Abstract
Lithium isotopic compositions of fluid inclusions and hosted gangue quartz from a giant volcanogenic massive sulfide deposit in China provide robust evidence for inputting of magmatic fluids into a Triassic submarine hydrothermal system. The δ7Li results vary from +4.5‰ to +13.8‰ for fluid inclusions and from +6.7‰ to +21.0‰ for the hosted gangue quartz(9 gangue quartz samples containing primary fluid inclusions). These data confirm the temperature-dependent Li isotopic fractionation between hydrothermal quartz and fluid (i.e., Δδ7Liquartz-fluid = –8.9382 × (1000/T) + 22.22(R2 = 0.98; 175 °C–340 °C)), which suggests that the fluid inclusions are in equilibrium with their hosted quartz, thus allowing to determine the composition of the fluids by using δ7Liquartz data. Accordingly, we estimate that the ore-forming fluids have a δ7Li range from −0.7‰ to +18.4‰ at temperatures of 175–340 °C. This δ7Li range, together with Li–O modeling , suggest that magmatic fluid played a significant role in the ore formation. This study demonstrates that Li isotope can be effectively used to trace magmatic fluids in a seafloor hydrothermal system and has the potential to monitor fluid mixing and ore-forming process.
Similar content being viewed by others
Introduction
The submarine hydrothermal fluids affect mass balance of the hydrosphere1 and form sulfide ores on the modern seafloor2 and their ancient analogues preserved on land3. However, their sources remain controversial4. A common view suggests that they are derived from pure seawater circulating through the hot rocks2,5. An alternative view argues that there may be admixture of magmatic fluids escaping from magma at depth4. This controversy reflects the fact that transitional isotope tracers (e.g., δD, δ18O) seldom provide a robust signature of the fluid sources. Lithium isotope has proven to be an important geochemical tracer for fluid-related processes, particularly aqueous fluids, volatiles and metals in magma-hydrothermal systems, because of chloride-complexed behavior of lithium in aqueous fluids6,7, strong fluid mobility8 and the large mass fractionation9. Li isotopic fractionation may be used to track mineral precipitation and/or diffusion in hydrothermal system. Quartz is the most common gangue mineral trapped ore-forming fluids as inclusions for hydrothermal deposits. Its lithium isotopic system may has potential for constraining the source of fluids and quantifying the flux of fluids from distinct sources.
In this study we first report Li isotopic compositions of the Gacun Zn-Pb-Cu deposit, a giant volcanogenic massive sulfide deposit in the Yindun arc-basin system10,11, southwest China (Fig. 1). Our data suggest that magmatic fluids escaping from a rhyolitic melt play an important role in ore formation. Additionally, we demonstrate that the Li isotopic measurements can be used to monitor the mixing of fluids associated with ore formation and could help to locate the particularly mineralized horizons.
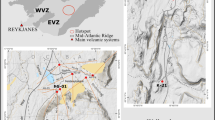
Geological map of the Gacun deposit (modified from refs. 10,12), showing sample locations and spatial distribution of the major orebodies on a 4100 m exploration plane.
The Triassic volcanic-sedimentary strata were folded and steeply-dipped eastwards due to later deformation, providing an ideal cross-section that shows a rhyolitic package and the hosted orebodies from the deep (west) to top (east). Inset map shows tectonic framework of the Yidun Arc, formed by westward subduction of the Ganzi-Litang oceanic lithposhere in Triassic10. The Gacun deposit is located in an intra-arc rifting zone within the Traissic arc and consists of three major orebodies. The location of U-Pb dating samples and the boundaries of a feeder zone of hydrothermal system10 are also shown. 53 samples for Li analyses were collected along 8 prospecting lines across orebodies at different heights (4050 m, 4100 m, 4150 m) and are shown on an exploration plane at 4100 m. Locations of all samples are marked by solid circles (at 4100 m) and open circles (at 4050 m and 4160 m). The minimal amount of seawater (Xseawater) in the ore-forming fluids for each sample was estimated by binary mixing modeling on Li-O isotopic data of 53 quartz samples (see Appendix II, Table S1 and Fig. 3). All data from Table S1.
