Abstract
The influence of carbon dioxide (CO2) and soil fertility on the physiological performance of plants has been extensively studied, but their combined effect is notoriously difficult to predict. Using Coffea arabica as a model tree species, we observed an additive effect on growth, by which aboveground productivity was highest under elevated CO2 and ammonium fertilization, while nitrate fertilization favored greater belowground biomass allocation regardless of CO2 concentration. A pulse of labelled gases (13CO2 and 15NH3) was administered to these trees as a means to determine the legacy effect of CO2 level and soil nitrogen form on foliar gas uptake and translocation. Surprisingly, trees with the largest aboveground biomass assimilated significantly less NH3 than the smaller trees. This was partly explained by declines in stomatal conductance in plants grown under elevated CO2. However, unlike the 13CO2 pulse, assimilation and transport of the 15NH3 pulse to shoots and roots varied as a function of interactions between stomatal conductance and direct plant response to the form of soil nitrogen, observed as differences in tissue nitrogen content and biomass allocation. Nitrogen form is therefore an intrinsic component of physiological responses to atmospheric change, including assimilation of gaseous nitrogen as influenced by plant growth history.
Similar content being viewed by others
Introduction
In recent decades, the influence of elevated CO2 on the physiological performance of terrestrial plants has been examined across a wide range of environments and species. Trees have been recognized as the most responsive functional type, consistently showing enhanced growth under CO2 enrichment1,2. For many species, however, growth stimulation under elevated CO2 is followed by a decline in plant nitrogen concentration and a subsequent shift in biomass and nutrient allocation among roots, stems and leaves3,4,5,6. Declines in plant nitrogen concentration have been attributed to CO2-induced inhibition of leaf nitrogen assimilation, which is influenced by soil fertility7,8,9, an effect possibly responsible for the absence of a long-term CO2 stimulation effect in many ecosystems dominated by trees10,11,12,13,14,15. Recent studies have attempted to describe interactions between the carbon and nitrogen cycles to better understand how management16,17,18, disturbance regime19,20,21 and atmospheric change22,23,24 affect soil processes and the productivity of terrestrial ecosystems. Common knowledge gaps in these distinct but interrelated lines of research stem from a lack of information on the combined effect of elevated CO2 and different sources of nitrogen during early tree growth. Investigating this effect was the motivation for the present study.
The primary sources of nitrogen for all terrestrial plants are the inorganic forms nitrate (NO3−) and ammonium (NH4+) and their relative abundance in soil is known to influence plant productivity25. Other sources of nitrogen include ammonia gas (NH3), the most abundant alkaline component of the atmosphere. Although atmospheric NH3 is a small pool compared to available soil nitrogen, there is compelling evidence to suggest that NH3, as well as other atmospheric nitrogen forms such as NOx, can affect tree growth26,27,28. Trees can acquire NH3 from and release NH3 into their surroundings, exhibiting a characteristic compensation point at which evolution of NH3 by leaves is equal to assimilation. This compensation point depends on the partial pressure of NH3 in the stomata and therefore on its partial pressure in the surrounding atmosphere, with linear increases in leaf uptake observed as its concentration rises29. As a point of reference, the concentration of NH3 in the atmosphere commonly varies between 1 and 10 ug/m3 (1.4 to 14 ppb or 0.15 to 1.5 mPa)30. Specific values, however, may range from around 0.03 ug/m3 in remote sites to concentrations up to four orders of magnitude higher near source hot spots30, the distribution of which is readily apparent in global datasets of atmospheric NH331. Given that most emitted NH3 is deposited downwind and assimilated by vegetation32,33 and considering recent findings showing that atmospheric CO2 enrichment decreases the NH3 compensation point34, it is likely that plants will become an increasingly stronger sink for atmospheric NH3.
To examine the effect of elevated CO2 and the form of soil nitrogen on foliar uptake of gases, we devised a dual-isotope (13C and 15N) labelling experiment to follow the assimilation and translocation of CO2 and NH3 among plant compartments. The experiment was imposed upon a longer history of growth under different conditions. The genus Coffea was ideal for this study, as it exhibits plastic morphophysiological features and has long been used as a model to investigate physiological mechanisms controlling productivity in woody plants. Members of the genus Coffea evolved as understory shrubs in tropical regions where rainfall seasonality gave rise to water conservation abilities, including strong regulation of leaf gas exchange, which is reflected in the productivity of the plant as a whole35,36. While these physiological responses are generally well understood, their effect on leaf CO2 and NH3 assimilation and transport has yet to be described.
