Abstract
Yindanxinnaotong (YD), a traditional Chinese medicine, has been introduced toclinical medicine for more than a decade, while its pharmacological properties arestill not to be well addressed. This report aimed to explore theanti-atherosclerosis properties and underlying mechanisms of YD. We initiallyperformed a computational prediction based on a network pharmacology simulation,which clued YD exerted synergistically anti-atherosclerosis properties by vascularendothelium protection, lipid-lowering, anti-inflammation and anti-oxidation. Theseoutcomes were then validated in atherosclerosis rats. The experiments providedevidences indicating YD’s contribution in this study included, (1)significantly reduced the severity of atherosclerosis, inhibited reconstruction ofthe artery wall and regulated the lipid profile; (2) enhanced antioxidant power,strengthened the activity of antioxidant enzymes and decreased malondialdhydelevels; (3) significantly increased the viability of umbilical vein endothelialcells exposed to oxidative stress due to pretreatment with YD; (4) significantlyreduced the level of pro-inflammatory cytokines; (5) significantly down-regulatedNF-kB/p65 and up-regulated IkB in the YD-treated groups. Overall, these resultsdemonstrated that YD intervention relieves atherosclerosis through regulatinglipids, reducing lipid particle deposition in the endothelial layer of artery,enhancing antioxidant power and repressing inflammation activity by inhibiting thenuclear factor-kappa B signal pathway.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide and evidencesuggests that half of all CVD cases occur in Asia1,2. Atherosclerosis(AS), the underlying syndrome of CVD, is a major pathogenic procession involving lipidmetabolism, inflammation, innate and adaptive immunity and many otherpathophysiological aspects. Clinical studies have demonstrated that AS may generate aseries of cardiovascular events (e.g., acute coronary syndrome and stroke). Secondary tolipid deposition in the vessel wall, AS is a chronic inflammatory disease of arteriesand oxidative stress participates in its pathogenesis2,3,4. First andthe most critical step in preventing AS involves regulation of the lipid profile. In theearly phase of AS, oxidative stress modifies low-density lipoprotein (LDL) to oxidizedLDL (ox-LDL), which is taken up by macrophages in the intima of the vascular wall andultimately leads to foam cell formation.
Another leading cause of AS is inflammation. AS is regarded as a chronic inflammatorydisease resulted from the production of cytokines such as interleukin-1β(IL-1β) and IL-105. Interestingly, IL-1β andIL-10 may promote expression of intercellular adhesion molecule-1 and vascular celladhesion molecule-1 in endothelial cells6 as well as interaction betweenmonocytes and endothelial cells, resulting in increased transmigration of circulatingmonocytes to the intima. Migrated monocytes mature to macrophages, which swallow lipidsand become foam cells, leading to inflammatory gene expression and atheromatic plaqueformation7.
In addition to critically participate in the development of AS, oxidative stress causesendothelial dysfunction, an early feature of AS8,9,10. Thus, theintimate links between lipid deposition, inflammation and oxidative stress playessential roles in AS. Elucidating the underlying mechanisms of AS may lead to novelprevention and treatment strategies with traditional Chinese medicine (TCM).
Systemic review and meta-analysis suggest that TCM may provide another treatment optionfor patients with CVD11. TCM herbal formulae can be valuable therapeuticstrategies and drug resources. Recent reports scientifically verify the clinical benefitof YD12,13. However, the identification of potent ingredients and theiractions are challenges in TCM research. Integrating network biology and polypharmacologypromises an expanded opportunity for druggable targets. Network biology may also aid theexploration of drug targets and identify potential active ingredients in TCMresearch14,15. The current study used a network pharmacologyapproach to help determine the active ingredients of YD. We also applied network targetprediction and experimental verification to evaluate the links between herbalingredients and pharmacological actions.
