products, functional tea and pharmaceutical products.
Similar content being viewed by others
Introduction
Medicinal food plants are widely utilized in dietary supplements as a source of bioactive compounds that have food additive properties1. Research has demonstrated that bioactive compounds extracted from plants are effective in food systems and can be used as antimicrobial and antioxidant additives in the food industry1,2. Given the increasing application and use of these bioactive compounds in food, cosmetic and pharmaceutical products as replacements for synthetic antioxidants, which are often constricted due to known carcinogenic effects3, there is an increased interest and emphasis on the discovery and utilization of traditional medicinal food plants.
The Astragalus spp. has a history of being widely used in traditional medicine, coffee, tea substitutes, food and cosmetics worldwide, particularly in places such as Europe, the Middle East and Asia4,5. Nearly 2000 species have been identified and distributed around largely subtropical and temperate regions with the exception of Oceania6,7. A. membranaceus Bunge, which is also known as Huangqi in Chinese and Radix Astragali in Latin, belongs to the Leguminosae family. It was first recorded in Shennong Bencao Jing and its dry roots have been used in traditional Chinese medicine since 18008,9,10,11. Currently, the products of Radix Astragali, such as Astragali tea and over-the-counter dietary supplement capsules, are available in U.S. health food markets12,13. In China, Astragalus-jujube-wolfberry tea is a well-known anti-cancer tea remedy that can enhance body vitality and immunity. The study of its chemical constituents demonstrated that more than 100 components of Radix Astragali have been identified and the main constituents are flavonoids, polysaccharides, saponins and amino acids14,15. Many pharmacology studies have revealed that the flavonoids of Radix Astragali exhibit noticeable antioxidant activity16,17 and pharmacological activities such as immunoregulatory effects18, a decrease in the damage caused by hypoxia19 and amelioration of chronic fatigue syndrome20.
A. taipaishanensis is a species native to China that belongs to the Astragalus genus, which is only distributed in the Taibai Mountain region. In the Taibai Mountains, teas, decoctions and infusions are prepared from the roots of A. taipaishanensis as folk medicine and functional tea to enhance immunity and to improve stamina and strength21; however, until now, there have been no reports on the phytochemicals or bioactive compounds of this plant. In the following study, the bioactive components present in the roots of A. taipaishanensis were isolated and extracted to discover how to better utilize this plant and also attempt to gain a deeper understanding of the correlation between the health benefits of the compounds and reduced risk of diseases. The antioxidant activities of these isolated compounds were tested using several in vitro assays (DPPH, ABTS, FRAP and lipid peroxidation inhibition assays) along with an additional test to assess their protective effects on Escherichia coli subjected to oxidative stress by hydrogen peroxide. The results indicated that the roots of A. taipaishanensis had similar constituents and antioxidant capacities to those of A. membranaceus, indicating that it may have the potential to be developed for use in supplementary medicine and pharmaceutical products and functional food ingredients.
Results
TPC, TFC and antioxidant activities of extracts and isolated fractions
The TPC and TFC of the ethanolic extracts (EE) and 4 isolated fractions (PEF, EAF, BF and WF) of A. taipaishanensis were analyzed (Table 1). The results demonstrated that the EAF had the highest content of TPC (46.41 ± 0.34 mmol equiv. GAE/100 g) and TFC (185.45 ± 1.04 mmol equiv. QUE/100 g). To identify the fraction with the greatest bioactivity, four in vitro antioxidant activities were observed in each fraction and the most bioactive fraction was then selected for further isolation and purification. The results are displayed in Table 1. For the ABTS•+ and FRAP assay, the EAF had the highest ABTS scavenging activity and reducing ability with values of 994.50 ± 4.21 μmol trolox/g and 685.67 ± 3.21 μmol trolox/g, respectively. For the DPPH and lipid peroxidation assays, the IC50 values of DPPH and lipid peroxidation ranged from 0.059 ± 0.002 mg/mL (EAF) to 0.99 ± 0.004 mg/mL (WF) and 1.95 ± 0.009 mg/mL (EAF) to 6.48 ± 0.039 mg/mL (WF), respectively. In conclusion, the EAF was determined to be the fraction with the greatest antioxidant activity; therefore, later isolation and purification efforts were focused on the EAF.
Structure elucidation of isolated compounds
Eight compounds were isolated and identified from the roots of A. taipaishanensis. The NMR data of the compounds are shown in Tables 2 and 3. The structures of compounds 1-8 and the key HMBC correlation of compounds 1 and 2 are illustrated in Figs 1 and 2.
Compound 1 was isolated as white needle-shaped crystals. Its molecular formula was determined to be C17H18O5 on the basis of HR-ESI-MS: m/z 301.1229 [M-H]− and ESI-MS: m/z 301.15 [M-H]−. The 13C-NMR and DEPT spectra exhibited signals for two OCH3, two CH2 (all aliphatic), six CH (five aromatic, one aliphatic) and seven quaternary carbons (four O-bearing and three aromatic), respectively. The 1H-NMR spectrum of compound 1 displayed two -OCH3 signals at δ 3.78 (3H,s) and δ 3.79 (3H,s), five aromatic proton signals at δ 6.22 (1H,s), δ 6.30 (1H,d,J = 8.0 Hz), δ 6.44 (1H,d,J = 8.4 Hz), δ 6.75 (1H,d,J = 8.8 Hz) and δ 6.85 (1H,d,J = 8.4 Hz), which represented the structure containing the AMX spin-coupling. When compared with published literature data, compound 1 was identified as 7, 2′-dihydroxy-3′, 4′-dimethoxy-isoflavane22.