Results
We have assessed a submarine hydrothermal system by a study of Li isotope on quartz and hosted fluid inclusions from the Gacun deposit, formed by Triassic submarine hydrothermal fluids10 (Fig. 1). This deposit is hosted by a 500-m thick, steeply-dipped, rhyolitic volcanic package exposed at Gacun (Fig. 1). It comprises three mineralized zones: (1) a sheet-like massive sulfide zone (UMO) with associated exhalites (barite, chert, jasper) covered by Triassic black phyllite, (2) a discordantly-underlying middle strata-bound stockwork zone (MSO) within a ~233 Ma rhyolitic tuff unit and (3) a semi-layered lower stringer zone (LSO) partly hosted in a ~221 Ma rhyolitic dome12. These three ore zones were formed by hydrothermal fluids discharged through a sub-seafloor feeder zone rooted into a rhyolitic dome10,13 (Fig. 1). The ~233 Ma rhyolitic volcanic rocks were derived from a single, relatively shallow, differentiating felsic magma14. The ~221 Ma rhyolitic dome, the lastest intrusive phase, acted as a “thermal engine”, driving the circulating of fluids through the overlying volcanic package12.
Nine rhyolite samples were collected for Li isotope analysis in this study (Table S1). The fresh rhyolites (n = 3) outside the Gacun deposit yield a narrow δ7Li range from +1.0‰ to +2.3‰, similar to continental crust (δ7Li = +1.2 ~ +1.7‰)15. The alterated rhyolites (n = 6) from a feeder zone show gradually increasing in δ7Li values from a rhyolitic dome (+1.2‰) and the LSO host rock (+3.4‰) to MSO host rock (+8.8‰) upwards (Table S1).
Fifty-three gangue quartz samples, collected across the main orebodies (Fig. 1) is divided into two groups: pure-quartz (n = 41) and quartz + sericite (n = 12). The former occurs in all three ore zones; the latter confines in the LSO and MSO. Li concentrations and isotopic compositions of 53 quartz samples and 29 fluid samples extraced from these host quartz are listed in Table S1 and plotted in Figs 2 and S1. The pure-quartz (n = 41) has Li concentrations of 0.03–1.61 μg/g and δ7Li varying from +4.1‰ to +22.5‰. The extracted fluids (n = 17) yield a range of δ7Li values from +3.7‰ to +15.0‰ (Fig. 2a). The quartz + sericite mixture has similar Li concentrations (0.02–1.75 μg/g) but lower δ7Li values (+0.8‰ to +4.2‰), the corresponding fluids (n = 12) have relatively high δ7Li varying from +1.3‰ to +10.2‰ (Fig. 2a). In general, the quartz samples from each ore zone have variable δ7Li values, laterally increasing outwards from a feeder zone (Table S1). The extracted fluids show average δ7Li values gradually increasing from +5.4‰ in the LSO to +9.8‰ in the UMO (Table S1).
(a) Variation in δ7Li of the host quartz and fluid inclusions with the measured homogeneous temperatures. (b) Relationship of Li isotopic fractionation factor (△quartz-fluid) with homogeneous temperatures (1000/T) of fluid inclusions hosted in nine pure-quartz samples (containing primary fluid inclusions). All data from Table S1.
All analyzed quartz samples (n = 53) were measured for O isotopic compositions, which yield δ18O values between +2.6‰ and +16.4‰ (Table S1). Based on homogenization temperatures (Th: 117°C–368°C) of fluid inclusions in analyzed quartz (Table S1), using the quation of Matsuhisa et al. (1979)16, the δ18O values of the fluids were estimated to vary from +0.9‰ to +8.7‰, showing a gradual decrease from a rhyolitic dome (av. +7.6‰), through the LSO (av. +7.1‰) and MSO (av. +5.4‰), to the UMO (av. +2.8‰).
Discussion
The δ7Li values of both fluid inclusions and host quartz were obtained for 17 pure-quartz samples, which yield Δδ7Liquartz-fluid values of +1.1 − +7.6‰ (Table S1). Extensive petrographic observations show that only 9 pure-quartz samples host primary fluid inclusions, evidenced by their alignment along quartz growth zones, regular forms (smooth grain, pillar and polygon) and variable sizes (usually 3–10 μm)10. These 9 samples yield Δδ7Liquartz-fluid values varying from +4.5‰ to +13.8‰ (Table S2) and show a negative correlation between Δδ7Liquartz-fluid and 1/T (Fig. 2b), which can be described by the following linear equation: Δδ7Liquartz-fluid = −8.9382 × (1000/T) + 22.22 (R2 = 0.98; 175 °C–340 °C). The Δδ7Liquartz-fluid shows a strong temperature- dependent fractionation.
