Abstract
Elevated emissions of sulfur dioxide, nitrogen oxides and ammonia in China have resulted in high levels of sulfur and nitrogen deposition, being contributors to soil acidification, especially in and near large cities. However, knowledge gaps still exist in the way that large cities shape spatial patterns of acid deposition. Here, we assessed the patterns of pH, sulfate, nitrate and ammonium in bulk precipitation and throughfall in southern China’s forests by synthesizing data from published literature. Concentrations and fluxes of sulfate, nitrate and ammonium in bulk precipitation and throughfall exhibited a power-law increase with a closer distance to the nearest large cities and accordingly pH showed a logarithmic decline. Our findings indicate the occurrence of urban ‘acid islands’ with a critical radius of approximately 70 km in southern China, receiving potential acid loads of more than 2 keq ha−1 yr−1. These urban acid islands covered an area of 0.70 million km2, accounting for nearly 30% of the land area in southern China. Despite a significant capacity to neutralize acids in precipitation, our analysis highlights a substantial contribution of ammonium to potential acid load. Our results suggest a joint control on emissions of multiple acid precursors from urban areas in southern China.
Similar content being viewed by others
Introduction
Elevated emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) have occurred during rapid industrialization and urbanization in China, especially in and near large cities1,2,3. Widespread areas in China, especially the southern regions, have been receiving considerable acid deposition4, which is likely to result in some key environmental issues, including acidification of terrestrial and aquatic ecosystems4,5,6, loss of biodiversity6 and damage of calcareous (e.g. concrete and marble) buildings and metal materials7. Although efforts have been made to improve the understanding of the status and effects of acid deposition in China4,6, knowledge gaps still exist in the way that large cities shape the spatial pattern of acid deposition.
Large cities with high population density are hotspots of anthropogenic emissions of acid precursors due to a combination of intensive energy production, motor traffic, waste treatment and industrial activities1,8,9. Concentrations and fluxes of nitrate (NO3−) in bulk precipitation have been found to exhibit a power-law increase with a closer distance to the center of the nearest large cities10. Because large cities also are hotspots of SO2 emissions, we hypothesized that sulfuric acid would behave the same way as nitrate. Assuming a proportional change in the neutralizing effect due to base cations and ammonia (NH3), we expected that hydrogen ion (H+) would also increase significantly the closer one gets to large cities. Based on the analysis above, we introduced a concept of urban ‘acid islands’, which implies that acid deposition increases with a closer distance to large cities. Therefore, the pH, defined as the negative logarithm of the activity of the hydrogen ion, is expected to show a logarithmic decline with a closer distance to large cities.
Although ammonia can neutralize sulfuric and nitric acids in cloud water and precipitation, subsequent ammonium (NH4+) deposition has a potential to generate significant acidification11. This is due to nitrification, causing the production of two protons (NH4+ + 2O2 → NO3− + H2O + 2H+). Note that one proton is due to ammonia deposition, while the other proton comes from acids (e.g. sulfuric acid and nitric acid) in precipitation, being neutralized in the atmosphere (NH3 + H+ → NH4+). The potential acid load thus equals the sum of the deposition of the hydrogen ion and twice the ammonium ion (H+ + 2 NH4+), being the value with which critical acid loads have been compared since several decades12. Due to a rapid increase in NH3 emission3, high levels of ammonium deposition have been observed in China’s forests, being more than twice of nitrate deposition10. Therefore, it is essential to include ammonium when testing the hypothesis of urban acid islands. Apart from acidifying effects, increased loadings of nutrient nitrogen in ecosystems have the potential to release nitrogen limitation or enhance eutrophication, either resulting in an increase in net primary production or leading to nutrient imbalance and biodiversity loss6.