Phase I – Changes in growth caused by CO2 enrichment and form of soil nitrogen
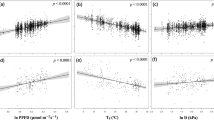
The first phase of the experiment was designed to test the combined effect of CO2 level and form of soil nitrogen on initial tree development under four different treatments: Ambient CO2 and NH4+ (A-NH4+); Ambient CO2 and NO3− (A-NO3−); Elevated CO2 and NH4+ (E-NH4+); Elevated CO2 and NO3− (E-NO3−). During five months, tree growth showed a significant additive effect of CO2 enrichment and form of soil nitrogen. Shoot growth was consistently higher under CO2 enrichment, with plants receiving NH4+ showing greater leaf area and total aboveground biomass than those receiving NO3− (Fig. 1). Tree productivity is generally expected to increase under elevated CO21,2 and here the positive effect of CO2 enrichment on the initial phase of tree development was enhanced by NH4+ fertilization. Despite this effect, no overall significant differences were observed for total plant biomass among all treatments; however, large contrasts in morphology occurred in response to soil nitrogen form, with twice as much biomass allocated to roots relative to shoots in NO3− treatments as compared to NH4+ treatments. Leaf area was also strongly affected by soil nitrogen form and, as a result, plants grown under A-NO3− and E-NH4+ treatments represented the low and high ends of the aboveground productivity spectrum, respectively (Fig. 1). The fact that these differences in structure and biomass allocation were largely independent of CO2 level but dependent on the form of soil nitrogen may be partially responsible for the observed effect of growth history on gaseous nitrogen uptake (discussed below).
Least square regressions describing initial plant growth (phase I), showing the effects of ambient and elevated CO2 on foliar area and dry biomass accumulation in shoots, roots and root to shoot ratio, in plants receiving nitrate (NO3−) or ammonium (NH4+) as the sole nitrogen source.
Shaded areas represent 95% confidence intervals of the average slope (solid lines). Significance levels correspond to the effect of treatments (fixed effects) as determined by repeated measure analysis of variance where time (day) is a random effect. Root to shoot ratios were not measured at time zero.
Differences in nitrogen content as a result of growth history provide further context for the observed differences in uptake of a pulse of isotopically labelled gas. As mentioned above, stimulation of growth by elevated CO2 was most clearly manifested as differences in aboveground biomass, maximized under NH4+ fertilization; at the same time, the foliar nitrogen concentrations of plants in this treatment (E-NH4+) were significantly greater than those of plants receiving NO3− (Fig. 2 and Supp Table 1). Plants grown under elevated CO2 generally had lower foliar nitrogen concentrations than those grown under ambient conditions, with the lowest levels of foliar nitrogen observed in the E-NO3− treatment (Fig. 2), which is consistent with a CO2-induced inhibition of NO3− assimilation into organic compounds shown in previous experiments8. Furthermore, differences in total aboveground biomass mirrored changes in nitrogen concentration in the plant tissue (Figs 1 and 2). This is diagnostic of nitrogen limitation6,37, an effect that was strongest under NO3− fertilization, despite the application of equal amounts of nitrogen during growth in all treatments.
Total nitrogen concentration in leaves, stems and roots tissue, determined at the end of phase I.
Horizontal lines within the boxes represent median values. The ends of the box represent the 75th and 25th quantiles and whiskers span the entire dataset including outliers. A full factorial analysis of main effects and interactions is presented in Supp Table 1. Letters show significant differences determined using Tukey HSD tests across treatments within each plant compartment (P < 0.05).
Phase II – The effect of growth history on foliar gas uptake
After 150 days, we assessed the legacy effect of growth conditions (i.e. atmospheric CO2 level and form of soil nitrogen) on leaf carbon and nitrogen uptake and subsequent allocation. Plants from each treatment were labelled with a simultaneous pulse of isotopically enriched gases (13CO2 and 15NH3). After one hour of exposure, analysis of leaf, stem and root tissue revealed that plants grown under a history of elevated CO2 assimilated significantly less 13CO2 and 15NH3 than those grown under a history of ambient CO2 (Fig. 3; Supp Table 2). Uptake of 15NH3 depended on the form of soil nitrogen, with highest uptake observed in the A-NO3− treatment. Carbon assimilation, on the other hand, was only affected by the CO2 treatment under which the plants had been previously grown. Surprisingly, plants grown under a history of elevated CO2 and NH4+, while larger, absorbed less of both labelled gases than smaller plants grown under ambient CO2 and NO3− (Fig. 3).