Compound 2 was obtained as white needle crystal. Its molecular formula was established as C16H12O4 on the basis of HR-ESI-MS: m/z 267.0930 [M-H]− and ESI-MS: m/z 267.17 [M-H]−. 13C-NMR and DEPT spectrum showed signals for one OCH3, eight CH (seven aromatic, one alkene) and seven quaternary carbons (one carbonyl, two O-bearing and four aliphatic), respectively. 1H-NMR spectrum of compound 2 exhibited one -OCH3 signal at δ 3.80(3H, s) and three aromatic proton signals at δ 6.88(1H, d, J = 2.5 Hz), δ 6.95(1H, dd, J = 2.0, 2.0 Hz), δ 7.98 (1H, d, J = 8.5 Hz), respectively. The signal at δ 8.34 (1H, s) was an obvious signal of isoflavone at the H-2 position. The AA′BB′ spin-coupling system, with the signals at δ 7.00 (2H, d, J = 9.0 Hz), δ 7.52 (2H, d, J = 8.5 Hz), was ascribed to the four protons of the B-ring. After being compared with published literature values, compound 2 was identified as formononetin23.
Compound 6 was isolated as a white amorphous powder. ESI-MS: m/z 431.32 [M+H]+. Its molecular formula was determined to be C22H22O9 based on the ESI-MS and NMR. Compared with compound 2, it had the same isoflavone skeleton. Additionally, the mass spectra and NMR spectra showed signals corresponding to a single sugar unit. The 1H-NMR spectrum of compound 6 displayed strong signals indicating anomeric protons at δ 5.16 (1H, d, J = 4.8 Hz). The sugar unit was examined by TLC analysis. Acid hydrolysis of compound 6 resulted in a product of D-glucose, which was identified by a comparison with an authentic sample. Based on the coupling constant J = 4.8 Hz greater than 4.0 Hz, the sugar configurations were identified as β-D-glucose. In addition, there was a remarkable upfield shift (Δδ 1.14) for the C-7 of compound 6 (161.91) compared to compound 2 (163.05), indicating that the sugar unit was attached to C-7. After comparison with published literature data, compound 6 was identified as ononin24.
Compound 3 was isolated as a yellow amorphous powder. ESI-MS: m/z 255.49 [M-H]−. The 13C-NMR and DEPT spectra exhibited signals for nine CH (seven aromatic, two alkene) and six quaternary carbons (one carbonyl, three O-bearing and two aliphatic), respectively. Its molecular formula was determined to be C15H12O4 based on the results from ESI-MS and NMR. The 1H-NMR spectrum of compound 3 exhibited three aromatic proton signals at δ 6.30 (1H, d, J = 2.4 Hz), δ 6.43 (1H, dd, J = 2.4, 2.4 Hz) and δ 7.99 (1H, d, J = 8.8 Hz) and it was ascribed to three protons of the A-ring of flavone. The signal at δ 7.63 (1H, d, J = 5.2 Hz) and δ 7.81(1H, d, J = 15.2 Hz) were obvious signals of conjugate protons; moreover, there was an AA′BB′ spin-coupling system with signals at δ 6.87 (2H, d, J = 8.8 Hz), δ 7.65(2H, d, J = 1.2 Hz), which was attributed to the four protons of the B-ring. When compared with published literature data, compound 3 was identified as isoliquiritigenin25.
Compound 4 was isolated as a yellow amorous powder. ESI-MS: m/z 301.29 [M-H]−. The 13C-NMR and DEPT spectra exhibited signals for five CH (all aromatic) and ten quaternary carbons (one carbonyl, five O-bearing, four aliphatic). Its molecular formula was determined to be C15H10O6 based on the results of ESI-MS and NMR. In the 1H-NMR spectrum, an ABX spin-coupling system was ascribed to the three protons of the B-ring of the flavone, with signals at δ 6.89 (1H, d, J = 6.8 Hz), δ 7.54 (1H, dd, J = 2.0 Hz, J = 2.0 Hz) and δ 7.65 (1H, d, J = 8.5 Hz) and with respect to published literature data, compound 4 was identified as quercetin26.
Compound 5 was isolated as a yellow amorous powder. ESI-MS: m/z 284.96 [M-H]−. The 13C-NMR and DEPT spectra displayed signals for six CH (all aromatic) and nine quaternary carbons (one carbonyl, four O-bearing, four aliphatic). Its molecular formula was determined to be C15H10O5 based on the ESI-MS and NMR spectra. Compound 5 was found to have the same skeleton as compound 4. In the 1H-NMR spectrum, the AA′BB′ spin-coupling system with signals at δ 6.92 (2H, d, J = 8.5 Hz) and δ 8.04 (1H, d, J = 8.5 Hz) was ascribed to the four protons of the B-ring. The signals at δ 6.19 (1H, d, J = 2.0 Hz) and δ 6.45 (1H, d, J = 2.0 Hz) were meta-substituted protons of the A-ring. When compared with published literature data, compound 5 was identified as kaempferol26.