Results
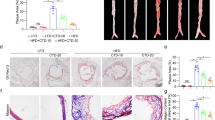
YD attenuates atherosclerotic lesions in rats
After 12 weeks of treatment with YD, light microscopy showed unimpaired integrityand intact layers in hematoxylin-eosin (HE)-stained abdominal aortas fromnon-atherosclerotic rats (Fig. 1A). In contrast, AS modelgroup (AS group) animals showed thicker and less smooth vessel walls and theelastic plates in intima and media were damaged (Fig. 1B);we also observed extensive atherosclerotic plaques containing foam cells,inflammatory cells, cholesterol crystals and tissues calcification(Fig. 1B). Comparing with AS group, pathological changesdecreased visibly in YD 0.5 g treated group (YD-0.5), YD1.0 g treated group (YD-1.0), YD 2.0 g treated group(YD-2.0) (Fig. 1D–F) and atorvastatin treatedgroup (Ator) (Fig. 1C) (i.e., vessel walls were slightlyrougher and thicker and there were fewer atherosclerotic plaques). Notably,both atorvastatin and YD alleviated lipid accumulation and foam cell formation.Comparing with AS group (Fig. 1B), YD intervention(YD-0.5, YD-1.0, YD-2.0 groups) significantly attenuated pathological changes(i.e., slightly rough vessel walls and fewer atherosclerotic plaques).
Rat carotid artery sections were subjected to histologicalexamination.
Representative photomicrographs of HE staining are shown. Originalmagnification: ×200. Normal control (A) showed noimpairment of the artery’s integrity and all layers remainedintact, whereas atherosclerotic rats exhibited atherosclerotic lesionformation (B). Animals receiving YD (D–F)and atorvastatin (C) intervention showed mild pathological changescompared with normal controls.
We measured intima-media thickness (IMT) to quantitatively assess the effect ofYD and atorvastatin intervention on the aortic arch. Comparing with normalcontrol group (con group), IMT of the aortic arch was significantly thicker inAS group(90.22 ± 7.78 μm vs.140.73 ± 16.32 μm,respectively, P < 0.01). After 12 weeks ofYD and atorvastatin intervention, IMT was significantly thinner (ator104.83 ± 19.33 μm,YD-2.0103.37 ± 12.28 μm,YD-1.0116.27 ± 16.73 μm,YD-0.5124.77 ± 20.03 μm),comparing with AS group(140.73 ± 16.32 μm).
Immunohistochemical analysis showed decreased expression of smooth muscle protein22 alpha (SM22α)16,17 in intimal smooth muscle cells(SMCs) in AS group (Fig. 2A and Fig.2B, respectively). Comparing with AS group, atorvastatin or YDtreatment yielded significantly augmented expression (Fig.2G).
Injecting vitamin D3 & ovalbumin and feeding with high-fat dietinduced reconstruction of the carotid artery.
Detection of smooth muscle protein 22 alpha (SM22α) expressiondenotes pathological change in the intima. Compared with thenormal-controlled group (A), there was low expression ofSM22α in intima of atherosclerotic rats (B). YD(D–F) and atorvastatin (C) interventionshowed relatively high expression of SM22α (G). Datadenote mean ± SD,n = 12*P < 0.05 vs. the atherosclerosisgroup, **P < 0.01 vs. theatherosclerosis group.
YD regulates the lipid profile in atherosclerotic rats
Comparing with normal-controlled group, cholesterol and TGs increasedsignificantly in AS group; YD reduced such elevation (Table1). In atherosclerotic rats, YD reduced serum concentrations oftotal cholesterol, LDL and TGs. Thus, YD treatment mediated the lipid profile,especially reducing TGs, cholesterol and LDL. Our data suggest that YD improvesthe serum lipid profile that associates with the pathogenesis ofatherosclerosis. As expected, atorvastatin improved the serum lipid profile aswell16.
YD elicits antioxidant action in vivo
Table 2 shows the parameters of redox behavior in rats.Compared to AS group, YD significantly (P < 0.05)modified oxidative stress markers after 12 weeks. Malondialdehyde (MDA), an endproduct of lipid peroxidation, increased significantly in atherosclerotic versusnon-atherosclerotic controls.
YD and atorvastatin treatments significantly reduced MDA levels. Concentrationsof glutathione (GHS), an antioxidant biomarker, were lower in atheroscleroticrats compared with normal-controlled group and YD significantly increased GSHlevels compared with AS group. Compared with normal-control group, antioxidantenzymes superoxide dismutase (SOD) and selenium-dependent glutathione peroxidase(GSH-px) were significantly less active(P < 0.05) in atherosclerotic rats.Moreover, YD treatment yielded significantly higher activity of antioxidantenzymes.