Isotopic data (phase II) reported as mass of carbon and nitrogen derived from 13CO2 and 15NH3 assimilated by leaves and present in plant compartments at one hour and five days after exposure to labelled gases.
Horizontal lines within the boxes represent median values. The ends of the box represent the 75th and 25th quantiles. Treatments applied during phase I significantly affected uptake and allocation of the pulse of labelled C and N. These treatments are: Ambient CO2 (A, 400 ppm); Elevated CO2 (E, 700 ppm); soil nitrogen supplied as NH4+ or as NO3−. A full factorial analysis of main effects and interactions is presented in Supp Table 2. Letters show significant differences determined using Tukey HSD tests across treatments within each plant compartment (P < 0.05). Where no letters are shown differences were not significant.
Differences in allocation during the five days after the labelling event further revealed physiological changes produced as a result of growth history. The amount of carbon and nitrogen translocated to stems and roots was proportional to that initially captured by leaves. In the case of carbon, significant differences emerged between plants grown under different CO2 levels, while for nitrogen the highest values were once again observed in plants grown under the A-NO3− treatment. These results show a clear legacy effect of atmospheric CO2 concentration on foliar gas uptake of young trees and soil nitrogen form on NH3 uptake in particular. The effect of soil nitrogen form disappears at high CO2, indicating that the CO2-induced decline in leaf nitrogen concentration (Fig. 2) is not only caused by inhibition of intercellular NO3− photoassimilation38, but is also a result of reduced uptake of NH3. This latter source of nitrogen proved noteworthy, comprising from 0.2% (E-NH4+) to 0.6% (A-NO3−) of total foliar nitrogen after only one hour of exposure, as calculated using average values of nitrogen derived from the 15NH3 pulse (Fig. 3), leaf nitrogen concentration (Fig. 2) and mass (Supp Table 3). Ammonia uptake could therefore be important in explaining growth responses in systems where the concentration of atmospheric NH3 is high. In fact, even at normal atmospheric concentrations, data from early research suggest that up to ten percent of the nitrogen requirement of a field crop could be satisfied by direct absorption of NH339. However, the factors that allow or limit continuous assimilation of NH3 by plants over extended periods of time remain to be determined.
Understanding soil-plant-atmosphere interactions
Our results represent an integrated measure of declines in foliar gas exchange induced by elevated CO2 and the additional influence of soil nitrogen form, on NH3 assimilation, an effect inversely correlated with plant size (Fig. 4). Notably, this effect was independent of foliar area and plant nitrogen content (Supp Fig 3). Furthermore, uptake of a pulse of 13CO2 was not significantly correlated with any allometric parameter, instead reflecting solely a decline in stomatal conductance (~23% on average) in plants subjected to CO2 enrichment (Supp Table 3). This result is consistent with earlier experiments performed using the same species under stress-free conditions (i.e. irrigated twice daily)36,40 and is comparable to CO2-induced declines in stomatal conductance recorded in a variety of other species and experimental settings2,41,42.
Relationship between whole plant mass measured at the end of phase I and total amount of labelled nitrogen and carbon assimilated by leaves during phase II.
The line shows a significant (P < 0.05) negative relationship between total biomass accumulation and foliar uptake of NH3, which was independent of foliar area and plant nitrogen content (Supp Fig 1). This relationship was not significant for assimilation of a pulse of CO2 (R2 = 0.74), which mainly responded to changes in stomatal conductance produced by a history of ambient or elevated CO2. Error bars represent standard errors of the mean.
While differences between the assimilation of a pulse of 13CO2 between ambient and elevated CO2 treatments reflect the expected influence of changes in conductance, differences in NH3 uptake among treatments were unexpected. The first step of NH3 assimilation by leaves is the simple absorption by leaf water, before any biochemical reaction has occurred. The second step involves the activity of the enzyme glutamine synthetase, which is affected by CO2 level and soil nitrogen form as well as other cell properties such as pH, which is also a major determinant of NH3 compensation point. Under optimal growth conditions, leaf uptake of NH3 would be affected by nitrogen demand, expected to increase under elevated CO234. However, shifts in soil nitrogen form can also alter demand gradients within plants, as NH4+ moves to the shoot via conversion into ureides, while NO3− is transported unaltered and then reduced by the enzyme nitrate reductase43. The observed differences in NH3 gas uptake associated with soil nitrogen form could thus be attributed to a decline in soil NO3− uptake, which involves its sequential conversion into NO2−, NH4+, glutamine and finally into other more complex compounds7.
