Abstract
Malaria parasites alter mosquito feeding behaviour in a way that enhances parasite transmission. This is widely considered a prime example of manipulation of host behaviour to increase onward transmission, but transient immune challenge in the absence of parasites can induce the same behavioural phenotype. Here, we show that alterations in feeding behaviour depend on the timing and dose of immune challenge relative to blood ingestion and that these changes are functionally linked to changes in insulin signalling in the mosquito gut. These results suggest that altered phenotypes derive from insulin signalling-dependent host resource allocation among immunity, blood feeding and reproduction in a manner that is not specific to malaria parasite infection. We measured large increases in mosquito survival and subsequent transmission potential when feeding patterns are altered. Leveraging these changes in physiology, behaviour and life history could promote effective and sustainable control of female mosquitoes responsible for transmission.
Similar content being viewed by others

Introduction
Transmission of malaria parasites is inextricably linked to vector survival and feeding behaviour. Mosquitoes infected with malaria parasites have been shown to exhibit changes in feeding persistence, duration and probing behaviour1. Intriguingly, changes in host-seeking behaviour are differentially associated with stages of parasite development, with decreased host-seeking associated with pre-infectious oocyst stages of the parasite2 and increased response with the infectious sporozoite stage2,3. Such behavioural changes are predicted to increase transmission success1,4 and have long been considered a classic example of parasitic manipulation in which malaria parasites actively alter the behaviour of the mosquito to increase their own fitness5,6. However, we recently reported that challenge with heat-killed Escherichia coli triggered similar time-specific changes in host-seeking propensity, suggesting that altered host-seeking behaviour is a response to immune challenge rather than a specific consequence of malaria parasite infection2.
Despite the potential importance of these behavioural phenomena in transmission, the underlying mechanisms remain unclear. Previous work has demonstrated that changes in the probing rates of some vectors could be attributed to parasite induced lesions in the salivary glands7 and one study reported altered protein expression in the heads of sporozoite infected females8. However, a mechanistic connection between the presence of malaria parasites in the midgut and stage-specific changes in mosquito feeding motivation and host-seeking rates is lacking. Elucidating this mechanism could help to both clarify the role of the parasite in driving the behavioural response and reveal potential novel targets for genetic manipulation of mosquito host-seeking behaviour.
Here, we sought to further investigate the role of the mosquito immune response in altered host-seeking behavior and to reveal potential mechanisms linking immune response to host-seeking propensity. Using heat-killed E. coli as a non-pathogenic immune elicitor, we found that the behavioural phenotype requires a challenge at the time of bloodmeal and also that it dynamically responds to dose. When we investigated the mechanism of this dynamic relationship between bloodmeal and immune challenge, we found that both heat-killed E. coli and the human malaria parasite, P. falciparum, trigger changes in insulin signaling in the mosquito midgut which are functionally linked to feeding propensity. Finally, we quantified the potential impact of altered feeding patterns on mosquito survival and hence, transmission potential.
Results and Discussion
We began by dissecting the relationship between immune challenge and bloodfeeding. As in previous work, we challenged female Anopheles stephensi by injecting heat-killed E. coli immediately following the first bloodmeal2. This technique has been shown to generate both the behavioural and neurophysiological phenotypes associated with malaria parasite infection2 and represents a more tractable experimental approach for evaluating mosquito behaviour in the lab. This technique was especially appropriate for our aim of investigating the role of the mosquito immune response to challenge in the absence of potentially confounding pathogen dynamics9.