YD regulates the secretion of pro-inflammatory cytokines and vascularendothelial function markers in vivo
AS is a chronic inflammatory disease that results in increased production ofinflammatory mediators18,19,20. Our results show that YD andatorvastatin treatment significantly decreased plasma levels of C-reactiveprotein, tumor necrosis factor alpha (TNF-α) and IL-1β(Table 3), compared with AS group.
Endothelial function is chronically disturbed in atherosclerosis and endothelialcell dysfunction is a critical step in the progression of the disease21,22. Our data (Table 4) showsignificantly decreased concentrations of endothelium-derived relaxing factor(NO) in atherosclerotic rats. Importantly, YD and atorvastatin inhibited suchdecreases. On the other hand, YD and atorvastatin treatment mediatedvasoconstrictor function markers (i.e., endothelin and TXB2).
Pretreatment with YD attenuated oxidative stress and increased cellviability in HUVECs
To confirm that YD protects against ox-LDL-induced injury, we conducted an MTTassay to check cell viability between groups. Compared with control Group, theox-LDL-exposed group exhibited significantly decreased cell viability (100% vs.49%, respectively). We also checked cell viability by pretreating with YD1.5–100 mg/L. When exposed to ox-LDL-induced oxidativeinjury, cell viability in groups pretreated with YD (1.5 and3.0 mg/L) differed little from control group (no pretreated group).Compared with control group, pretreatment with YD(6.25–100 mg/L) significantly increased cell viability(54% ~ 96% vs. 49%) afterox-LDL-induced injury. The maximum non-toxicity concentration was100 mg/L.
Expression of IkB and NF-kB/p65
As shown in Fig. 3, Western blotting revealed increasedexpression of IkB and significantly decreased levels of nuclear factor-kappa B(NF-kB)/p65 in the YD group compared with AS group. As expected, atorvastatinincreased IkB expression and decreased NF-kB/p65 expression (Group Ator).
Discussion
Network pharmacology offers a new approach of the drug-target exploring and thepotential active ingredients identification in TCM research. The present study usednetwork pharmacology to analyze and to predict main drug targets, mechanism ofcardiovascular protection. Our experiments verified that YD reduces serum lipids,lowers oxidative stress and effectively inhibits inflammation in an established ratAS model. To the best of our knowledge, the present study is the first to show thatYD attenuates atherosclerosis. Interestingly, atorvastatin and YD offered similarprotection in terms of lipid-lowering, anti-inflammation and anti-oxidationeffects.
A previous report showed that high fat diet (HFD) triggers changes in thecardiovascular oxidative state, independent of obesity-induced co-morbidities23. We aimed to test the hypothesis that YD can prevent thepathogenesis of AS by improving the blood lipid profile and suppressing oxidativestress and inflammatory processes. Our pathological findings suggest significantlyincreased plaque formation and lipid deposition in atherosclerotic animals comparedwith normal controls. YD treatment can alleviate plaque formation and lipiddeposition in the artery and can significantly attenuate pro-atherogenicpathological changes.
SM22α—a 22-kDa protein also known as transgelin or WS3-10 andconsidered a marker of contractile smooth muscle cells (SMCs)—isexclusively and abundantly expressed in SMCs of adult animals. During atherogenesis,SM22-α restricts plaque growth by inhibiting the phenotypic modulationof SMCs from contractile to synthetic/proliferative cells19,20,24.As key pathogenic factor in AS, phenotypic modulation of SMCs affects therecruitment and proliferation of other cells in the lesion. We usedimmunohistochemistry to elucidate SM22α expression, the inner mechanismthat attenuates AS. Our results show that YD treatment inhibited vascular remodelingby increasing SM22-α expression.
CVD is the leading cause of morbidity and mortality worldwide and sedentarylifestyle and HFD may contribute to increased risk of CVD1,3.Hyperlipidemia and oxidative stress are among the known risk factors of AS, which isthe key and common pathology of CVD. Importantly, recent studies showed that naturaland herbal medicines might prevent atherosclerosis by lowering plasma lipid levelsand blocking the oxidation of LDL25,26,27. Our data suggest that YDimproves the serum lipid profile associated with the pathogenesis of atherosclerosis(Fig. 2). Additionally, YD prevents atherosclerotic lesiondevelopment by decreasing TGs and LDL without changing high-density lipoprotein(HDL), yielding a decreased LDL/HDL ratio.