In plants with C3 metabolism, elevated CO2 has been shown to decrease photorespiration, inhibiting shoot assimilation of NO3− 38,44. Divergent patterns of carbon and nitrogen translocation also integrate the effect of plant nutrition driven by the stoichiometry of biomass production, which determines a stronger sink for nitrogen in plants receiving only NO3−45. Consistent with this interpretation, plants grown under NO3− fertilization had the lowest tissue nitrogen content (Fig. 2). Furthermore, the highest and lowest amounts of NH3 uptake were observed, respectively, in plants receiving NO3− at ambient CO2 and plants receiving NH4+ at elevated CO2. Nevertheless, some general responses were common across all treatments. For example, after five days had elapsed following exposure to the pulse of labelled gases, most of the carbon assimilated had been distributed among plant organs (Fig. 3), while most of the nitrogen remained within leaves, where the majority of protein synthesis for photosynthesis occurs. Foliar uptake of NH3, therefore, occurs as a result of immediate metabolism (one hour) following exposure, while subsequent allocation represents a slower response (5 days) that is dependent on the form of nitrogen present in the soil during initial growth. Over 20% of the ammonia-derived nitrogen assimilated during the pulse moved into the stem and roots by the end of our 5-day observation period. This is consistent with earlier work using similar atmospheric NH3 levels46, in which, just as in the present study, metabolism and translocation of NH3 did not depend on plant nitrogen status.
Broad implications
Higher productivity due to decreased photorespiration and enhanced photosynthesis has been observed with rising CO2 levels, but this effect diminishes over time as a result of nutritional constraints, most commonly detected as a decline in foliar nitrogen concentration5,6,13. Several biochemical mechanisms have been proposed to explain CO2-induced decreases in plant nitrogen concentration7,8,25, none of which include decreased nitrogen uptake from the atmosphere. While NH3 uptake in the present study occurred via plant stomata, deposition of NH3 as well as ammonium compounds directly onto leaf surfaces can also supply nitrogen to vegetation by way of cuticular uptake28, although the importance of such deposition is debated, as several studies have shown it to be only a minor pathway47. At the plant level, previous experiments have shown that following exposure to NH3, a two-phase pool corresponding to assimilated and reversible storage may occur7,46. At the ecosystem level, a distinction must be made between canopy and foliar compensation points, which result from competition between cuticular and stomatal assimilation pathways, with cuticular uptake (especially in moist conditions) recapturing NH3 emitted by stomata48. In the present study, foliar NH3 uptake varied with the distinct stomatal conductances observed in plants in the elevated and ambient CO2 treatments, but its subsequent allocation to shoots and roots was strongly influenced by soil nitrogen form. Although the uptake of atmospheric nitrogen is expected to vary among different tree species owing to contrasts in foliar attributes and gas assimilation abilities49, we suspect that the effect of CO2 and soil nutrient histories is important in determining the contribution of gaseous nitrogen at the plant and ecosystem levels.
It is notable that results obtained from the first phase coupled with those obtained in the second phase reveal a clear association between plant productivity and assimilation NH3 (Fig. 4). Assimilation and translocation differed as a function of interactions between changes in stomatal conductance, recognized as a major determinant of NH3 uptake47 and the direct effects of soil nitrogen form, including obvious differences in tissue nitrogen content and biomass allocation. Our findings thus have important implications. Since the chemical form of soil nitrogen directly affects root growth as well as the uptake and distribution of NH3, it is critical to account for the effects of soil nutrients when predicting the impact of atmospheric change on tree species and tree-dominated ecosystems. Furthermore, leaf gas exchange and carbon and nitrogen assimilation in young trees reflect the legacy effect of atmospheric CO2 level and form of soil nitrogen during early tree growth. Widespread patterns of growth decline have been observed across biomes where CO2 stimulation was previously expected to occur, suggesting that nitrogen availability or form has constrained productivity23,50. The present study shows that nitrogen limitation can be caused, at least in part, by a decline in leaf assimilation of gaseous nitrogen. Exploring absorption factors for NH3 and other reactive gases is a promising direction for future research, as is investigating physiological thresholds that limit canopy sinks of atmospheric nitrogen emitted from fertilized and unfertilized lands.