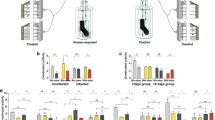
First, we examined possible interactions between the timing of immune challenge and the bloodmeal. Female mosquitoes were given an uninfected bloodmeal and were then challenged with 200,000 heat-killed E. coli administered either immediately following the bloodmeal, 2 days, or 4 days post-bloodmeal2. The behavioural response of these mosquitoes to human host cues was compared with mosquitoes that received either a bloodmeal or immune challenge alone, or were completely unmanipulated. Behaviour was assayed at two time points coinciding with non-infectious (6–8 days post infection) and infectious (14–16 days post infection) stages of parasite development in malaria-infected mosquitoes. Behavioural changes only occurred when the bloodmeal and immune challenge were synchronous (treatment x test period, Wald χ2 = 11.753, d.f. = 5, P = 0.04, Fig. 1a). Neither immune challenge alone nor a bloodmeal alone altered host-seeking propensity compared to unmanipulated controls and only females challenged directly after the bloodmeal exhibited a significantly different phenotype from bloodfed control females (Fig. 1a, Wald χ2 = 10.91, d.f. = 11, P < 0.01).
Effects of timing and dose of immune challenge on host-seeking behaviour.
A. Immune challenge coinciding with the bloodmeal generates the mosquito host-seeking patterns associated with malaria parasite infection. The previously described ‘manipulation’ phenotype is only recapitulated when females are challenged immediately following the bloodmeal (HK-0, n = 163). Immune challenge alone (HK, n = 156) or occurring later (HK-2 days, n = 145 and HK-4 days, n = 148) do not generate the same phenotype. Controls included unmanipulated females (UC, n = 78) and females which only received a bloodmeal (BC, n = 157) This experiment was replicated twice. B. The effect of dose of heat-killed E. coli on behaviour. Dose had no effect on the duration or time of decreased response period (Fig. S2). Control, n = 391, Low, n = 227, Medium, n = 209, High, n = 221. This experiment was replicated three times. Error bars represent 1 SE and * indicates P < 0.05 between first and second test period within a treatment.
To further investigate the role of the mosquito immune response, we examined whether the magnitude of immune response altered host-seeking patterns. We challenged bloodfed females directly after an uninfected bloodmeal with low, medium and high doses of heat-killed E. coli. These doses were chosen based on previous work demonstrating that challenge with 200,000 Heat-killed E. coli (the “high” treatment here) generated a behavioural and neurophysiological response equivalent to that elicited by challenge with the rodent malaria parasite, P. yoelii2. We confirmed that these doses triggered corresponding variation in immune response by measuring total body expression of the immune gene defensin (DEF1) from 6–48 h post-challenge. We observed a dose effect on immune gene expression at the peak of DEF1 expression, 12 hr post-immune challenge (Fig. S1, Table S1). Corresponding analysis of behavioural responses of females (total of 1453 independent observations) revealed that the dose of immune challenge received at the time of the bloodmeal was an important determinant of host-seeking patterns (dose x stage, χ2 = 20.041, d.f. = 11 , P < 0.001, Fig. 1b). The higher the dose of heat-killed E. coli a treatment group received, the lower its response to the host 6–8 days post-challenge. When mosquitoes from the same treatment groups were assayed on days 14–16 post bloodmeal we observed the opposite trend, with the response of mosquitoes to host odour increasing with the dose of heat-killed E. coli. Thus, behavioural change depended on both the dose and timing of immune challenge relative to the bloodmeal, suggesting possible trade-offs or constraints between immune response, digestion and potentially reproduction.
With this new insight, we next set out to characterize the regulation of the altered host-seeking behaviour. Insulin-like peptides (ILPs) have been reported to regulate the sensitivity of odorant receptor neurons (ORNs) in Drosophila10. We previously reported that changes in the sensitivity of the ORNs in mosquito maxillary palps are associated with rodent malaria parasite infection and challenge with heat-killed E. coli2. Further, the expression levels of ILP3 and ILP4 in the An. stephensi midgut and head are altered in response to the human malaria parasite, P. falciparum in the ingested bloodmeal11. Therefore, we hypothesized that changes in ILP expression might be associated with observed changes in host-seeking behaviour.