The complex process of atherosclerosis begins when LDL molecules are deposited in thearterial walls and undergo oxidation by reactive oxygen species (ROS) or enzymessuch as myeloperoxidase or lipoxygenase. This study focused mainly on elucidatingthe mechanisms and pathways through which oxidative stress modulates cellular andmolecular processes and affects atherosclerotic pathologies. In vivo, YDtreatment increases the activity of antioxidant enzymes SOD and GSH-px, elevatesGSH and decreases MDA, resulting in significant elevation of total antioxidantcapacity. In vitro, we used an MTT assay to detect cell viability andconfirmed that YD exerted antioxidant activity when exposed to ox-LDL-inducedoxidative stress.
Early-stage atherosclerosis associates closely with the inflammatory response ofblood vessels that occurs at the beginning of atherosclerotic plaque formation28. Thus, an inhibited inflammation response benefits CVD, especiallyin the early stages of AS. Because transcription factor NF-kB critically inducescytokine-associated genes that participate in the pathogenesis ofatherosclerosis29,30, an activated or inhibited NF-kB signalpathway may play a key role in regulating inflammation and the pathologicalprogression of AS29,31. In vivo, elevated concentrations ofplasma inflammation cytokines such as CRP, TNF-α and IL-1βdecreased significantly in atherosclerotic rats undergoing YD intervention. Invitro, western blotting showed increased levels of IkB and significantlyreduced levels of NF-kB/p65 YD-treated rats. Thus, the anti-inflammation effect ofYD may result partly from inhibition of the NF-kB signal pathway.
In summary, our findings demonstrate that YD attenuates AS and inhibits vascularremodeling by reducing wall thickness and the wall thickness-to-diameter ratio.Underlying mechanisms may include regulation of the lipid profile, reduceddeposition of lipid particles in the endothelial layer of the artery, enhancedantioxidant power and anti-inflammation activity via inhibition of NF-kB signalpathway. Our results demonstrate that YD’s ability to inhibit AS mayoffer a potential therapy for CVD.
Materials and methods
Preparation and quality control of YD
In 2002, the China Food and Drug Administration approved YD for the treatment ofCVD and later included YD in the National Essential Medicine List (2012edition). Guizhou Bailing Pharmaceutical Co., Ltd, provided YD comprised mainlyof ginkgo leaves (0.5 g crude drug per capsule), salvia miltiorrhiza(0.5 g crude drug per capsule), herba erigeromtis (0.3 gcrude drug per capsule), gynostemma pentaphyllum (0.3 g crude drugper capsule), hawthorn (0.4 g crude drug per capsule), alliumsativum (0.4 g crude drug per capsule), panax notoginseng(0.2 g crude drug per capsule) and borneol (0.01 gcrude drug per capsule). The principal pharmacologically active components of YDinclude ginkgo leaves and salvia miltiorrhiza and its main bioactiveconstituents include flavonoids (0.59%) and the terpene lactones (0.31%) fromginkgo leaves, salvianolic acid A (0.29%), tanshinone IIA (0.11%), gynostemmatotal saponins (0.51%), panax notoginseng saponins (5.42%) and borneol (1.21%),which may be responsible for YD’s cardiovascular pharmacologicalactivity. To reduce variation of YD capsules in different batches, GuizhouBailing strictly standardizes the species, origin, harvest time, medicinalcomponents and concocted methods for each component. For quality control,Guizhou Bailing uses high-performance liquid chromatography with ultra-violetabsorbance optical detector to establish the fingerprint of YD soft capsules(Supplementary Fig. S1).
Prediction analysis of pharmacological mechanism based on networkpharmacology
YD mainly comprises ginkgo leaves, salvia miltiorrhiza, herba erigeromtis,gynostemma pentaphyllum, hawthorn, allium sativum, panax notoginseng andborneol. The active chemical components of YD were collected and extracted fromthe article database (Chinese Pharmacopoeia 2010 edition, web of science,http://www.wanfangdata.com.cn/, http://www.cnki.net/, www.ncbi.nlm.nih.gov/pubmed/). After reviewing the database, wetried to extract a higher relative content of various medicinal ingredients,taking into account the relevant activity reports. The 173 compounds wecollected encompassed the main components of YD.