Final considerations
It has long been known that atmospheric loading of NH3 has risen continually over the past century as a result of anthropogenic activities39 and is projected to further increase as these activities continue33. A recent estimate incorporating the dependence of emissions on climatic factors suggests that global annual NH3 emissions could increase from 65 Tg N in 2008 to 132 Tg by 210051. It is also known that uptake of NH3 by plants may increase as its concentration in the atmosphere increases29,52. It follows, then, that plant uptake of NH3 will become increasingly more important to terrestrial productivity in the future, although it is perhaps more appropriate to recognize that it has already been important for a long time. Indeed, some of the first researchers to demonstrate absorption of NH3 by plants expressed the opinion that “the importance of atmospheric NH3 as an agent for the transport and redistribution of nitrogen has been vastly underestimated” and that NH3 “can contribute significantly to the nitrogen budget of a growing plant community and could exert a prodigious influence on the long-term behavior of an ecosystem”39. Four decades later, having established that the amount of NH3 absorbed by a tree species is dependent on the combined history of CO2 and soil nutrients, the present study reveals more of the connection between soils, plants and the atmosphere, a connection that is especially pertinent today, as environmental changes persist and the long-term behaviour of ecosystems comes under greater scrutiny.
Materials and methods
The experiment was conducted in two phases. The first was designed to test the combined effect of CO2 level and form of soil nitrogen on initial tree development. We monitored plants during a period of approximately five months to determine growth patterns in each of the following four treatments: Ambient CO2 and NH4+ as the sole nitrogen source (A-NH4+); Ambient CO2 and NO3− as the sole nitrogen source (A-NO3−); Elevated CO2 and NH4+ as the sole nitrogen source (E-NH4+); Elevated CO2 and NO3− as the sole nitrogen source (E-NO3−). The second phase was designed to measure changes in uptake of 13CO2 and 15NH3 as influenced by the legacy effect of elevated and ambient atmospheric CO2 and soil nitrogen form imposed during the previous five months. We traced isotopic signals in leaves, stems and roots, calculating the total amount of each gas assimilated as well as their relative contribution to plant carbon and nitrogen pools. Differences in gas uptake were then compared with changes in above and below ground biomass allocation. Details of the experimental approach and sampling conditions in both phases are as follows:
Phase I
The first phase of the experiment was conducted in the controlled environment facilities of the University of California, Davis, using two chambers (3.3 m2 floor area by 1.8 m high) with metal halide and high-pressure sodium lamps (700 μmol s−1 m−2 PAR) and high-resolution controls to generate ambient (400 ppm) and elevated CO2 (700 ppm) conditions, under identical photoperiod (12 h), temperature (~20 °C at night and ~25 °C during daytime) and relative humidity (70%). Plants were grown in 0.65-liter pots (Supp Fig 2) containing the same mass and volume of a fine sand substrate. All plants were irrigated individually and rotated in the chambers twice a day, receiving a daily total of 200 ml of modified Hoagland nutrient solution53, diluted to a final concentration of 1.6 mM nitrogen as either NH4+ or NO3− and adjusted to the same pH. To obtain baseline data for isotopic composition and nutrient content we used control plants growing under ambient CO2 and receiving only deionized water. Since water and nutrient stress can affect photosynthesis and gas exchange, thereby altering responses to treatments, we performed three preliminary experiments with ~30 plants each for approximately 60 days to determine optimal pot size, water and nutrient supply. The main experiment was then initiated with 124 plants grown from seeds obtained from the same plant; six replicates from each treatment were destructively sampled at 0, 20, 79 and 145 days for determination of biomass in different plant compartments. Photosynthesis and stomatal conductance were measured weekly during this phase using a LiCor 6400 system (LiCor Inc., Lincoln, NE, USA) and three replicate plants of each treatment. Physiological parameters in all treatments proved consistent with earlier characterizations of coffee plants under stress-free conditions irrigated daily17.