Compound 7 and compound 8 were identified by TLC analysis and a comparison with an authentic sample. Compound 7 was identified as p-hydroxy benzoic acid and compound 8 was identified as vanillic acid27.
To the best of our knowledge, this is the first report of the characterization of phenolic compounds 1–8 isolated from A. taipaishanensis; furthermore, the 2D-NMR data of compounds 1 and 2 were presented for the first time in this study.
In vitro antioxidant assays of isolated compounds
Phenolic compounds possess biologically beneficial activities and are frequently utilized as antioxidant, antiallergic and antidiabetic reagents28. In our study, the compounds 1–6 were tested for antioxidant by using four in vitro antioxidant assays and protective effect on E. coli under peroxide stress. The results showed in the Table 4. For the ABTS assay, all the tested compounds showed strong antioxidant capacity compared to BHA and TBHQ. In particular, compounds 4 and 5 had comparative higher antioxidant capacity with ABTS values over 30 mmol trolox/g. FRAP assay, compounds 4, 5 and 1 had higher antioxidant activity compared to BHA (5.83 ± 0.08 mmol trolox/g) and TBHQ (6.57 ± 0.13 mmol trolox/g) and the values were 14.18 ± 0.13, 14.13 ± 0.16 and 10.77 ± 0.11 mmol trolox/g, respectively. The results of DPPH assay demonstrated that all the tested compounds had stronger DPPH radical-scavenging ability than the standards (BHA and TBHQ). Compounds 4, 5, 1 and 3 in particular had relatively higher antioxidant activity, in which the IC50 values were lower than 1 μM. The lipid peroxidation assay was carried out using egg yolk as a lipid-rich medium. For this assay, all the tested compounds exhibited a particular capacity for lipid peroxidation inhibition. In particular, compounds 4, 5 and 1 had comparatively higher lipid peroxidation potential with IC50 values 1.65 ± 0.08, 1.30 ± 0.09 and 1.87 ± 0.07 μM, respectively, all of which were lower than 2 μM.
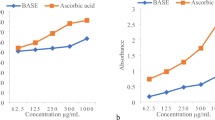
Protective effect on E. coli under peroxide stress
Six of the isolated compounds were tested on E. coli growth under peroxide stress. We evaluated and compared the specific growth rate (μ) of E. coli cells by pretreating them with the 6 compounds before subjecting them to oxidative stress damage caused by H2O2 for 30 min. The absorbance (OD600) and μ values are displayed in Figs 3 and 4, respectively. The higher values of the μ and the OD600 were indicative of a higher antioxidant potential for the compounds. The results demonstrated that all the compounds had the ability to increase the cell growth rate under oxidative stress by 3.86- to 5.56-fold compared to untreated cells subjected to oxidative stress for 30 min. The compound with the highest activity was compound 4, which resulted in a growth rate of 5.56, followed by compound 3 and compound 1, which resulted in growth rates of 4.14 and 4.09, respectively.
Discussion
A. membranaceus and A. membranaceus var. mongholicus are mainly grown in North, Northeast and Northwest China and have been widely used for 2000 years29. Flavonoids, triterpene saponins and polysaccharides are the main constituents of this plant. Currently, almost 30 flavonoids have been isolated from A. membranaceus30,31, most of them being isoflavones, isoflavans and flavonols. Isoflavones in particular are major active ingredients in A. membranaceus32 and were selected as marker compounds to evaluate the quality of A. membranaceus. Due to its known multiple health benefits, the natural sources of A. membranaceus are diminishing yearly33. In an attempt to address this shortage by exploring alternative plant options, we carried out a study using bio-guided isolation to isolate 8 phenolic compounds from the roots of A. taipaishanensis for the first time, examining and evaluating their bioactivities by in vitro radical scavenging, ferric reducing power and lipid peroxidation in addition to assessing their protective ability on E. coli cells subjected to oxidative stress damage caused by H2O2.
We isolated 7, 2′-dihydroxy-3′,4′-dimethoxy isoflavan (1), formononetin (2), isoliquiritigenin (3), quercetin (4), kaempferol (5), ononin (6), p-hydroxy benzoic acid (7) and vanillic acid (8) from the roots of A. taipaishanensis. When compared with published literature, it was discovered that these 8 compounds isolated from A. taipaishanensis were all present in A. membranaceus, particularly isoliquiritigenin, formononetin, ononin, kaempferol, quercetin, which are the main beneficial flavonoids in A. membranaceus; therefore, it is possible to infer that A. taipaishanensis would contain similar flavonoid constituents to those of A. membranaceus. This could also explain the popular use the roots of A. taipaishanensis as folk medicine and functional tea to enhance the immune system and improve stamina and strength.