Recent experiments on distinct mineral-fluid systems give conflicting results on Li isotopic fractionation between fluids and minerals. Lynton et al. (2005)17 studied Li isotopic fractionation in a quartz-muscovite-fluid system and found that fluids are isotopically lighter than minerals. A study on natural samples (e.g., granitic pegmatite) also reaches a similar conclusion18. By contrast, an experiment on a synthetic spodumene-hydrothermal fluid system confirms a temperature-dependent fractionation, but found that fluids are isotopically heavier than coexisting spodumene19. This result coincides with a few empirical studies, which suggest that fluids are isotopically heavier than altered basalts at low to moderate temperatures20,21,22. Our result is consistent with the studies of Lynton et al. (2005)17 and Teng et al. (2006)18.
The fractionation driven by diffusion predicts that δ7Li difference should exist in both hosted quartz and fluid after the entrapment of fluid inclusions and the measured results (i.e., Δδ7Liquartz-fluid) should be consistent with the traditional theory of stable isotopes: heavier isotope tending to enter into the liquid phase, due to diffusive transport in millions of years. Moreover, as this difference is often uncertain, a good correlation with the temperature is lacking. However, our data indicate that fluid inclusions were strongly lighter than their hosted quartz and show a strong correlation between Δδ7Liquartz-fluid and 1/T (Fig. 2b). We, therefore, argue that the result is mainly an equilibrium fractionation that occured when quartz was forming.
The empirical equation above indicates that the fluids in equilibrium with quartz have +2.1‰ to +7.6‰ lighter than their hosted quartz for these 9 pure-quartz samples. For other pure-quartz samples (n = 32) that contain secondary inclusions and failed in δ7Lifluid measurement, their δ7Liquartz data could be allowed to indicate the variable composition of the fluids. Based on measured Th (Table S1), using above equation, we estimate their δ7Lifluid to vary between –0.7‰ and +18.4‰ (Table S1).
It is noteworthy that the quartz + sericte group (n = 12) yields distinct Δδ7Liquartz-fluid, varying from −6.7‰ to −0.5‰ (Table S1). The ‘δ7Liquartz’ of quartz + sericte group (n = 12) is a mixing value, due to intermingling with minor sericite. The ‘δ7Liquartz’ tends to lighter than that of pure-quartz, because δ7Li of micas tends to lighter23. However, we grinded quartz + sericte samples to >200 mesh (<44 μm) during the extraction runs for fluid inclusions and centrifuged 30 mL leachates (distilled and deionized water: 5 rounds of 6 mL) and finally filtered using nylon filters with 0.22 μm pores (see Appendix I), which has avoided mixing of residual mineral powders. The δ7Li (+1.3 to +10.2‰) of the fluids extraced from quartz-sercite samples, therefore, could represent the δ7Li values of the ore-forming fluids at Gacun.
Our data above indicate that the Gacun ore-forming fluids are estimated to have a wider range of δ7Li (–0.7‰ to +18.5‰) than active vent fluids in 11°–13° EPR (+8.1‰ to +10.9‰)20, 21° EPR (+6.8‰ to +8.9‰)20, Endeavour in Judan de Fuca Ridge (+7.2‰ to +8.9‰)21 and Guyamas (~+10‰)22.
The sources of these vent fluids have been attributed to (1) progressively interacting seawater, passed through the hot rocks downward and heated by magma chamber20,21, (2) admixture of seawater-derived hot brine with cold seawater24 and (3) admixture of a magmatic fluid and seawater21. Our Li isotopic data can be used to test above hypothesis. A reaction progress model suggests that the reacted seawater gradually decrease its δ7Li with increasing in interaction depths and fluid temperatures and predicts that the seawater reacted at the lowest water/rock ratios has the lightest δ7Li values20. However, this model can not explain the low-δ7Li signature (<4‰) of the Gacun fluids, because experiment25, modeling24 and study on natural samples26 indicate that the seawater reacted at <450 °C seldom yields much light δ7Li down to <4‰. Moreover, the lightest δ7Lifluid values mostly cluster within or near a feeder zone at Gacun (Fig. S1), where no any organic-rich sediments were penetrated by circulating fluids (Fig. 1) and th heat transfer from a rhyolitic dome likely produced high water/rock ratios27, also arguing against this model. Phase separation of heated seawater could form a brine and a vapor phase28,29, however, this process did not make the δ7Li value decrease21. At Gacun, the δ7Li value of strongly-altered dome rhyolite (+1.2‰, GC4100-9-1, Table S1) is similar to that of the fresh rhyolites (~ + 1.2‰), which requires an extremely low δ7Li (≤1.2‰) hot fluid or brine interacted with the subvolcanic rock. The formation of such low-δ7Li brine (fluid) requires an anomalously high temperature (>500 °C)30 during seawater/rock interaction, which is inconsistent with microthermomatric results for fluid inclusions (Table S1). The last candidate is the injection of magmatic fluid escaping from a felsic magma, which resulted in releasing of buoyant vapor-rich fluids, localized heating and supercritical phase separation21. This scenario is supported by high-temperature (>350 °C) and high-salinity (41.0 wt% NaCl) fluid inclusions hosted by phenocryst quartz in the dome rhyolite13 and also consistent with close spatial association of ores with rhyolitic rocks13 and the relatively high gas content, high salinity and 18O enrichment of the Gacun hydrothermal system10.