Phase II
This short pulse labeling experiment was conducted once plants had achieved a stature that corresponded to a high survival rate under field conditions (>25 cm height; the typical transplantation size). For this experiment, all remaining plants (7 replicates per treatment; 28 plants total) were placed into an enclosed chamber with a fan inside to circulate air and sodium vapour lights above (Supp Fig 3). Immediately prior to the labelling event, the soil was isolated by sealing a plastic bag around the base of each individual stem, leaving only the upper stem and leaves exposed. The chamber was sealed and two pulses of gas (both at 99% atom percent enrichment) were injected simultaneously into the chamber: 300 ml of 13CO2, giving an initial concentration in the chamber of ~600 ppmv CO2 and 80 ml of a mixture of 15NH3 and air, giving an initial concentration of ~40 ppmv NH3 (28 mg/m3). Gas samples were taken regularly for the duration of the labelling event (one hour) from a small port in the chamber, in order to monitor the absorption of gases by plants. The temperature during the labelling event was ~25 °C and a previous test had shown that there was negligible leakage of the chamber. Labelled CO2 was used as received from Cambridge Isotope Laboratories, Inc. (Andover, MA) and labelled ammonia was prepared by gently heating a mixture of labelled ammonium sulphate and magnesium oxide and capturing the evolved ammonia in a small gas sampling bag.
Data analysis and interpretation
Before the labelling event, nitrogen treatments had been continuously maintained under ambient and elevated CO2 conditions. Thus, the responses observed in phase II represent an integrated measure of the legacy effect of elevated or ambient CO2 levels and of soil NH4+ or NO3− applied during phase I. During the pulse labelling event, the concentration of CO2 decreased approximately 150 ppm over the course of the hour, but remained above ambient concentrations and thus did not become limiting. The concentration of NH3 was intentionally chosen to be higher than in unpolluted air, greatly surpassing the typical NH3 compensation point (~0.003 ppmv), beyond which only strong differences in leaf NH3 absorption capacity would be able to affect absorption29. This allowed us to confidently assess the effects of growing conditions (treatments) on foliar CO2 and NH3 uptake and subsequent allocation. After one hour in the labelling chamber, all plants were removed and three plants from each treatment were immediately separated into leaves, stems and roots. Five days later, the four remaining plants in each treatment were processed in the same way, to assess translocation of labelled carbon and nitrogen among plant organs after initial uptake. Plant samples were dried at 65 °C to constant mass, ball milled and analyzed for C and N content and isotopic composition using an Elementar Vario EL Cube or Micro Cube elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) at the Stable Isotope Facility of the University of California, Davis. The amount of carbon and nitrogen in each plant component which was derived from the pulse of gas was calculated using standard label recovery equations54 and the background isotopic composition of control plants not exposed to the labelled gases.
Statistical Analysis
In phase I two different chambers were used to impose ambient and elevated CO2 treatments. A potential chamber effect is therefore incorporated into the analysis of initial growth. However, this effect does not influence the analysis of data generated during phase II, as a single chamber was used to simultaneously label replicates from all treatments. Accordingly, we used a bivariate line-fitting method for allometric comparisons in phase I, comprised of a mixed model and repeated measures analyses of variance, in which sampling time is considered a random effect nested within the fixed effects of CO2 and N source. Levene’s test confirmed that variances were homogeneous across treatments for all response variables measured in phase I. This was not the case for the data generated during phase II, which was log transformed prior to analyses of variance, followed by post hoc Tukey tests of honest significant difference to compare the recovery of carbon and nitrogen derived from the pulse of gas in each plant component. This approach was applied to both sampling events (one hour and 5 days after labelling) and statistical results are presented alongside the original (untransformed) data.
Additional Information
How to cite this article: Silva, L. C. R. et al. Carbon dioxide level and form of soil nitrogen regulate assimilation of atmospheric ammonia in young trees. Sci. Rep. 5, 13141; doi: 10.1038/srep13141 (2015).
References
Ainsworth, E. A. & Long, S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol. 165, 351–371 (2005).
Ainsworth, E. A. & Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant. Cell Environ. 30, 258–70 (2007).
Iversen, C. M., Keller, J. K., Garten, C. T. & Norby, R. J. Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2 enrichment. Glob. Chang. Biol. 18, 1684–1697 (2012).
Rogers, H. H., Prior, S. A., Runion, G. B. & Mitchell, R. J. Root to Shoot Ratio of Crops as Influenced by CO2 . Plant Soil 187, 229–248 (1996).
Poorter, H. & Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust. J. Plant Physiol. 27, 595–607 (2000).
Reich, P. B. & Hobbie, S. E. Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat. Clim. Chang. 3, 278–282 (2012).
Bloom, A. J. et al. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 93, 355–67 (2012).
Bloom, A. J., Burger, M., Rubio Asensio, J. S. & Cousins, A. B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899–903 (2010).
Myers, S. S. et al. Increasing CO2 threatens human nutrition. Nature 510, 139–142 (2014).