When we measured expression of ILP3 and ILP4 in the midgut and head of mosquitoes challenged with the same three doses of heat-killed E. coli, we found that ILP3 (F1,12 = 7.56, P = 0.02) and ILP4 (F1,124.67, P = 0.05) expression levels were low in the midgut at days 6–8 post bloodmeal when host-seeking was reduced and elevated at 14–16 days (Fig. 2a,b) when host-seeking was enhanced2. In contrast to the midgut, ILP3 expression in the head was elevated at day 6 relative to day 14, (d.f = 1, F = 6.35, P = 0.03; Fig. S3), but there was no significant pattern of ILP4 expression in the head in response to any E. coli challenge. These results suggest that ILP expression in the midgut, not the head, regulates behavioural changes.
Immune challenge alters time-specific ILP expression in the mosquito midgut.
A-B. The expression of ILP 3 (A) and ILP 4 (B) in the midgut in response to the high, medium and low doses of heat-killed E.coli over 14 days. Horizontal dashed lines represent control levels of expression. C-D. Expression of ILP 3 (C) and ILP 4 (D) in the midgut is downregulated in P. falciparum-infected An. stephensi during the period of oocyst development and upregulated during the period of sporozoite development. Mosquitoes were dissected 7, 10, or 14 days after infection with P. falciparum and expression of ILP 3 or ILP 4 was analyzed by qRT-PCR. Both experiments were replicated independently 3 times with pools of 25 female mosquitoes. * indicates significant difference in mean expression between test periods relative to control levels (ANOVA with Tukey’s multiple comparisons) and all error bars represent 1 SE.
To confirm whether insulin signalling also plays a role in the Plasmodium parasite-triggered behavioural changes, we measured expression levels of ILP3 and ILP4 in the midguts of P. falciparum-infected An. stephensi at times consistent with altered host-seeking behaviour. We used the NF54 strain of P. falciparum, which regularly produces low-intensity infection similar to those observed in field populations12 (39–67% prevalence and intensity of 1.36–2.00 oocyst/midgut, Table S2). We found a sequential under- and overexpression of these ILPs coinciding with the sequential changes in host-seeking behaviour (Fig. 2). ILP3 expression was lowest at 7 days post-infection, but began to rise by 10 days and was significantly elevated at 14 days post-infection (Fig. 2c; ANOVA, d.f = 2, F = 6.31, P = 0.03; Tukey’s multiple comparison test, day 7 vs day 14, P < 0.05). ILP4 expression was lowest at day 10 post-infection and significantly increased on day 14 post-infection (Fig. 2d; ANOVA, d.f = 2, F = 5.18, P = 0.05; day 10 vs day 14, P < 0.05). Therefore, these rather diverse challenges (bacteria/transient, parasite/infective) both trigger similar changes in the expression of these two ILPs in the mosquito midgut.
To determine whether the association of reduced midgut ILP expression with host-seeking behaviour was functionally significant, peptide levels of target ILPs were knocked down by treating females with antisense ILP3 and ILP4 morpholino oligomers or with a control morpholino11. This minimally invasive method allows for targeted, efficient knockdown of midgut expression. We confirmed knockdown efficiency using a Western blot (Fig. S4). These females were then provided access to an uninfected bloodmeal. Females treated with either ILP3 or ILP4 morpholinos were significantly less likely to engorge on available blood (43.2% feeding and 56.3% feeding, respectively) than those treated with a control morpholino (67.1% feeding) (χ2 = 16.65 , d.f. = 2 , P < 0.05). Hence, reduced ILP3 and ILP4 expression is functionally associated with reduced feeding behaviour, a pattern consistent with the oocyst-stage phenotype in parasite-infected females and also females after a transient activation of the immune response with heat-killed E. coli2.