To date, a few experiments have been performed to determine the Li isotopic fractionation between magmatic fluids and melts30,31. A theoretic model shows that the δ7Li of both residual melts and exsolving fluids do not change significantly with progressive fluid exsolution17. Based on Li isotopic compositions of the Gacun rhyolites (δ7Li: +1.0‰ to +2.3‰), we estimate that the δ7Li values of initial fluid exsolving from a rhyolitic melt vary from +1.0‰ to +3.0, close to the lowest measured δ7Li values for the LSO. This means that variation in δ7Lifluid (- 0.7 to +18.5‰) observed at Gacun may be caused by other processes, such as interacting with marine sediments (δ7Li = −1.0 ∼ +24‰)32,33 and mixing with seawater (δ7Li = +31.5‰)24,26. However, the lack of hydrothermal alteration in the hanging-wall (Triassic shales) and the absence of marine sediments in the host package at Gacun (Fig. 1) rule out the first possibility. All δ7Lifluid data may be interpreted by a binary mixing between magmatic fluid and seawater, which is supported by Li–O isotopic system at Gacun (Fig. 3).
Oxygen-lithium isotopic compositions of the ore-forming fluids at Gacun, which can be reproduced by mixing of variable amounts of seawater (δ18O = +0‰; δ7Li = +31.5‰) with a magmatic fluid (δ18O = +8‰; δ7Li = +1.5‰) with variable Limagmatic/Liseawater mass mixing ratios.
Dot-lines with open-circles (10% interval) show a binary mixing between magmatic fluid and seawater. Lim/Lic refers Li concentration ratios in magmatic fluid and seawater. Modeling calculation sees Appendix II.
Figure 3 shows that δ18O–δ7Li data partially overlap with magmatic fluids, suggesting a significant role of magmatic fluids in the ore formation. However, their majority show a systemic shift toward seawater with a general negative correlation between δ7Li and δ18O for each ore zone (Fig. 3). The UMO has relatively high δ7Lifluid (+7.4‰ to +18.4‰) and low δ18Ofluid (+0.9‰ to +4.9‰), implying more seawater contribution, whereas the LSO within the dome yields lower δ7Lifluid (0 to +6.5‰) and higher δ18Ofluid (+6.9‰ to +8.7‰), recording the footprint of magmatic fluids (Fig. 3). Li–O mixing modeling (see Appendix II) indicates that all sample data can be reproduced by the mixing of variable amounts of seawater with a magmatic fluid. The best-fit model curves linking each ore zone can be obtained by using variable Limagmatic/Liseawater mass mixing ratios (Fig. 3). This two-component modeling requires Limagmatic/Liseawater mixing ratio for the UMO (1.5:1 to 5:1), much lower than those for the LSO (5:1 to 20:1). The highly variable ratios are probably controlled by the chemical compositions of the degassed magmatic fluids, which changes with the magma evolution4. This is because that Li partition coefficient (Dfluid/melt) increases with temperature, the mole fraction of H2O and the concentration of Cl in the fluids6,34. A previous study has shown that the quartz from the LSO (av. 320 °C) host CO2-rich multiphase inclusions, whereas ones from the UMO (av. 200 °C) host liquid-vapor two-phase aqueous inclusions at Gacun10. This indicates that the ore-forming fluids at Gacun vary from a mixture of CO2 with H2O to H2O-dominated with time, which is consistent with compositional trend produced by successively degassing of the evolving magma4,35. At similar temperatures and pressures, one can predict that relatively high CO2/H2O ratios in the fluid for the LSO likely resulted in low Li concentrations in the magmatic fluid, whereas higher concentrations of H2O and Cl in the fluid for the UMO probably lead to stronger Li partitioning into the fluid (Fig. 3). Assuming that a magmatic fluid has δ7Li of +3‰ and δ18O of +8‰36 and Triassic seawater is same as modern seawater (+31.5‰), about 80–90% and <50% magmatic fluids are estimated to account for the formation of the LSO and UMO, respectively (Table S1). Considering possible lighter δ7Li (~ + 26‰) of Mesozoic seawater than modern seawater37,38, our estimated results are the maxima quantitative proportions of magmatic fluids in the ore-forming hydrothermal system at Gacun.