We put the 173 chemical compounds input TCMSP (http://sm.nwsuaf.edu.cn/lsp/index.php) database to simulate thedrug-likeness results by ADME Sico TCMSP database model. Given the integral roleof multi-components in TCM, we set at bioavailability (OB) >10%,drug-like >0.04 as the threshold for further extraction and optimizationof the medicinal ingredients. To build a compound-medicine network (C-Mnetwork), we extracted 63 compounds covering the main drug-likeness component ofthe YD and put them into the Cytoscape 2.8 software.
Next, we used Target-Bank, DrugBank, BindingDB and PDTD to validate 123 CVDtargets. We put the 63 chemical compounds into Cytoscape 2.8 software to build acompound-target network (C-T network, Fig. 4).
To explain target participation in cardiovascular activity, we used Cytoscape 2.8to build a network between target and function (T-F network, Fig.5). Multiple targets indicated an integral role of YD in CVD andcerebrovascular disease, sharing synergy targets of the different compounds.
The main 110 targets included the following functions: anti-inflammation,coagulation and thrombosis, anti-apoptotic, insulin mediation, glucosemediation, cardiac energy metabolism manipulation, ion channel mediation,vascular protection, blood lipids regulation, endothelium protection, andanti-oxidation. To verify this predication, the next experiment was performedaccordingly.
Animal experiments for verification
Sprague Dawley rats (150–200 g) obtained from BeijingVital River Laboratories Co., Ltd. (Beijing, China) were housed in a controlledenvironment (temperature23° ± 2 °C,relative humidity 50% ± 10%,12 h light/dark cycle) and allowed free access to standard diet. Allanimals were acclimatized for 5 days prior to the initiation of our experiment.All experimental procedures were conducted in accordance with the GuidingPrinciples for the Care and Use of Laboratory Animals. The Ethics Committee forAnimal Experiments (Institute of Medicinal Plant Development, Chinese Academy ofMedical Sciences, Beijing, China) approved our experimental protocol.
We used an AS rat model to evaluate the attenuation of AS and reveal the possiblemechanism of YD. Following a procedure established in related reports31,32,33,34, we induced AS by injecting the rats with vitamin D3and ovalbumin and feeding them a high-fat diet (HFD). normal control group ratsconsumed a regular diet for 21 weeks, whereas rats in AS groups consumed a HFD(1% cholesterol, 0.2% pig bile salts, 10% lard, 10% egg yolk powder, 78.8% basaldiet). Initially, rats in the AS model received an intraperitoneal injection ofvitamin D3 (600 K IU/kg), followed by a hypodermicallyinjected antigen emulsion (ovalbumin [3 mg/kg] and completeFreund’s adjuvant) to their backs on day 2. After 3 weeks,atherosclerotic rats received weekly intraperitoneal booster injections ofovalbumin (2.5 mg/kg) for 3 consecutive weeks, whereas controlsconsumed a normal diet and received isovolumic saline injections. At week 9, werandomly divided atherosclerotic rats into five groups for a 12-week treatmentregimen, using normal rats as control group (con group). con group andatherosclerotic model controls (AS group) received an equal volume of vehicles.The other atherosclerotic rats received atorvastatin (anti-hyperlipidemics,Ator, 0.01 g/kg b.w., i.g [Ator Group]) or YD treatment (YD2.0 g/kg b.w. i.g. [YD-2.0 group]); YD 1.0 g/kg BW i.g.[YD-1.0 group]; or YD 0.5 g/kg b.w. i.g. [YD-0.5 group]). Normalcontrol rats (con group) consumed a normal diet throughout the experiment.
After 21 weeks, we deprived all animals of food (but not water) overnight, andthen anesthetized them. Next, we drew blood samples from the abdominal aorta,and obtained serum and plasma via blood centrifugation (3,000 rpmfor 10 min at 4° C). Prior to histopathologicexamination and immunohistochemical analysis, we harvested and weighed theabdominal aorta.
Histology lesion analysis and immunohistochemical staining
After taking blood samples from the abdominal aorta, we quickly removed the aortabetween the aortic valve cusps and the iliac bifurcation, storing them in 4%paraformaldehyde for histological studies. Next, we cut consecutivecross-sections (4 μm thick) of the aorta and stained withhematoxylin-eosin (HE). Pathological changes were observed with an opticalmicroscope and intima-media thickness (IMT) was measured using Image-ProExpress 5.0 software.