Gedalof, Z. & Berg, A. A. Tree ring evidence for limited direct CO2 fertilization of forests over the 20th century. Glob. Biogeochem Cycles 24, GB3027 (2010).
Peñuelas, J., Canadell, J. G. & Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 20, 597–608 (2010).
Silva, L. de C. R. & Horwath, W. R. Explaining global increases in water use efficiency: Why have we overestimated responses to rising atmospheric CO2 in natural forest ecosystems? PLoS One 8, e530 (2013).
Finzi, A. C. et al. Progressive nitrogen limitation of ecosystem processes under elevated CO2 in a warm-temperate forest. Ecology 87, 15–25 (2006).
Silva, L. & Anand, M. Historical links and new frontiers in the study of forest-atmosphere interactions. Community Ecol. 14, 208–218 (2013).
Gómez-Guerrero, A. et al. Growth decline and divergent tree ring isotopic composition (δ13C and δ18O) contradict predictions of CO2 stimulation in high altitudinal forests. Glob. Chang. Biol. 19, 1748–1758 (2013).
Qiao, Y., Miao, S., Silva, L. C. R. & Horwath, W. R. Understory species regulate litter decomposition and accumulation of C and N in forest soils: A long-term dual-isotope experiment. For. Ecol. Manage. 329, 318–327 (2014).
Townsend, A. R., Vitousek, P. M. & Houlton, B. Z. The Climate Benefits of Better Nitrogen and Phosphorus Management. Issues Sci. Technol. 28, 85–91 (2012).
Baldocchi, D. Biogeochemistry : Managing land and climate. Nat. Clim. Chang. 4, 330–331 (2014).
Franco, A. C., Rossatto, D. R., De Carvalho Ramos Silva, L. & Da Silva Ferreira, C. Cerrado vegetation and global change: the role of functional types, resource availability and disturbance in regulating plant community responses to rising CO2 levels and climate warming. Theor. Exp. Plant Physiol. 26, 19–38 (2014).
Moncrieff, G. R., Scheiter, S., Bond, W. J. & Higgins, S. I. Increasing atmospheric CO2 overrides the historical legacy of multiple stable biome states in Africa. New Phytol. 201, 908–15 (2014).
Paiva, A. O., Silva, L. C. R. & Haridasan, M. Productivity-efficiency tradeoffs in tropical gallery forest-savanna transitions: linking plant and soil processes through litter input and composition. Plant Ecol. 216, 775–787 (2015).
Adriaenssens, S. et al. Foliar Nitrogen Uptake from Wet Deposition and the Relation with Leaf Wettability and Water Storage Capacity. Water, Air, Soil Pollut. 219, 43–57 (2010).
Fernández-Martínez, M. et al. Nutrient availability as the key regulator of global forest carbon balance. Nat. Clim. Chang. 4, 471–476 (2014).
Silva, L. C. R., Gómez-Guerrero, A., Doane, T. A. & Horwath, W. R. Isotopic and nutritional evidence for species- and site-specific responses to N deposition and rising atmospheric CO2 in temperate forests. J. Geophys. Res. Biogeosciences (2015) 10.1002/2014JG002865.
Epstein, E. & Bloom, A. Mineral Nutrition of Plants: Principles and Perspectives. (Sinauer Associates, 2004).
Pinder, R. W. et al. Climate change impacts of US reactive nitrogen. Proc. Natl. Acad. Sci. USA 109, 7671–5 (2012).
Gessler, A., Rienks, M. & Rennenberg, H. Stomatal uptake and cuticular adsorption contribute to dry deposition of NH3 and NO2 to needles of adult spruce (Picea abies) trees. New Phytol. 156, 179–194 (2002).
Sutton, M. A., Erisman, J. W., Dentener, F. & Möller, D. Ammonia in the environment: from ancient times to the present. Environ. Pollut. 156, 583–604 (2008).
Farquhar, G. D., Firth, P. M., Wetselaar, R. & Weir, B. On the Gaseous Exchange of Ammonia between Leaves and the Environment: Determination of the Ammonia Compensation Point. Plant Physiol. 66, 710–714 (1980).
Krupa, S. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environ. Pollut. 124, 179–221 (2003).
Clarisse, L., Clerbaux, C., Dentener, F., Hurtmans, D. & Coheur, P.-F. Global ammonia distribution derived from infrared satellite observations. Nat. Geosci. 2, 479–483 (2009).
Vitousek, P. M., Menge, D. N. L., Reed, S. C. & Cleveland, C. C. Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 368, 20130119 (2013).