Spatial variation in the minimum percentages of seawater (Xseawater) in the ore-forming fluid system shows the outline of a Triassic submarine hydrothermal system at Gacun (Figs 1 and 4), in which the convective circulation of fluids through ~233 Ma volcanic units was driven by a rhyolitic dome emplaced at ~221 Ma. The initial fluid is dominated by magmatic water, escaping from the rhyolitic dome, which firstly formed the LSO and subsequently mixed with seawater circulating through the hot rocks and discharged upward via a sub-vertical feeder zone (Fig. 4). Lateral migration of seawater mixed with magmatic fluid along permeable layers within the rhyolitic package formed the MSO orebodies (Fig. 4). The episodically inputting of fluids dominated by seawater into a seafloor brine pool within a submarine basin10 led to the formation of the UMO10,11.
Two-dimentional compositional variation of a Triassic submarine hydrothermal system on a geological cross-section at Gacun.
The map shows that the initial fluid dominated by magmatic vapors escaping from a rhyolitic dome discharged upwards via a sub-vertical feeder zone and drived the convective circulation of seawater-dominated fluids through volcanic units. The contours with tick of seawater quantitative proportion (number) for each mineralized zone were outlined, based on all point data on Xseawater for each sample in Fig. 1.
Conclusions
The Gacun deposit yields δ7Li varying from +4.5‰ to +13.8‰ for fluid inclusions and from +6.7‰ to +21.0‰ for the hosted gangue quartz(9 gangue quartz samples containing primary fluid inclusions). The estimated δ7Li values (−0.7‰ to +18.4‰) for the ore-forming fluids suggest a significant role of magmatic fluids in the ore formation. Our data show that combination of δ7Li with δ18O data could monitor the mixing of fluids associated with ore formation and help to locate the particularly mineralized horizons.
Methods
Li isotopic analysis
Li concentrations and isotopic ratios of quartz and hosted fluid inclusions were measured using an atomic absorption spectrophotometer (AAS) and a double focusing multicollector inductively coupled plasma mass spectrometer (MC–ICP–MS) at the MLR Key Laboratory of Metallogeny and Mineral Assessment, Institute of Mineral Resources, CAGS, China, following Rudnick et al. (2004)39 and Tian et al. (2012)40. The details of sample processing and analytical methods and results of microtherometric measurement and Li-O isotopic analyses are given in Appendix I and Tables S1 and S2.
Fluid inclusions were extracted by the crush and leach method at the MLR Key Laboratory of Metallogeny and Mineral Assessment, Insititute of Mineral Resources, CAGS. Before extracting fluid inclusions, approximately 4 g of handpicked quartz grains (60 ~ 80 mesh , 178 ~ 250 μm) were heated in chloroazotic acid (a ~3:1 mixture of HCl-HNO3) on a hot plate (T < 120 °C) for 3 hours. Then distilled-deionized water is used to clean quartz grains, until the conductivity of leachates is consistent with that of deionized water (>18.2 MΩ cm resistivity). The cleaned quartz grains were dried and grinded to be fine powders (>200 mesh, <44 μm) using an agate mortar in ultraclean cabinet. The leachates were extracted by fine powders in 30 ml of deionized water (each 6 ml, 5 times), centrifuged and then filtered using nylon filters with 0.22 μm pores (remove silica powder). Based on traditional textural analysis of fluid inclusion populations and homogenization temperatures, primary inclusions of magmatic origin dominate the quartz samples selected for the grind-leach analysis (>90%). The fluid inclusion leachates were dried and re-dissolved in 4 M HCl, in preparation for chromatographic separation.