Immunohistochemical staining were conducted using standard techniques, asdescribed previously35. Briefly, we inhibited endogenousperoxidase activity via incubation with 3% H2O2. Afterblocking the sections with 5% albumin from bovine serum and incubating them for10 min, we added primary antibodies and incubated the sections for60 min before preserving them overnight at 4° C.Following a PBS wash, we incubated the sections with a secondary antibody for60 min at 37° C. Immunohistochemical staining wasvisualized using a DAB kit (Boster Biotechnology, LTD, Wuhan, China), accordingto the manufacturer’s instructions. Immunohistochemical staining(relative positive expression) was quantified using integral optic density
Biochemical analysis
We collected blood samples and measured serum levels of endothelium-derivedrelaxing factor (NO), cholesterol, triglycerides (TGs), LDL and high-densitylipoprotein (HDL) using chemical colorimetry assay kits (Nanjing JianchengBioengineering Institute). Rat serum IL-1β, tumor necrosis factoralpha (TNF-α), endothelin, 6-keto-PGF1α and thromboxaneB2 (TXB2) concentrations were measured byradio-immunity assay, using commercial kits according to manufacturerinstructions.
Measurement of the antioxidative activity in vivo
We used total superoxide dismutase (SOD, hydroxylamine method), glutathioneperoxidase (GSH-PX, colorimetric method), lipid peroxidation malondialdehyde(MDA) and reduced GSH (spectrophotometric method) assay kits to determineanti-oxidative activity in serum. These commercially available assay kits wereprovided by Nanjing Jiancheng Bioengineering Institute.
Culture, proliferation and cytotoxity assay of human umbilical veinendothelial cells
For in vitro experiments, we used human umbilical vein endothelial cells(HUVECs) obtained from the Cell Resource Center of the Institute of BasicMedical Sciences, Peking Union Medical College/Chinese Academy of MedicalSciences (Beijing, China) and cultured them in a humidified atmosphere inDulbecco’s Modified Eagle Medium (DMEM, Thermo US) with 20% fetalbovine serum (20% FBS) at 37° C under 5% CO2. The 6thpassage cells were used for experiments.
HUVECs (1 × 105) were plantedin a 96-well plate, then incubated for 24 h with YD (at the seriesconcentration of 1.5, 3, 6.25, 12.5, 25, 50, 100, 200 mg/L) forprotection. After 24 h pretreatment, the supernatant was discarded.Cells then were exposed to 100 mg/L ox-LDL for 24 h tocause oxidative injury, at 37 °C in a 5% CO2atmosphere10,34,35. The viability of HUVEC cells wasdetermined by 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT, sigma) assay to express the protection of YD. The culture medium wasreplaced with serum-free medium containing 0.5 g/L MTT tetrazoliumsalt and incubated at 37 °C for 4 hours36,37. At the end of the incubation with MTT, the cells werescraped and solubilized into DMSO solution. The absorbance was measured at570 nm by microplate reader (Thermo Scientific). The viability ofthe cells was expressed as the percentage of the absorbance measured in controlcells.
Western blotting
Using lysis buffer (1%Triton X-100; 50 mmol/L Tris–HCl,pH 7.6; 150 mmol/L NaCl; and 1% protease inhibitor cocktail), weextracted tissue homogenates from the resected left common carotid artery. Forwestern blot, proteins were separated by SDS–PAGE and transferred topolyvinylidene difluoride membranes. After blocking, the membranes wereincubated at room temperature for with the indicated antibodies, includinganti-p65, anti-IkB and anti-actin (1:1000 dilution). Next, wetreated the membranes with horseradish peroxidase-conjugated secondaryantibodies, diluted to 1:2000 (Santa Cruz Biotechnology)38,39.The blots were developed using ECL western blotting reagents and quantifiedusing optical densitometry. We quantified the bands according to the averageratios of integral optic density of anti-p65/β-actin andanti-IkB/β-actin.