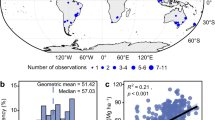
Anthropogenic emissions of acid precursors (SO2, NOx and NH3) generally are low from forests and therefore concentrations and fluxes of sulfate, nitrate and ammonium in bulk precipitation at forested sites well indicate the ambient status of acid deposition. In northern China, acid deposition rarely become problematic due to neutralization by high levels of base cation deposition13, while acid deposition in southern China can lead to significant acidification14,15. Therefore, we focused our study on southern China (Fig. 1). Here, we tested the hypothesis of urban acid islands by synthesizing data from published literature on pH, nitrate, sulfate and ammonium in bulk precipitation and throughfall in southern China’s forests. Throughfall is a sum of bulk deposition, canopy captured dry deposition and canopy exchange16,17. Previous evidence indicates that throughfall deposition is a reasonable estimate of total adeposition10,18, although it may underestimate acid deposition due to canopy uptake of nitrogen compounds and hydrogen ion19,20,21. We then estimated the critical radius of the urban acid islands, based on a maximum critical load of acidification for soils in southern China and discussed the policy implications for a better control of acid deposition in China.
Results
Concentrations and deposition of hydrogen ion, sulfate, nitrate and ammonium
Concentrations and fluxes of hydrogen ion, sulfate, nitrate and ammonium in bulk precipitation and throughfall generally were high in southern China, but large spatial heterogeneity existed (Figs 2, 3, 4, 5). Highest levels of acid deposition occurred around several hotspots, including Yangtze River delta, Changsha-Zhuzhou-Xiangtan city cluster, Wuhan city cluster, Chengdu-Chongqing city cluster and Pearl River delta.
Values of pH in bulk precipitation showed a geometric mean of 5.5 (n = 28) and increased to 5.8 in throughfall. Geometric mean concentrations of sulfate, nitrate and ammonium in bulk precipitation were estimated at 101.2 (n = 30), 19.1 (n = 33) and 47.6 (n = 33) μeq L−1, respectively. Bulk deposition of sulfate, nitrate and ammonium showed geometric means at 1.55 (n = 30), 0.30 (n = 33) and 0.73 (n = 33) keq ha−1 yr−1, respectively. Throughfall concentrations of sulfate, nitrate and ammonium showed elevated geometric means at 184.2, 30.1 and 64.2 μeq L−1, leading to throughfall deposition of sulfate, nitrate and ammonium at 2.83, 0.47 and 0.98 keq ha−1 yr−1, respectively.
Testing the hypothesis of urban acid islands
Values of pH in bulk precipitation showed a logarithmic decline with a closer distance to the center of the nearest large cities (Fig. 6a). Accordingly, concentrations of sulfate, nitrate and ammonium showed a power-law increase with a closer distance to the nearest large cities (Fig. 6b–d). Moreover, bulk deposition of hydrogen ion, sulfate, nitrate and ammonium all showed a power-law increase with a closer distance to the nearest large cities (Fig. 6e–h). Concentrations and fluxes of hydrogen ion, sulfate, nitrate and ammonium in throughfall behaved similar as those in bulk precipitation (Fig. 7).
Changes in (a) pH, concentrations (μeq L−1) of (b) sulfate (SO42−), (c) nitrate (NO3−) and (d) ammonium (NH4+) in bulk precipitation and bulk deposition (keq ha−1 yr−1) of (e) hydrogen ion (H+), (f) sulfate, (g) nitrate and (h) ammonium with a closer distance (km) to the center of the nearest large cities.
Changes in (a) pH, concentrations (μeq L−1) of (b) sulfate (SO42−), (c) nitrate (NO3−) and (d) ammonium (NH4+) in throughfall and throughfall deposition (keq ha−1 yr−1) of (e) hydrogen ion (H+), (f) sulfate, (g) nitrate and (h) ammonium with a closer distance (km) to the center of the nearest large cities.