Dried quartz fine powders were dissolved in a mixture of concentrated HNO3-HF (about 1g sample in 0.5 mL HNO3 +5 mL HF) in savillex screw-top beakers overnight on a hot plate (T < 120 °C), followed by replenishment of the dried residua with concentrated HNO3 overnight and dried again, then picked up in concentrated HCl until solutions were clear. The solutions were then dried down and re-dissolved in 4M HCl, in preparation for chromatographic separation. Purification procedures with three columns were described by Tian et al. (2012)40. Lithium isotopic compositions were determined at the Institute of Mineral Resources using a standard bracketing method and a Thermo Finnigan Neptune MC-ICP-MS instrument. The δ7Li value of each unknown was calculated relative to the average of two bracketing IRMM-016 runs. Results of IRMM-016 (δ7Li = +0.2 ± 0.4‰, 2σ, n = 6) agree with previously published data (e.g., Rudnick et al., 200439; Halama et al., 200741, 200842). During the course of this study, two international rock standards were analyzed to evaluate the accuracy of the measurements, with the basaltic BHVO-2 standard yielding a δ7Li value of +4.7‰ ± 1.0‰ (2σ, n = 53; Zhao et al., 201543) and the andesitic AGV-2 standard yielding a δ7Li value of +6.1 ± 0.4‰ (2σ, n = 14; Zhao et al., 201543). The external precision of Li isotopic analyses was based on 2σ values of repeat runs of pure Li standard solutions and rock solutions over a three-year period and is less than or equal ±1.0‰ (Zhao et al., 201543). The accuracy of this method was established by Teng et al. (2006a)18 as being ±5% based on isotope dilution methods and the precision is less than ±10% (Teng et al., 2006b)44.
O isotopic analysis
The O isotopic compositions of quarz were analyzed at the Laboratory of Isotope, CAGS (Beijing). Single quartz grains were separated for oxygen isotope measurements. The analytical procedures of O isotopes are following Clayton and Mayeda (1963)45 and Hou et al. (2014)46, respectively. At ~550 °C, the quartz reacted with BrF5 to produce O2, SiF4 and BrF5 were separated from O2 by liquid nitrogen. At 700 °C, the O2 reacted with kryptol and was converted into CO2, which was collected in a sample tube. Oxygen isotope ratios were measured using a Thermo Finnigan MAT 253 mass spectrometer. The results are expressed in δ with V-SMOW as standards, as follows: δ18OV-SMOW = [(18O/16O)sample/(18O/18O)V-SMOW −1] × 1000. The analytical precisions of δ18O measurements were 0.2‰ (1SD), based on repeated measurements of standard samples46.
Additional Information
How to cite this article: Yang, D. et al. Lithium isotope traces magmatic fluid in a seafloor hydrothermal system. Sci. Rep. 5, 13812; doi: 10.1038/srep13812 (2015).
References
Von Damn, K. L. et al. Chemistry of submarine hydrothermal solutions at 21°, East Pacific Rise. Geochim Cosmochim Acta 49, 2197–2220 (1985).
Rona, P. A. et al. Major active and relict sea-floor hydrothermal mineralization: TAG hydrothermal field, Mid-Atlantic Ridge 26°N, 45°W. Economic Geology 88, 1989–2017 (1993).
Franklin, J. M., Gibson, H. L., Jonasson, I. R. & Galley, A. G. Volcanogenic massive sulfide deposits. Economic Geology 100, 523–560 (2005).
Yang, K. & Scott, S. D. Magmatic degassing of volatiles and ore metals into a hydrothermal system on the modern sea floor of the eastern Manus back-arc basin, western Pacific. Economic Geology 97, 1079–1100 (2002).
Lydon, J. W. Ore deposit models #14. Volcanogenic massive sulfide deposits Part 2: Genetic models. Geoscience Canada 15, 43–65 (1988).
Webster, J. D., Holloway, J. R. & Hervig, R. L. Partitioning of lithophile trace-elements between H2O and H2O + CO2 fluids and topaz rhyolite melt. Economic Geology 84, 116–134 (1989).
Candela, P. A. & Piccoli, P. M. Model ore-metal partitioning from melts into vapor and vapor/brine mixtures. In Thompson, J. F. H., Ed., Mineralogical Association of Canada, Quebec. Granites, Fluids and Ore Deposits 23, 101–128 (1995).
You, C. F., Castillo, P. R. & Gieskes, J. M. Trace element behavior in hydrothermal experiments: Implications for fluid processes at shallow depths in subduction zones. Earth Planet Sci Lett 140, 41–52 (1996).
Tomascak, P. B. Developments in the understanding and application of lithium isotopes in the earth and planetary sciences. In: Johnson C., Beard B. & Albarede F., eds. Geochemistry of Non-Traditional Isotopes Systems. America. Mineral Soc of Amer Geochem Soc 153–195 (2004).
Hou, Z. Q. et al. Origin of the Gacun volcanic-hosted massive sulfide deposit in Sichuan, China: Fluid inclusion and oxygen isotope evidence. Economic Geology 96, 1491–1512 (2001).