By comparing the results of four in vitro antioxidant assays, it was evident that quercetin and kaempferol demonstrated comparatively higher levels of antioxidant activity in the assays, whereas the formononetin and ononin had the lowest antioxidant potential and the 7, 2′-dihydroxy-3′,4′-dimethoxy isoflavan and isoliquiritigenin had moderate antioxidant capacity. Compared with BHA and TBHQ, all the tested compounds demonstrated higher radical scavenging abilities against the ABTS and DPPH assays, whereas for the lipid peroxidation assay, all the compounds had lower activity than BHA and TBHQ. This result demonstrated that all the compounds isolated from the A. taipaishanensis had good radical scavenging activity. In particular, the results of the antioxidant assays indicated that all the tested compounds had certain antioxidant properties although their activity levels were significantly different. Among the 6 compounds that were tested, we found that the compounds with a flavonol skeleton, such as quercetin (4) and kaempferol (5), always had the highest antioxidant activities. In contrast, 7, 2′-dihydroxy-3′,4′-dimethoxy isoflavan (1) had a slightly lower antioxidant activity than flavonol followed by chalcone (isoliquiritigenin, 3) and the isoflavone (formononetin, 2) and isoflavone glucoside (ononin, 6) had the lowest antioxidant capacities. These results were consistent with previous reports stating that the number and position of hydroxyl groups and the different flavonoid skeletons would largely influence the radical scavenging ability. Previous reports stated that the 3-OH group would significantly increase the radical scavenging power34,35 and among all the tested compounds, only quercetin and kaempferol had the 3-OH group, both of which were found to have the highest anti-radical activities. In contrast, the formononetin and ononin, which had no 3-OH group, had the lowest radical scavenging power36; moreover, the presence of ortho-dihydroxy groups on the B-ring or A-ring could have also enhanced the radical scavenging potential, which is a possible explanation as to why quercetin had slightly higher antioxidant activity in comparison to kaempferol. In addition, the presence of an α, β-double bond in isoliquiritigenin made its anti-radical potential evident36,37.
In conclusion, the present study, to the best of our knowledge, was the first time a comprehensive study was carried out on the phytochemicals and antioxidant capacity of the A. taipaishanensis. We isolated 6 flavonoid compounds and 2 phenolic compounds from A. taipaishanensis, all of which were isolated for the first time from this species. Additionally, we found that the main chemical components and the bioactivity of A. taipaishanensis were similar to those found in A. membranaceus, which is recorded in the pharmacopoeia as a medicinal and food plant. The antioxidant activities and structure-activity relationships of these isolated compounds were also evaluated and the results revealed that all the tested compounds exhibited noticeable antioxidant activity compared to BHA and TBHQ. These findings demonstrate the potential of A. taipaishanensis to serve as a valuable natural resource in the medicinal and food industry and its ability to be used as a possible alternative in response to the diminishing supply of A. membranaceus.
Methods
General
ESI-MS was performed on an Agilent 1200 HPLC with the 6130 SQD system and a Waters XBridge C18 column (50 × 4.6 mm, 3.5 μm, Waters). HR-ESI-MS was performed on 1260 HPLC combined with 6250 ESI-Q-TOF MS (Agilent, USA). HPLC separation was performed on an Agilent 1260 HPLC with a Zobax Eclipse Plus C18 column (4.6 × 250 mm, 5 μm, Agilent). 1H-and 13C-spectra were measured with a Bruker AVANCE III spectrometer (500 MHz). Thin layer silica gel and column gel were both purchased from Qingdao Ocean Company (China). Sephadex LH-20 was purchased from GE Healthcare Bioscience AB (Sweden).
Chemicals and reagents
The following chemicals and reagents were used: 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), 2,2-azino-bis(3-ethyl-benzothiazoline-6-sulphonic acid) di-ammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), (Sigma-Aldrich Co., St. Louis, USA); thiobarbituric acid (TBA) (Guangdong Guanghua Chemical Factory Co., Ltd. PR China); sodium borohydride (NaBH4), vanillin, aluminum chloride, acetic acid, hydrochloric acid, sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride, potassium chloride, potassium dihydrogen phosphate, sodium carbonate, sodium acetate, ferric trichloride hexahydrate (FeCl3·6H2O), potassium persulfate (Tianjin Bodi Chemical Co., Ltd, PR China); tetrahydrofuran (THF) (Shenzhen Guanghua Technology Co., Ltd, PR China); quercetin, formononetin, glucose (Shanghai Source Leaf Biological Technology Co., Ltd. PR China); yeast extract, tryptone (Oxoid Ltd., Basingstoke, Hampshire, England). All the chemicals used including the solvents were of analytical grade.
Plant material
Five-year-old roots of A. taipaishanensis were collected on July 2013 at the Taibai Mountains of Shaanxi province, China. The specimen was kept in the herbarium of Northwest A&F University, Yangling, China.
Extraction and isolation
The dried roots of A. taipaishanensis (3.0 kg) were extracted five times each with 95% ethanol (10 L) for 24 h at room temperature. The ethanol extracts (EE, 488 g) were evaporated in vacuum at 45 °C and dried to solid at 35 °C. The EE (420 g) was further partitioned with petroleum ether-water, ethyl acetate-water and n-BuOH-water to obtain the 4 fractions: petroleum ether fraction (PEF, 24 g), ethyl acetate fraction (EAF, 18 g), n-butanol fraction (BF, 40 g) and water fraction (WF, 374 g), respectively. The yield of EE and the four fractions was 16.27%, 5.71%, 4.29%, 9.52% and 80.48%, respectively.