Statistical analysis
All data are expressed by mean values and standard deviations. Quantitative datawere assessed by one-way analysis of variance (ANOVA). If the F distribution wassignificant, we used a t-test to specify the differences between groups.Differences with a probability value < 0.05 wereconsidered statistically significant. We performed statistical analysis using anSPSS 11.0 software package (SPSS Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Cheng, L. et al. Yindanxinnaotong, a Chinesecompound medicine, synergistically attenuates atherosclerosis progress. Sci.Rep.5, 12333; doi: 10.1038/srep12333 (2015).
References
Ohira, T. et al. Cardiovascular disease epidemiology in Asia: an overview. Circ J. 77, 1646–52 (2013).
Mendis, S., Puska, P. & Norrving, B. Global atlas on cardiovascular disease prevention and control . 8–13 (World Health Organization, Geneva, 2011).
Weber, C. et al. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 17, 1410–22 (2011).
Bleijerveld, O. B. et al. Proteomics of plaques and novel sources of potential biomarkers for atherosclerosis. Proteomics Clin Appl. 7, 490–503 (2013).
Choi, J. H. et al. Hematein inhibits atherosclerosis by inhibition of reactive oxygen generation and NF-kappa B-dependent inflammatory mediators in hyperlipidemic mice. J Cardiovasc Pharmacol. 42, 287–95 (2003).
Lee, G. et al. 4-O-methylgallic acid down-regulates endothelial adhesion molecule expression by inhibiting NF-kappaB-DNA-binding activity. Eur J Pharmacol. 551, 143–51 (2006).
Libby, P., Ridker, P. M. & Hansson, G. K. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 54, 2129–38 (2009).
Davignon, J. et al. Role of endothelial dysfunction in atherosclerosis. Circulation . 109, III27–32 (2004).
Libby, P. et al. Progress and challenges in translating the biology of atherosclerosis. Nature . 473, 317–25 (2011).
Chen, X. P. et al. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul Pharmacolo. 7, 1–97 (2004).
Wang, J. Y. et al. Potential effectiveness of traditional Chinese medicine for cardiac syndrome X (CSX): a systematic review and meta-analysis. BMC Complement Altern Med. 13, 62 (2013).
Wang, W. et al. Protective effects of yindanxinnaotong capsule in a rat model of myocardial ischemia/reperfusion injury. J Tradit Chin Med. 34, 699–709 (2014).
Wang, W. et al. Protection of yindan xinnao tong capsule and main compositions compatibility on myocardial ischemia/reperfusion injury Zhongguo Zhong Yao Za Zhi. 39, 1690–4 (2014). In Chinese.
Hopkins, A. L. et al. Network pharmacology. Nat Biotechnol. 25, 1110–1 (2007).
Li, S. et al. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 11, 110–20 (2013).
Park, J. H. et al. The clinical significance of the atrial subendocardial smooth muscle layer and cardiac myofibroblasts in human atrial tissue with valvular atrial fibrillation. Cardiovasc Pathol. 22, 58–64 (2013).
Han, M. et al. Smooth muscle 22 alpha maintains the differentiated phenotype of vascular smooth muscle cells by inducing filamentous actin bundling. Life Sci. 84, 394–401 (2009).
Jamkhande, P. G. et al. Therapeutic approaches to drug targets in atherosclerosis. Saudi Pharm J. 22, 179–90 (2014).
Kalz, J. et al. Thrombin generation and atherosclerosis. J Thromb Thrombolysis . 37, 45–55 (2014).
Wolak, T. Osteopontin - A multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis . 236, 327–37 (2014).
Lee, P. S. et al. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells . 6, 355–66 (2014).
Shaw, A. et al. Endothelial cell oxidative stress in diabetes: a key driver of cardiovascular complications. Biochem Soc Trans. 42, 928–33 (2014).
Chalkiadaki, A. et al. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16, 180–8 (2012).
Feil, S. et al. SM22alpha modulates vascular smooth muscle cell phenotype during atherogenesis. Circ Res. 94, 863–5 (2004).
Zhang, J. Y. et al. Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. fruit on experimental atherosclerosis in rats. J Ethnopharmacol. 148, 563–9 (2013).
Su, W. et al. Tongxinluo inhibits oxidized low-density lipoprotein-induced maturation of human dendritic cells via activating peroxisome proliferator-activated receptor gamma pathway. J Cardiovasc Pharmacol. 56, 177–83 (2010).