Estimating the critical radius of urban acid islands
Based on a critical acid load at 2 keq ha−1 yr−1 and an assessment of the potential acid load using the empirical equations on throughfall deposition of hydrogen ion and ammonium (Fig. 7e,h), we estimated a critical radius at 67 km for the urban acid islands in southern China. We further estimated that the total area of these urban acid islands was approximately 0.70 million km2 (Fig. 8), accounting for 29% of the land area in southern China.
Distribution of urban acid islands in southern China.
Large cities (nonagricultural population > 0.5 million) are illustrated as acid islands (in red, receiving potential acid loads of more than 2 keq ha−1 yr−1) with a critical radius of 67 km. The figure is generated by ArcGIS Desktop (version 9.3, ESRI, Redlands, USA).
Discussion
In line with the hypothesis of urban acid islands, our results demonstrate a power-law increase of concentrations and fluxes of hydrogen ion, sulfate, nitrate and ammonium in bulk precipitation and throughfall with a closer distance to urban hotspots. Accordingly, pH values showed a logarithmic decline with a closer distance to large cities, which was consistent with a power-law increase of the hydrogen ion. In comparison with sulfate and ammonium, the less significant effect of urban areas on nitrate concentrations and nitrate deposition (Figs 6 and 7) is most likely caused by an intermixing effect of road networks10. Well-developed road network systems have severely fragmented the landscape in China22 and automobile NOx emissions can substantially increase NOx concentrations near roadside areas23, resulting in an intermixing effect of road networks on spatial patterns of nitrate deposition. Nevertheless, the concept of urban acid islands is an important approach to describe the way in which large cities shape the spatial pattern of acid deposition.
Uncertainties might exist in our analysis due to the close proximity of several cities in strongly urbanized regions, which could simultaneously contribute to the levels of acid deposition at a specific site. In that case, a larger critical radius would be expected. Here, we defined all the cities with nonagricultural population > 0.5 million as ‘large cities’ and this might also lead to uncertainties. Larger cities, having a wider spatial extent of acid precursors in the atmosphere8, would result in larger urban acid islands. Furthermore, spatial extent of acid precursors in the atmosphere generally varies with wind direction and wind velocity8 and therefore the radius of the urban acid islands is likely shorter in the upwind direction than in the downwind direction. The heterogeneity in spatial patterns of base cation deposition13 may also lead to uncertainties. Overall, our analysis most likely underestimated the critical radius of the urban acid islands because throughfall deposition is less than total deposition, especially for nitrogen compounds, due to significant foliar uptake19,21. This is also evident from the ratio of throughfall deposition versus bulk deposition, which is much lower for nitrogen compounds (1.56 for nitrate and 1.34 for ammonium) than for sulfate (1.82). Therefore, further assessments are needed to integrating detailed datasets on these factors as discussed above.
Our analysis shows that acid deposition in southern China was dominated by sulfuric acid, which contributed five to six times that of nitric acid, as determined by a comparison of sulfate to nitrate in bulk precipitation and throughfall. National average emissions of SO2 (14.4 TgS yr−1)2 and NOx (5.0 TgN yr−1)3 during the period 2000 to 2009 lead to a ratio of acidification capacity near 2.5, being half of the estimates in southern China. As indicated by ammonium in bulk precipitation and throughfall, NH3 roughly neutralized 35% of the total acidity caused by sulfuric and nitric acids, while national average emissions of NH3 during the 2000s (12.7 TgN yr−1)3 suggest a potential to naturalize 72% of the precipitation acidity due to SO2 and NOx emissions. Without the neutralization capacity of NH3 in southern China, the pH in precipitation would be significantly lower especially near large cities.
Despite a neutralization capacity of NH3 in the atmosphere, deposited ammonium has a potential to generate substantial soil acidification. By comparing ammonium deposition with sulfate and nitrate deposition, the net acidification capacity of ammonium deposition was estimated to contribute 25% to deposition-induced soil acidification in southern China. Integrating the contribution of nitric acid (11%), nitrogen deposition generally accounted for 36% of deposition-induced potential soil acidification, while sulfur deposition made a much higher contribution (64%). However, emission assessments2,3 lead to a lower contribution of SO2 (42%) and higher contributions of NOx (16%) and NH3 (42%) at national scale.