Galloway, J. N. et al. Nitrogen Cycles: Past, Present and Future. Biogeochemistry 70, 153–226 (2004).
Wang, L., Pedas, P., Eriksson, D. & Schjoerring, J. K. Elevated atmospheric CO2 decreases the ammonia compensation point of barley plants. J. Exp. Bot. 64, 2713–24 (2013).
Alvim, P. D. T. Moisture Stress as a Requirement for Flowering of Coffee. Science. 132, 354 (1960).
Meinzer, F. C., Saliendra, N. Z. & Crisosto, C. H. Carbon isotope discrimination and gas exchange in Coffea arabica during adjustment to different soil moisture regimes. Funct. Plant Biol. 19, 171–184 (1992).
LeBauer, D. S. & Treseder, K. K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 (2008).
Bloom, A. J., Smart, D. R., Nguyen, D. T. & Searles, P. S. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA. 99, 1730–5 (2002).
Hutchinson, G. L., Millington, R. J. & Peters, D. B. Atmospheric ammonia: absorption by plant leaves. Science 175, 771–2 (1972).
Meinzer, F. C., Grantz, D. A., Goldstein, G. & Saliendra, N. Z. Leaf Water Relations and Maintenance of Gas Exchange in Coffee Cultivars Grown in Drying Soil 1. Plant Physiol. 94, 1781–1787 (1990).
Tricker, P. J. et al. Stomatal conductance and not stomatal density determines the long-term reduction in leaf transpiration of poplar in elevated CO2 . Oecologia 143, 652–60 (2005).
Franks, P. J. & Beerling, D. J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA. 106, 10343–7 (2009).
Raven, J. A. & Smith, F. A. Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytol. 76, 415–431 (1976).
Rachmilevitch, S., Cousins, A. B. & Bloom, A. J. Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA. 101, 11506–10 (2004).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry. Ecol. Stoichiom. Biol. Elem. from Mol. to Biosph. 84, 439 (2002).
Porter, L. K., Viets, F. G. & Hutchinson, G. L. Air Containing Nitrogen-15 Ammonia: Foliar Absorption by Corn Seedlings. Science. 175, 759–761 (1972).
Pearson, J. & Stewart, G. R. The deposition of atmospheric ammonia and its effects on plants. New Phytol. 125, 283–305 (1993).
Sutton, M. A. et al. Plant-Atmosphere Exchange of Ammonia [and Discussion]. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 351, 261–278 (1995).
Adriaenssens, S. et al. Canopy Uptake of 15NH3 by Four Temperate Tree Species and the Interaction with Leaf Properties. Water, Air, Soil Pollut. 223, 5643–5657 (2012).
Cleveland, C. C. et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol. Lett. 14, 939–947 (2011).
Sutton, M. A. et al. Towards a climate-dependent paradigm of ammonia emission and deposition. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 368, 20130166 (2013).
Rogers, H. H. & Aneja, V. P. Uptake of atmospheric ammonia by selected plant species. Environ. Exp. Bot. 20, 251–257 (1980).
Hoagland, D. R. & Arnon, D. I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 347, 1–32 (1950).
Kramer, A. W., Doane, T. A., Horwath, W. R. & Kessel, C. van. Combining fertilizer and organic inputs to synchronize N supply in alternative cropping systems in California. Agric. Ecosyst. Environ. 91, 233–243 (2002).
Acknowledgements
We wish to acknowledge the continued support provided by the J.G. Boswell Endowed Chair in Soil Science and Fulbright Exchange Program (Colombia-USA). We also wish to thank Jose Gutierrez Lopez and Lisa Auchincloss for their help with greenhouse work, James Richards for providing laboratorial space and Leonel Sternberg, Wendy Silk and Arnold Bloom for valuable comments during the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.C.R.S. and A.S.-J. conceived the experiment; A.S.-J., L.C.R.S. and T.A.D. performed the experiment; W.R.H. provided materials; L.C.R.S. wrote the first version of the manuscript; all authors contributed to the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Silva, L., Salamanca-Jimenez, A., Doane, T. et al. Carbon dioxide level and form of soil nitrogen regulate assimilation of atmospheric ammonia in young trees. Sci Rep 5, 13141 (2015). https://doi.org/10.1038/srep13141
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep13141
This article is cited by
-
Soil-plant-atmosphere interactions: structure, function, and predictive scaling for climate change mitigation
Plant and Soil (2021)
-
Temperature gradients assist carbohydrate allocation within trees
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.