The EAF (18 g) was purified using a silica gel column (200–300 mesh, 1000 × 60 mm) and successively eluted with a petroleum ether/ethyl acetate gradient elution (90:10 to 0:100) to yield 5 fractions (Fr. 1-Fr. 5). Fr. 1 (2.2 g, 80:20) was subjected to a silica gel column (200–300 mesh, 600 × 35 mm) using a petroleum ether/ethyl acetate gradient elution (90:10 to 0:100) to yield 3 fractions (Fr. 1.1–1.3). Fr. 1.3 (1.5 g, 1:1) was subjected to a silica gel column (200–300 mesh, 600 × 20 mm) using a dichloromethane/benzene gradient elution (100:0 to 0:100) to obtain 3 fractions (Fr. 1.3.1–1.3.3). Fr. 1.3.2 (920 mg, 2:1) was purified by silica gel column (200–300 mesh, 600 × 15 mm) and eluted with a petroleum ether/ethyl acetate gradient elution (90:10 to 0:100) to obtain compound 1 (110 mg). Fr. 3 (3.5 g, 1:1) was subjected to a silica gel column (200–300 mesh, 600 × 35 mm) using a petroleum ether/acetone gradient elution (100:0 to 0:100) to yield 4 sub-fractions (Fr. 3.1–3.4). Fr. 3.2 (950 mg, 4:1) was further isolated by using a silica gel column (200–300 mesh, 600 × 15 mm) and an ethyl acetate/benzene gradient elution (100:0 to 0:100) to yield 3 fractions (Fr. 3.2.1–3.2.3). Fr. 3.2.2 (410 mg, 2:1) was further purified by HPLC (Agilent Zobax Eclipse Plus C18, 4.6 × 250 mm, 30% MeOH, 70% H2O, 0.8 mL/min, UV 254 nm) to obtain compound 2 (35 mg). Fr. 3.4 (1.9 g, 1:2) was subjected to a silica gel column (200–300 mesh, 600 × 20 mm) using a petroleum ether/acetone gradient elution (100:0 to 0:100) to yield 3 sub-fractions (Fr. 3.4.1–3.4.3). Fr. 3.4.2 (1.3 g, 2:1) was subjected to a silica gel column (200–300 mesh, 600 × 15 mm) to further isolation by eluting with dichloromethane/benzene (100:0 to 0:100) and yield 3 fractions (Fr. 3.4.2.1-3.4.2.3). Fr. 3.4.2.2 (830 mg, 2:1) was purified by using a silica gel column (200–300 mesh, 600× 15 mm) and a petroleum ether/ethyl acetate gradient elution (100:0 to 0:100) to yield 2 fractions (Fr. 3.4.2.2.1–3.4.2.2.2). Fr. 3.4.2.2.1 was further purified by HPLC (Agilent Zobax Eclipse Plus C18, 4.6 × 250 mm, 30% MeOH in H2O, 0.8 mL/min, UV 254 nm) to obtain compound 3 (25 mg). Compound 7 (22 mg) was purified by HPLC (Agilent Zobax Eclipse Plus C18, 4.6 × 250 mm, 30% MeOH in H2O, 0.8 mL/min, UV 254 nm) from Fr. 3.4.2.2.2. Compound 8 (23 mg) was purified by HPLC (Agilent Zobax Eclipse Plus C18, 4.6 × 250 mm, 30% MeOH, 70% H2O, 0.8 mL/min, UV 254 nm) from Fr. 3.4.2.3. Fr. 4 (3.9 g, 1:4) was subjected to a silica gel column (200–300 mesh, 600 × 35 mm) using a petroleum ether/acetone gradient elution (100:0 to 0:100) to yield 4 sub-fractions (Fr. 4.1–4.4). Fr. 4.2 (1.4 g, 3:1) was subjected to a silica gel column (200–300 mesh, 600 × 20 mm) using a dichloromethane/acetone gradient elution (100:0 to 0:100) to yield 3 fractions (Fr. 4.2.1–4.2.3). Fr. 4.2.2 (210 mg, 2:1) was applied to a Sephadex LH-20 column (1000 × 15 mm) and eluted with MeOH to obtain compound 4 (21 mg). Fr. 4.3 (1.2 g, 2:1) was subjected to a silica gel column (200–300 mesh, 600 × 20 mm) using a dichloromethane/acetone gradient elution (100:0 to 0:100) to obtain 2 fractions (Fr. 4.3.1–4.3.2). Fr. 4.3.1 (320 mg, 2:1) was further purified by HPLC (Agilent Zobax Eclipse Plus C18, 4.6 × 250 mm, 25% MeOH, 75% H2O, 0.8 mL/min, UV 254 nm) to obtain compound 5 (24 mg). Fr. 5 (2.3 g) was subjected to a silica gel column (200–300 mesh, 600 × 35 mm) using a petroleum ether/acetone gradient elution (100:0 to 0:100) to yield 4 fractions (Fr. 5.1–5.4). Fr. 5.4 (1.1 g, 1:2) was subjected to a silica gel column (200–300 mesh, 600 × 15 mm) using a dichloromethane/acetone gradient elution (100:0 to 0:100) to yield 2 fractions (Fr. 5.4.1–5.4.2). Fr. 5.4.2 (630 mg, 1:2) was applied to a Sephadex LH-20 column (1000 × 15 mm) and eluted with MeOH to obtain Fr. 5.4.2.1 (250 mg). Compound 6 (36 mg) was purified by HPLC (Agilent Zobax Eclipse Plus C18, 4.6 × 250 mm, 25% MeOH, 75% H2O, 0.8 mL/min, UV 254 nm) from Fr. 5.4.2.1.