Hou, Z. Q. et al. Gacun VHMS deposit in southwest China: from field observation to ore model. Mineral Deposits 20, 44–56 (2001) (in Chinese with English abstract).
Yang, D. Source and evolution of ore-forming fluids in a volcanogenic massive sulfide (VMS) deposit: New constraint from lithium isotope on the genesis of the Gacun deposit, Sichuan. PhD dissertation of China University of Geosciences (Beijing) 47–57 (2014).
Xu, M. J. et al. Gacun Ag-rich polymetalic deposit in Sichuan province, China. Publishing House of Chengdu University of Science and Technology 1–164 (Chinese with English abstract) (1993).
Hou, Z. Q. et al. Collisional orogenesis of the Yidun arc, southwest China: evidence from the Triassic granitoid rocks. Acta Geologica Sinica 75, 484–497 (2001).
Teng, F. Z., Rudnick, R. L., McDonough, W. F. & Wu, F. Y. Lithium isotopic systematics of A-type granites and their mafic enclaves: Further constraints on the Li isotopic composition of the continental crust. Chem. Geol 262, 370–379 (2009).
Matsuhisa, Y. et al. Oxygen isotope fractionation in the system quartz-albite-anorthite-water. Geochimica et Cosmochimica Acta 43, 1131–1140 (1979).
Lynton, S. J., Walker, R. J. & Candela, P. A. Lithium isotopes in the system Qz-Ms-fluid: An experimental study. Geochim. Cosmochim. Acta 69, 3337–3347 (2005).
Teng, F. Z. et al. Lithium isotopic systematics of granites and pegmatites from the Black Hills, South Dakota. Am Mineral 91, 1488–1498 (2006a).
Wunder, B. et al. Temperature dependent isotopic fractionation of lithium between clinopyroxene and high-pressure hydrous fluids. Contributions to Mineralogy and Petrology 151, 112–120 (2006).
Chan, L. H., Edmond, J. M. & Thompson, G. A. lithium isotope study of hot springs and metabasalts from mid-ocean ridge hydrothermal systems. Jour Geophys Res 98, 9653–9659 (1993).
Foustoukos, D. I., James, R. H., Berndt, M. E. & Seyfried, W. E. Jr. Lithium isotopic systematics of hydrothermal vent fluids at the Main Endeavour Field, northern Juan de Fuca ridge. Chem Geol 212, 17–26 (2004).
Chan, L. H. et al. Lithium isotope geochemistry of sediments and hydrothermal fluids of the Guaymas basin, Gulf of California. Geochim Cosmochim Acta 58, 4443–4453 (1994).
Wunder, B. et al. Lithium isotope fractionation between Li-bearing staurolite, Li-mica and aqueous fluids: An experimental study. Chem Geol 238, 277–290 (2007).
Millot, R., Scaillet, B. & Sanjuan, B. Lithium isotopes in island arc geothermal systems: Guadeloupe, Martinique (French West Indies) and experimental approach. Geochim Cosmochim Acta 74, 1852–1871 (2009).
Seyfried, Jr., W. E., Chen, X. & Chan, L. H. Trace element mobility and lithium isotope exchange during hydrothermal alteration of seafloor weathered basalt: An experimental study at 350C, 500 bars. Geochim Cosmochim Acta 62, 949–960 (1998).
Chan, L. H. & Edmond, J. M. Variation of lithium isotope composition in the marine environment: A preliminary report. Geochim Cosmochim Acta 52, 1711–1717 (1988).
Cann, J. R., Strens, M. R. & Rice, A. A. simple magma-driven thermal balance model for the formation of volcanogenic massive sulfides. Earth Planet Sci Lett 76, 122–136 (1986).
Butterfield, D. A. et al. Gradients in the composition of hydrothermal fluids from the Endeavor segment vent field: phase separation and brine loss. Jour Geophys Res 99, 9561– 9583 (1994).
Seyfried Jr. et al. Chemistry of hydrothermal vent fluids from the Main Endeavour Field, Northern Juan de Fuca Ridge: geochemical controls in the aftermath of June 1999 seismic events. Jour Geophys Res 108 (B9), 2429 (2003).
Chacko, T., Cole, D. R. & Horita, J. Equilibrium oxygen, hydrogen and carbon isotope fractionation factors applicable to geological systems. In J. W. Valley & D. R. Cole, Eds., Stable Isotope Geochemistry. Reviews in Mineralogy and Geochemistry Mineralogical Society of America, Chantilly, Virginia. 43, 1–82 (2001).