Transient activation of the mosquito immune response has now been shown to trigger the same behavioural, neurophysiological2 and midgut insulin signalling as active infection with malaria parasites. Parasites can manipulate their host through induction of host trade-offs13. However, we would argue that a more parsimonious interpretation is that the observed relationships between immune response and feeding behaviour are a mosquito response to physiological or resource-based constraints14. Without any mechanism specific to Plasmodium parasites, it seems most reasonable to conclude that changes in host-seeking behaviours in malaria parasite-infected females are a mosquito-mediated response to infection rather than parasite-driven manipulation. In fact, the development times of malaria parasites (extrinsic incubation period) might have been shaped via selective forces associated with altered feeding to maximize their likelihood of transmission.
These conclusions do not alter the fact that the malaria parasites might benefit from these changes. Therefore, we quantified the potential impact of altered feeding behaviour for malaria transmission potential. Specifically, we examined the significance of reduced mosquito feeding and associated mortality during the early, non-infectious stages for malaria transmission potential. Bloodfeeding is known to be costly to uninfected mosquitoes15,16, but its effect on infected or immune challenged mosquitoes remains unexplored. Immune-challenged females were split into two groups, one that was bloodfed at a regular 4-day cycle and one that was not offered bloodmeals during the second and third cycles (days 4 and 8, consistent with the ‘manipulation’ phenotype). We found that skipping bloodmeals led to a significant increase in survival (Cox Regression, z = 2.37, P = 0.02), doubling the number of mosquitoes alive at the fourth and fifth feeding cycles when, in infected mosquitoes, parasites would have completed development and could potentially be transmitted (Fig. 3). Given that feeding and reproductive costs (e.g. feeding-associated mortality from defensive host behaviour and flights to shuttle between hosts and oviposition sites) are likely higher in the field than under benign laboratory conditions, these results confirm the potential importance of the altered feeding phenotype for malaria transmission. Future work quantifying the effect of altered feeding using local vector-parasite pairings under field conditions, will further clarify effect on transmission. However, this simple experiment demonstrates the need to better understand this phenomenon and its mechanisms.
The effect of blood feeding on the cumulative proportional survival of female mosquitoes challenged with heat-killed E. coli.
Control females received bloodmeals every 4 days while treatment females skipped the 2nd and 3rd bloodmeals during the period associated with down-regulated feeding behaviour. When not on bloodmeals, females were maintained on 2.5% sugar solution. Female survival was individually tracked. Each treatment group contained 100 females and the experiment was replicated twice (mean shown for total of 400 females, 200 females per treatment group). Red shading indicates the extrinsic incubation period of P. falciparum at 27 °C36. Stars below control and above treatment line indicate that treatment received a bloodmeal. Error bars represent 1 SE. Survival was compared using a Cox Proportional Hazards Test.
We demonstrate that the interactions between immunity and behaviour appear, at least in part, to be mediated by alterations in ILP biology in the midgut. In Caenorhabditis elegans and in D. melanogaster, the intestine or midgut is considered an “insulin signalling center,” directing physiological responses among midgut cells and between cells of the midgut and other tissues to regulate lifespan and oxidative stress17. Studies in D. melanogaster suggest that ILPs produced in neurosecretory cells act as endocrine signals to control hunger, physical activity and mating18,19. In addition to regulating these important processes, ILPs also appear to coordinate trade-offs between these processes20. For example, experiments in fruit flies have revealed activation of Toll signalling in the fat body inhibits insulin signalling activity in other tissues19. Conversely, activation of insulin signalling in An. stephensi can inhibit NF-κB-dependent immunity in the midgut21. Previous work in mosquitoes has demonstrated that ILPs have been shown to play a role in the regulation of vitellogenesis and bloodmeal digestion22,23. It appears that in An. stephensi there is also an inhibition of insulin signalling activity in the migut in association with immune activation and additionally, that this suppression dramatically alters mosquito life history and transmission potential. We have observed that genetic manipulation of midgut insulin signalling can significantly affect both lifespan and immunity. Now we extend this phenotypic control to behaviour24,25.