Acid hydrolysis of compound 6
Compound 6 (5.0 mg) was dissolved in 5.0 mL of 2 N HCl, heated at 90 °C for 2 h and then partitioned between ethyl acetate and water. A glycon formononetin was recovered from the ethyl acetate layer and identified by direct comparison with an authentic sample. The water layers were identified by comparison with an authentic sample using TLC analysis38. Sugars liberated from compound 6 were identified as glucose.
Total phenolic content
The total phenolic content (TPC) was determined using a modified version of the Folin-Ciocalteau colorimetric method39. The phenolic content was calculated as a gallic acid equivalent from the calibration curve of gallic acid standard solutions (20–300 μg/mL) and was expressed as millimoles of gallic acid equivalent per 100 g of dry weight (mmol equiv. GAE/100 g). The data are reported as the mean ± SD for three replicates.
Total flavonoid content
The total flavonoid content (TFC) was determined using a sodium borohydride/chloranil-based (SBC) assay40. A calibration curve was constructed to create a standard using different concentrations of quercetin (0.1–10.0 mM). The total flavonoid content was expressed as millimoles of quercetin equivalents per 100 g of dry weight (mmol equiv. QUE/100 g). The data are reported as the mean ± SD for three replicates.
DPPH radical-scavenging assay
The radical scavenging activity of extracts and compounds were measured by a slightly modified version of the method described by Brand-Williams41. All the extracts and compound samples were tested in triplicate. The inhibition rate (I%) of the radical-scavenging capacity was calculated using the following equation:

where ADPPH is the absorbance of the DPPH solution, Ablank is the absorbance of methanol instead of DPPH, As-DPPH is the absorbance of the DPPH solution in the presence of the sample and As-blank is the absorbance of methanol in the presence of the sample.
ABTS•+ radical cation scavenging assay
The ABTS assay was performed as previously described in published literature with some modifications42. The results are expressed in terms of micromoles of trolox equivalents per g of dry weight of extracts (μmol eq. trolox/g) or millimoles of trolox equivalent per gram of dry weight of compounds (mmol eq. trolox/g). All determinations were carried out in triplicate.
Ferric reducing power (FRAP) assay
The FRAP assay used was a modified version of that reported by Benzie and Strain43. The FRAP results are expressed in terms of micromoles of trolox equivalents per g of dry weight of extracts (μmol eq. trolox/g) or millimoles of trolox equivalent per gram of dry weight of compounds (mmol eq. trolox/g). All experiments were carried out in triplicate.
Inhibition of lipid peroxidation
In this assay, a modified thiobarbituric acid reactive species (TBARS) assay was used to measure the amount of lipid peroxide formed. The egg yolk homogenates were used as a lipid-rich medium44,45. Percentage inhibition was calculated with the following formula:

where C is the absorbance of fully peroxidized control and T is the absorbance of test sample. BHA and TBHQ were used as reference standards. The IC50 value was calculated from the regression equation obtained from plotting the sample concentration and rate of inhibition.
Protective effect on H2O2-induced E. coli
The microbial test system used in the manuscript was an effective method to evaluate the antioxidant properties of medicinal plant extracts and polyphenols. This method is easier in terms of experimental operation, lower in cost compared to cellular antioxidant activity assays and more biological relevant than the popular chemistry antioxidant activity measures. In this study, E. coli (ATCC No. 25922) was used to determine the antioxidant activity of compounds in vivo based on a modified version of the method described by Smirnova46,47. Bacteria were incubated in Luria-Bertani medium at 37 °C in a 150-mL flask with shaking at 50 r/min overnight until they reached the mid-log phase. The optical density at 600 nm was used to monitor the cell growth. A 1.5-mL cell suspension was added to 8.5 mL of LB medium and resulted in a final OD600 of 0.25 ± 0.004. Then, 100 μL of the compounds (0.1 mM), a 1-mL cell suspension and 8.9 mL of LB medium were mixed and incubated at 37 °C with shaking at 180 r/min for 1.5 h. After the 1.5 h incubation, 6.5 mM of hydrogen peroxide was added and the absorbance at 600 nm was then measured immediately. The sample was then incubated at 37 °C with shaking at 180 r/min for an additional 30 min before the absorbance was measured again. The OD600 is used to indicate the growth of E. coli, with a higher value of the OD600 indicating a higher growth rate of E. coli. The isolated compounds have the ability to reduce and eliminate the oxidation of H2O2 and protect the growth of E. coli; therefore, the higher antioxidant ability of the compounds indicates that the specific growth rate will be higher.