Marschall, H. R. et al. The lithium isotopic composition of orogenic eclogites and deep subducted slabs. Earth Planet Sci Lett 262, 563–580 (2007).
Chan, L. H. et al. Lithium isotopic composition of Central American Volcanic Arc lavas: implications for modification of subarc mantle by slab-derived fluids. Chem Geol 160, 255–280 (1999).
Hoefs, J. & Sywall, M. Lithium isotope composition of Quaternary and Tertiary biogene carbonates and a global lithium isotope balance. Geochim Cosmochim Acta 61, 2679–2690 (1997).
London, D., Hervig, R. L. & Morgan, G. B. Melt-vapor solubilities and elemental partitioning in peraluminous granite-pegmatite systems—experimental results with Macusani glass at 200 MPa. Contrib Mineral Petrol 99, 360–373 (1988).
Yang, K. & Scott, S. D. Possible contribution of metal-rich magmatic fluid to a seafloor hydrothermal system. Nature 383, 410–413 (1996).
Taylor, H. P. Jr. & Sheppard, S. M. F. Igneous rocks: I. Processes of isotope fractionation and isotope systematic. Rev. Mineralogy 16, 227–272 (1986).
Misra, S., Philip N. & Froelich, P. N. Seawater: Changes in silicate weathering and reverse weathering. Science 335, 818–822 (2012).
Pogge von Strandmann, P. A. E., Jenkyns, H. C. & Woodfine, R. G. Lithium isotope evidence for enhanced weathering during Oceanic Anoxic Event 2. Nature Geoscience 10.1038/NGEO1875 (2013).
Rudnick, R. L., Tomascak, P. B., Njoa, H. B. & Gardnerb, R. Extreme lithium isotopic fractionation during continental weathering revealed in saprolites from South Carolina. Chem Geol 212, 45–57 (2004).
Tian, S. H., Hou, Z. Q. & Su, A. N. Separation and Precise Measurement of Lithium Isotopes in Three Reference Materials Using MC-ICPMS. Acta Geologica Sinica 86, 1297–1305 (2012).
Halama, R. et al. The Li isotopic composition of Oldoinyo Lengai: Nature of the mantle sources and lack of isotopic fractionation during carbonatite petrogenesis. Earth Planet Sci Lett 254, 77–89 (2007).
Halama, R. et al. Tracking the lithium isotopic evolution of the mantle using carbonatites. Earth Planet Sci Lett 265, 726–742 (2008).
Zhao, Y. et al. Study on measurements of lithium isotopic compositions for common standard reference materials using MC–ICP–MS. Rock Miner Analy. 34, 28–39 (in Chinese, with English abstract) (2015).
Teng, F. Z. et al. Diffusion-driven lithium isotopic fractionation in country rocks of the Tin Mountain pegmatite. Earth Planet Sci Lett 243, 701–710 (2006b).
Clayton, R. N. & Mayeda, T. K. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim Cosmochim Acta 27, 43–45 (1963).
Hou, Z. Q., Liu, Y., Yang, Z. M., Tian, S. H. & Xie, Y. L. Formation of carbonatite-related giant rare-earth-element deposits by the recycling of marine sediments. Scientific Reports 5, 10231, 10.1038/srep10231 (2015).
Acknowledgements
This work was funded by IGCP/SIDA-600 project and the NSFC (41103005, 41573038). We are grateful to Chen Weishi for her help with fluid inclusion measurement and Wan Defang for oxygen isotopic analyses.
Author information
Authors and Affiliations
Contributions
Z.Q.H. designed and initiated the research and interpreted data and wrote this draft. D.Y. contributed to the field investigation and Li isotopic analyses and data interpretation. Y.Z., K.H. and S.T. contributed to the isotopic measurements and Z.Y. and Q.F. for field-work and microthermomatric measurements.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, D., Hou, Z., Zhao, Y. et al. Lithium isotope traces magmatic fluid in a seafloor hydrothermal system. Sci Rep 5, 13812 (2015). https://doi.org/10.1038/srep13812
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13812
This article is cited by
-
Lithium and oxygen isotopic constraints on the source and evolution of ore-forming fluids: a case study from the Shuiyindong Carlin-type gold deposit, SW China
Mineralium Deposita (2024)
-
Multi-stage metal enrichment and formation of gold mineralization in black shales: the role of high heat flow in a rift setting
Mineralium Deposita (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.