In recent decades, China has made considerable efforts in curbing the problem of acid deposition, such as shifting the coal-dominated energy structure, developing desulfurization technologies and adopting stricter vehicular emission standards24. Due to these efforts, SO2 emissions have been reduced continuously since the middle 2000s but currently are still above the levels of the late 1990s2,25. In contrast, anthropogenic emissions of NOx have increased from 1.3 TgN yr−1 in 1980 to more than 6 TgN yr−1 in 2010 (Ref. 3), but fortunately the government has recently set goals to reduce NOx emissions by 10% in 2015 against the 2010 levels (Twelfth Five-year Plan) (A full version of the plan is available at http://news.xinhuanet.com/politics/2011-03/16/c_121193916.htm). However, our analysis indicates that sulfuric acid still dominates the acid deposition in southern China, while the contribution of nitric acid is likely growing in importance due to the stricter control of SO2 emissions and dramatic increase in NOx emissions3,25, showing a same trend as that in Europe and USA in the early 1980s11. In addition, NH3 emissions have been more than doubled since 1980s (from 5.6 to 14.5 Tg N yr−1)3 and need urgent regulation in view of the significant potential of ammonium deposition in soil acidification11. There will also be a co-benefit from a long-term target to reduce emissions of CO2 and other pollutants for China24,26.
On the basis of our analysis, urban acid islands with a radius of approximately 70 km in southern China (Fig. 8), receiving potential acid loads of more than 2 keq ha−1 yr−1, are at high risk to soil acidification. Most of these urban acid islands are located around several hotspots, including Yangtze River delta, Changsha-Zhuzhou-Xiangtan city cluster, Wuhan city cluster, Chengdu-Chongqing city cluster and Pearl River delta. The occurrence of urban acid islands may significantly contribute to acidification of arable lands (as lime application is not common in China’s cropland), forests and water bodies in suburbs of large cities5,27,28, potentially threatening agricultural production, forest health and water quality. Since the late 1990s, control zones of SO2 emissions and acid deposition have been designed as the first-generation approach to set priority regions for acid-rain reduction15. Our findings suggest that a second-generation policy for the reduction of acid deposition in China should also focus on the urban acid islands.
In conclusion, our analysis indicates that large cities in southern China are ‘acid islands’. Concentrations and fluxes of sulfate, nitrate, hydrogen ion and ammonium in bulk precipitation and throughfall exhibited a power-law increase with a closer distance to large cities and therefore the pH showed a logarithmic decline. The concept of urban acid islands is useful in predicting acid deposition at regional scales. Although SO2 has been controlled for two decades in China15, sulfur deposition still contributes largest to soil acidification in southern China. Despite a significant capacity of NH3 to neutralize acids in precipitation, high levels of ammonium deposition have a substantial contribution to soil acidification in southern China. Our results also have important implications for the control of acid deposition via a joint control on emissions of multiple acid precursors, including not only SO2 and NOx but also NH3, especially in and near large cities.
Methods
Data set
The hypothesis of urban acid islands was tested by collecting data from published literature on pH and concentrations of sulfate, nitrate and ammonium in bulk precipitation and throughfall in typical forests in southern China (Fig. 1), as well as information on site location (latitude and longitude) and annual mean precipitation. Chemical data were selected only when precipitation and throughfall were measured simultaneously. Targeted data were either taken directly from tables or digitized from figures. Volume-weighted means of pH and concentrations of nitrate, sulfate and ammonium were calculated for the whole sampling period. Bulk and throughfall deposition was calculated based on the multiplication of annual precipitation and volume-weighted mean concentration. The distance between the sampling site and the center of the nearest large city (nonagricultural population > 0.5 million) was derived using Google Earth for Microsoft Windows (Version 7.1.2.2041, Google Inc., Mountain View, USA). Our database included information on pH for 28 sites and on concentrations of sulfate, nitrate and ammonium for 30, 33 and 33 sites, respectively. Detailed information on each site, including annual mean precipitation, observation year(s) and the related references is given in the Supplementary Material (Table S1).