Specific growth rate was calculated according to the following equation:

where μ is the specific growth rate and N0 and N are the optical density at time zero and t, respectively.
Statistical analysis
All results are expressed as the mean ± standard deviation (SD). SPSS (Statistical Package for the Social Sciences) software was used to determine significant differences via one-way ANOVA followed by Duncan’s test; values < 0.05 were considered to be significant.
Additional Information
How to cite this article: Pu, W. et al. Structural Characterization and Evaluation of the Antioxidant Activity of Phenolic Compounds from Astragalus taipaishanensis and Their Structure-Activity Relationship. Sci. Rep. 5, 13914; doi: 10.1038/srep13914 (2015).
References
Ortega-Ramirez, L. A. et al. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: a hypothesis. J Food Sci. 79, 129–137 (2014).
Li, H. B., Wong, C. C., Cheng, K. W. & Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 41, 385–390 (2008).
Ito, N., Fukushima, S., Hasegawa, A., Shibata, M. & Ogiso, T. Carcinogenicity of butylated hydroxy anisole in F344 rats. J Natl Cancer Inst. 70, 343–347 (1983).
Movafeghi, A., Djozan, D., Razeghi, J. & Baheri, T. Identification of volatile organic compounds in leaves, roots and gum of Astragalus compactus Lam. using solid phase microextraction followed by GC-MS analysis. Nat Prod Res. 24, 703–709 (2010).
Yang, Z. G ., Sun, H. X . & Fang, W. H. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice. Vaccine. 23, 5196–5203 (2005).
Rios, J. & Waterman, P. A review of the pharmacology and toxicology of Astragalus. Phytother Res. 11, 411–418 (1997).
Chaudhary, L. B. & Srivastava, S. K. Taxonomic and distributional notes on some Astragalus L. (Fabaceae) in India. Taiwania. 52, 25–48 (2007).
McKenna, D. J., Jones, K., Hughes, K. & Humphrey, S. Astragalus. In: Botanical Medicines. The Desk Reference for Major Herbal Supplements. seconded. 1–17. The Haworth Herbal Press, New York, 2002.
WHO (World Health Organization). Radix Astragali. In: WHO monographs on selected medicinal plants. 50–58. World Health Organization, Geneva, Switzerland, 1999.
Wang, Y. P., Li, X. Y., Song, C. Q. & Hu, Z. B. Effect of astragaloside F on T, В lymphocyte proliferation and peritoneal macrophage function in mice1. Acta Pharmacol Sin. 23, 263–266 (2002).
Lei, H. et al. Anti-aging effect of astragalosides and its mechanism of action. Acta Pharmacol Sin. 24, 230–234 (2003).
Ganzera, M., Bedir, E., Calis, I. & Khan, I. Separation of Astragalus saponins by reversed phase high performance liquid chromatography and evaporative light scattering detection. Chromatographia. 53, 131–134 (2000).
Xiao, W. L. et al. Chemical and Genetic Assessment of Variability in Commercial Radix Astragali (Astragalus spp.) by Ion Trap LC-MS and Nuclear Ribosomal DNA Barcoding Sequence Analyses. J Agr Food Chem. 59, 1548–1556 (2011).
Huang, X., Liu, Y., Song, F., Liu, Z. & Liu, S. Studies on principal components and antioxidant activity of different Radix Astragali samples using high performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta. 78, 1090–1101 (2009).
Shao, B. M. et al. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Bioph Res Co. 320, 1103–1111 (2004).
Toda, S. Antioxidant effects of Ogikeishigomotsuto, Astragali Radix (Ogi) and isoflavones in Astragali Radix on oxidative stress in vitro. J. Tradit. Med. 22, 162–166 (2005).
Toda, S. & Shirataki, Y. Inhibitory effects of Astragali Radix, a crude drug in Oriental medicines, on lipid peroxidation and protein oxidative modification by copper. J Ethnopharmacol. 68, 331–333 (1999).
Cui, R. et al. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemoth Pharm. 51, 75–80 (2003).
Fan, Y., Wu, D. Z., Gong, Y. Q., Zhou, J. Y. & Hu, Z. B. Effects of calycosin on the impairment of barrier function induced by hypoxia in human umbilical vein endothelial cells. Eur J Pharmacol. 481, 33–40 (2003).
Kuo, Y. H., Tsai, W. J., Loke, S. H., Wu, T. S. & Chiou, W. F. Astragalus membranaceus flavonoids (AMF) ameliorate chronic fatigue syndrome induced by food intake restriction plus forced swimming. J Ethnopharmacol. 122, 28–34 (2009).
Xu, W. Y. & Luo, J. N. Study of Astragalus taipaishanensis in pharmacognosy. J Xi’an Medical University. 9, 24–28 (1988).
Tong, X. et al. Studies on the chemical constituents of leaves and twigs of Taxus cuspidata. Acta Pharmacol Sin. 26, 510–514 (1994).
Quetin-Leclercq, J., Ancenot, L., Dupont, L. & Bisset, N. Isolation and purification of panarine, A. Alkaloid from a Venezuelan Curare. Phytochemistry. 27, 4002–4004 (1988).