Cheng, L. et al. Evaluation of anxiolytic-like effect of aqueous extract of asparagus stem in mice. Evid Based Complement Alternat Med. 2013, 587260 (2013).
Ross, R. Atherosclerosis—an inflammatory disease. N Engl J Med. 340, 115–26 (1999).
Choi, J. H. et al. Hematein inhibits atherosclerosis by inhibition of reactive oxygen generation and NF-kappaB-dependent inflammatory mediators in hyperlipidemic mice. J Cardiovasc Pharmacol. 42, 287–95(2003).
Kim, J. H. et al. Functional dissection of Nrf2-dependent phase II genes in vascular inflammation and endotoxic injury using Keap1 siRNA. Free Radic Biol Med. 53, 629–40 (2012).
Gocmen, A. Y. et al. Effect of atorvastatin on atherosclerotic plaque formation and platelet activation in hypercholesterolemic rats. Can J Physiol Pharmacol. 91, 680–5 (2013).
Li, Y. et al. Rosuvastatin attenuates atherosclerosis in rats via activation of scavenger receptor class B type I. Eur J Pharmacol. 723, 23–8 (2014).
Zhou, B. R. et al. Fibrinogen facilitates atherosclerotic formation in Sprague-Dawley rats: A rodent model of atherosclerosis. Exp Ther Med. 5, 730–34 (2013).
Xu, X. et al. Amelioration of lipid profile and level of aAntioxidant activities by epigallocatechin-gallate in a rat model of atherogenesis. Heart Lung Circ. 23, 1194–201 (2014).
Torzewski, M. et al. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler Thromb Vasc Biol. 18, 369–78 (1998).
Guo, H. et al. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc Drugs Ther. 27, 189–98 (2013).
Huang, C. S. et al. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependentantioxidation and suppressing NF-κB activation. Mol Nutr Food Res. 57, 1918–30 (2013).
Gareus, R. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 8, 372–83 (2008).
Matsumoto, T. et al. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol Meas. 23, 635–38 (2002).
Acknowledgements
The authors thank scientific editor Karen Williams (Kwills Editing Services,Weymouth, MA, USA) and Dr. Li Yong for providing professional English languageediting of this paper. This work has been funded by China Ministry of Science and Technology (theNational Science and Technology Projects No: 2012ZX09201201-003, 2013BAI11B01) andChina Postdoctoral Science Foundation (No: 2014M552546XB).
Author information
Authors and Affiliations
Contributions
Conceived and designed the protocol: L.C., R.-x.L., X.-b.S. and G.-f.P. Performed theexperiments: J.-l.W., W.-d.W., J.-y.Z. and H.W. Analyzed the data: R.-x.L., J.-y.Z.and X.-d.Z. Wrote and revised the paper: X.-d.Z. and L.C. All authors reviewed andapproved the submitted version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0International License. The images or other third party material in this article areincluded in the article’s Creative Commons license, unless indicatedotherwise in the credit line; if the material is not included under the CreativeCommons license, users will need to obtain permission from the license holder toreproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cheng, L., Pan, Gf., Zhang, Xd. et al. Yindanxinnaotong, a Chinese compound medicine, synergistically attenuatesatherosclerosis progress. Sci Rep 5, 12333 (2015). https://doi.org/10.1038/srep12333
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12333
This article is cited by
-
Therapeutic efficacy and pharmacological mechanism of Yindan Xinnaotong soft capsule on acute ischemic stroke: a meta-analysis and network pharmacology analysis
Metabolic Brain Disease (2023)
-
Systems pharmacology and transcriptomics reveal the mechanisms of Sanhuang decoction enema in the treatment of ulcerative colitis with additional Candida albicans infection
Chinese Medicine (2021)
-
Lipingshu capsule improves atherosclerosis associated with lipid regulation and inflammation inhibition in apolipoprotein E–deficient mice
Lipids in Health and Disease (2018)
-
Chinese herbal formula Fuzheng Huayu alleviates CCl4-induced liver fibrosis in rats: a transcriptomic and proteomic analysis
Acta Pharmacologica Sinica (2018)
-
Systems Pharmacology-based strategy to screen new adjuvant for hepatitis B vaccine from Traditional Chinese Medicine Ophiocordyceps sinensis
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.