Statistical analysis
We tested the hypothesis of urban acid island by assessing whether both concentrations and fluxes of hydrogen ion, sulfate, nitrate and ammonium in bulk precipitation and throughfall show a power-law increase with a closer distance to large cities, according to equation (1),

where y is volume-weighted mean concentration (μeq L−1) or annual flux (keq ha−1 yr−1), x is the distance (km) between the sampling site and the center of the nearest large city, a and b are parameters. In addition, we tested whether the value of pH shows a logarithmic decline with a closer distance to large cities, as described in equation (2),

where x is the distance (km) between the sampling site and the center of the nearest large city, c and d are parameters.
In comparison with bulk deposition, throughfall deposition has been used as a more precise estimate of total deposition10,18. Based on a recent assessment29, critical loads of acidification for soils are always lower than 2 keq ha−1 yr−1 in southern China, except for some insensitive systems. Therefore, we estimated the critical distance of urban acid islands based on the empirical equations on the potential acid load (H+ + 2NH4+) via throughfall deposition and a maximum critical acid load at 2 keq ha−1 yr−1. All statistical analysis was performed using R software (version 3.1.0; R Development Core Team, 2014, http://www.r-project.org/) with a significance level of P < 0.05.
Additional Information
How to cite this article: Du, E. et al. Spatial boundary of urban ‘acid islands’ in southern China. Sci. Rep. 5, 12625; doi: 10.1038/srep12625 (2015).
References
Chan, C. K. & Yao, X. Air pollution in mega cities in China. Atmos. Environ. 42, 1–42 (2008).
Lu, Z. F., Zhang, Q. & Streets, D. G. Sulfur dioxide and primary carbonaceous aerosol emissions in China and India, 1996–2010. Atmos. Chem. Phys. 11, 9839–9864 (2011).
Liu, X. J. et al. Enhanced nitrogen deposition over China. Nature 494, 459–462 (2013).
Larssen, T. et al. Acid rain in China. Environ. Sci. Technol. 40, 418–425 (2006).
Zhao, Y. et al. Soil acidification in China: Is controlling SO2 emissions enough? Environ. Sci. Technol. 43, 8021–8026 (2009).
Liu, X. J. et al. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 159, 2251–2264 (2011).
Maeda, Y. et al. Material damage caused by acidic air pollution in East Asia. Water Air Soil Poll. 130, 141–150 (2001).
Beirle, S., Boersma, K. F., Platt, U., Lawrence, M. G. & Wagner, T. Megacity emissions and lifetimes of nitrogen oxides probed from space. Science 333, 1737–1739 (2011).
Reche, C. et al. Urban NH3 levels and sources in six major Spanish cities. Chemosphere 119, 769–777 (2015).
Du, E. Z., Jiang, Y., Fang, J. Y. & De Vries, W. Inorganic nitrogen deposition in China’s forests: Status and characteristics. Atmos. Environ. 98, 474–482 (2014).
Galloway, J. N. Acid deposition: perspectives in time and space. Water Air Soil Poll. 85, 15–24 (1995).
Sverdrup, H. & De Vries, W. Calculating critical loads for acidity with the simple mass balance method. Water Air Soil Poll. 72, 143–162 (1994).
Larssen, T. & Carmichael, G. R. Acid rain and acidification in China: the importance of base cation deposition. Environ. Pollut. 110, 89–102 (2000).
Lu, X. K., Mao, Q. G., Gilliam, F. S., Luo, Y. Q. & Mo, J. M. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Global Change Biol. 20, 3790–3801 (2014).