Lee, Y. W. et al. Application of countercurrent chromatography/thermospray mass spectrometry for the identification of bioactive lignans from plant natural products. Analy Chem. 62, 244–248 (1990).
Ma, C. J., Li, G. S., Zhang, D. L., Liu, K. & Fan, X. One step isolation and purification of liquiritigenin and isoliquiritigenin from Glycyrrhiza uralensis Risch. using high speed counter current chromatography. J Chromatogr A. 1078, 188–192 (2005).
Wawer, I. & Zielinska, A. 13C CP/MAS NMR studies of flavonoids. Magn Reson Chem. 39, 374–380 (2001).
Aziz, N., Farag, S., Mousa, L. & Abo-Zaid, M. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 93, 43–54 (1997).
Pandey, K. B. & Rizvi, S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2, 270–278 (2009).
Ma, X. Q., Shi, Q., Duan, J., Dong, T. T. & Tsim, K. W. Chemical analysis of Radix Astragali (Huangqi) in China: a comparison with its adulterants and seasonal variations. J Agr Food Chem. 50, 4861–4866 (2002).
Chen, G. H. & Huang, W. F. Progress in pharmacological effects of compositions of Astragalus membranaceus. Chinese J New Drugs. 17, 1482–1485 (2008).
Aldarmaa, J. et al. Anti-convulsant effect and mechanism of Astragalus mongholicus extract in vitro and in vivo: protection against oxidative damage and mitochondrial dysfunction. Neurochem Res. 35, 33–41 (2010).
Zheng, K. Y. et al. Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: a signaling mediated via the accumulation of hypoxia-inducible factor-1α. J Agr Food Chem. 59, 1697–1704 (2011).
Ma, X., Duan, J., Zhu, D., Dong, T. & Tsim, K. Chemical comparison of Astragali Radix (Huangqi) from different regions of China. Nat Med. 54, 213–218 (2000).
Amić, D., Davidović-Amić, D., Bešlo, D. & Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat Chem Acta. 76, 55–61 (2003).
Seyoum, A., Asres, K. & EI-Fiky, F. K. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry. 67, 2058–2070 (2006).
Cai, Y. Z., Sun, M., Xing, J., Luo, Q. & Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 78, 2872–2888 (2006).
Burda, S. & Oleszek, W. Antioxidant and antiradical activities of flavonoids. J Agr Food Chem. 49, 2774–2779 (2001).
Wang, D. M., Pu, W. J., Wang, Y. H., Zhang, Y. J. & Wang, S. S. A new isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules. 17, 4595–4603 (2012).
Mocan, A. et al. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules. 19, 15162–15179 (2014).
He, X., Liu, D. & Liu, R. H. Sodium borohydride/chloranil-based assay for quantifying total flavonoids. J Agr Food Chem. 56, 9337–9344 (2008).
Brand-Williams, W., Cuvelier, M. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 28, 25–30 (1995).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorizationassay. Free Radic Bio Med. 26, 1231–1237 (1999).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. J Analyt Biochem. 239, 70–76 (1996).
Miguel, G. et al. Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chem. 86, 183–188 (2009).
Dhar, P. et al. Chemical composition and antioxidant capacities of phytococktail extracts from trans-Himalayan cold desert. BMC Complement Altern Med. 13, 259–289 (2013).
Smirnova, G. V., Samoylova, Z. Y., Muzyka, N. G. & Oktyabrsky, O. N. Influence of polyphenols on Escherichia coli resistance to oxidative stress. Free Radic Bio Med. 46, 759–768 (2009).
Smirnova, G. V. et al. Evaluation of antioxidant properties of medical plants using microbial test systems. World J Microb Biot. 26, 2269–2276 (2010).
Acknowledgements
This work was supported by the program from the special fund for forestry scientific research in the public interest of China (200904004).
Author information
Authors and Affiliations
Contributions
Experiment conception and design: D.M.W. and W.J.P. Experiment Performance: W.J.P. and D.Z. Data Analysis: W.J.P. and D.M.W. Writer: W.J.P. and D.M.W.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Pu, W., Wang, D. & Zhou, D. Structural Characterization and Evaluation of the Antioxidant Activity of Phenolic Compounds from Astragalus taipaishanensis and Their Structure-Activity Relationship. Sci Rep 5, 13914 (2015). https://doi.org/10.1038/srep13914
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13914
This article is cited by
-
Alleviation of liver cirrhosis and associated portal-hypertension by Astragalus species in relation to their UPLC-MS/MS metabolic profiles: a mechanistic study
Scientific Reports (2022)
-
Simultaneous determination of six active metabolites in Astragalus mongholicus (Fisch.) Bge. under salt stress by ultra-pressure liquid chromatography with tandem mass spectrometry
SpringerPlus (2016)
-
Astragaloside IV improves lipid metabolism in obese mice by alleviation of leptin resistance and regulation of thermogenic network
Scientific Reports (2016)
-
Bioactivity evaluation of cudraxanthone I, neocyclomorusin and (9βh)-3β-acetoxylanosta-7,24-diene isolated from Milicia excelsa Welw. C. C. Berg (Moraceae)
Medicinal Chemistry Research (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.