Hao, J. M., Wang, S. X., Liu, B. J. & He, K. B. Control strategy for sulfur dioxide and acid rain pollution in China. J. Environ. Sci-China 12, 385–393 (2000).
Lindberg, S. E., Lovett, G. M., Richter, D. D. & Johnson, D. W. Atmospheric deposition and canopy interactions of major ions in a forest. Science 231, 141–145(1986).
Lovett, G. M., Nolan, S. S., Driscoll, C. T. & Fahey, T. J. Factors regulating throughfall flux in a New Hampshire forested landscape. Can. J. For. Res. 26, 2134–2144 (1996).
Draaijers, G. P. J., Erisman, J. W., Sprangert, T. & Wyers, G. P. The application of throughfall measurements for atmospheric deposition monitoring. Atmos. Environ. 30, 3349–3361 (1996).
Sparks, J. P. Ecological ramifications of the direct foliar uptake of nitrogen. Oecologia 159, 1–13 (2009).
Langusch, J. J., Borken, W., Armbruster, M., Dise, N. B. & Matzner, E. Canopy leaching of cations in Central European forest ecosystems – a regional assessment. J. Plant Nutr. Soil Sci. 166, 168–174 (2003).
Potter, C. S., Ragsdale, H. L. & Swank, W. T. Atmospheric deposition and foliar leaching in a regenerating southern Appalachian forest canopy. J. Ecol. 79, 97–115 (1991).
Li, T. et al. Fragmentation of China’s landscape by roads and urban areas. Landscape Ecol. 25, 839–853 (2010).
Redling, K., Elliott, E., Bain, D. & Sherwell, J. Highway contributions to reactive nitrogen deposition: Tracing the fate of vehicular NOx using stable isotopes and plant biomonitors. Biogeochemistry 116, 261–274 (2013).
Wang, S. X. & Hao, J. M. Air quality management in China: Issues, challenges and options. J. Environ. Sci-China 24, 2–13 (2012).
Mao, X. Q., Zhou, J. & Corsetti, G. How well have China’s Recent Five-Year Plans been implemented for energy conservation and air pollution control? Environ. Sci. Technol. 48, 10036–10044 (2014).
Tollefson, J. US–China climate deal raises hopes for Lima talks. Nature 515, 473–474 (2014).
Singh, A. & Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 29, 15–24 (2008).
Guo, J. H. et al. Significant soil acidification in major Chinese croplands. Science 327, 1008–1010 (2010).
Duan, L., Zhao, Y. & Hao, J. M. in Critical Loads and Dynamic Risk Assessments: Nitrogen, Acidity and Metals in Terrestrial and Aquatic Ecosystems (eds De Vries, W. et al.) Ch. 16, 419–438 (Springer, 2015).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 31400381, 41171067 & 40425007).
Author information
Authors and Affiliations
Contributions
E.D. conceived the idea. E.D. collected and analysed the data. E.D., Y.J. and W.D.V. wrote the manuscript. X.L., J.F. and J.G. reviewed and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Du, E., de Vries, W., Liu, X. et al. Spatial boundary of urban ‘acid islands’ in southern China. Sci Rep 5, 12625 (2015). https://doi.org/10.1038/srep12625
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12625
This article is cited by
-
Increased interactions between iron oxides and organic carbon under acid deposition drive large increases in soil organic carbon in a tropical forest in southern China
Biogeochemistry (2022)
-
Sulfur deposition still contributes to forest soil acidification in the Pearl River Delta, South China, despite the control of sulfur dioxide emission since 2001
Environmental Science and Pollution Research (2019)
-
Spatial pattern and seasonal variation of alkaline precipitation observed in the Gansu Province, NW China
Environmental Earth Sciences (2019)
-
A database of annual atmospheric acid and nutrient deposition to China’s forests
Scientific Data (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.