Overall, our results further support the idea that the subset of mosquitoes responsible for malaria transmission exhibit altered foraging phenotypes1,26,27,28 leading to changes in mortality schedules and enhanced transmission potential4. Further mechanistic characterization of behavioural modifications could improve our ability to control malaria transmission in three key ways. First, incorporation of these altered behaviours into standard epidemiological models could improve our understanding and ability to predict transmission dynamics. Second, altered behavioural ecology of the subset of mosquitoes responsible for transmission provides opportunities for focusing control tools to target transmission instead of entire mosquito populations. Increased specificity of control efforts could be used to effectively control malaria transmission, while weakening the strong selection for resistance that accompanies population-wide control measures29. Finally, this study identifies possible new targets for genetic manipulation. Insulin signalling pathways may be used to target the parasite both directly via mosquito immunity and indirectly by altering mosquito feeding behaviour to curtail transmission potential.
Methods
Mosquito Rearing
All experiments were conducted using an Indian wild type strain of An.stephensi originally sourced from the Walter Reed Army Institute of Medical Research. An.stephensi used in timing, dose and survival assays were reared at the PSU insectary at a larval density of 400 larvae per 1 L tray of distilled water. Eggs from over 1000 colony females were placed in plastic trays (25 × 25 x 7 cm) filled with 1.5 L of distilled water and held at 26 °C rearing temperature. Upon reaching 2nd instar larvae were transferred to fresh trays at a density of 400 larvae per 1.5 L of distilled water. We fed larvae 10 mg of ground fish flakes (TetraFin, Melle, Germany) a day. We collected pupae and placed them in cages for emergence. Larvae for the ILP 3/4 expression work and engorgement assays were reared at UC Davis and were provided with a 2% solution of 2:1 Sera Micron® powdered fish-fry food (Sera North America, Montgomeryville, PA) through day 4 post-hatching and then were reared on high protein, low fat Game Fish Chow pellets (Purina Mills, St. Louis, MO) until pupation. All adults were held at 26 °C , 80% RH with a 12:12 hr light:dark lighting cycle. Emerged adults were maintained on cotton pads soaked in a 10–20% sucrose solution.
Timing of Immune Challenge
We investigated the effect of immune challenge timing relative to the bloodmeal using two control and four treatment groups (Fig. 1a). Controls included unmanipulated females that were not bloodfed or challenged (UC) and females that were not bloodfed, but challenged with heat-killed E. coli (HK, tetracycline resistant GFP expressing dh5 alpha strain2). Treated females were offered a bloodmeal on uninfected anesthetized mice (C57 BL/6, Animal Care and Use Committee of the Pennsylvania State University, permit no: 27452), then divided into the following four treatment groups: (1) no immune challenge (BC), (2) challenged with heat-killed E. coli immediately following bloodmeal (HK 0), (3) challenged with heat-killed E. coli 2 days after the bloodmeal (HK 2) and (4) challenged with heat-killed E. coli on day 4 post-bloodmeal (HK 4). All females receiving bacterial challenge were injected with 200,000 heat-killed E. coli using a microcapillary glass needle inserted intrathoracically into the anepisternal cleft2,30. Prior to challenge all groups were anesthetized on ice. Previous experiments established that injury alone did have a significant effect on this set of behaviours2.
The female behavioural response to a host was tested using the short range assay described in2. Briefly, females were aspirated individually into a release cage. This cage was connected to a cage containing the hand of a human host (LJC) with a plastic tube (12 cm diameter, 48 cm length). The 4 minute trial began when a flap blocking entry to the tube from the release cage was lifted. A positive response was counted if females exited the release cage, entered the host cage and attempted to feed on the host. Females that did not respond within the 4 minute trial period were counted as not responding. Reponses were tested on days corresponding with oocyst (days 6–8) and sporozoite (13–15) stages of Plasmodium yoelii infection at this temperature. The responses of 40 individual females per treatment group were tested for each stage (80 females total per treatment group). This experiment was replicated twice with the exception of the unmanipulated control group, which was added for only the second replicate of the experiment. The Pennsylvania State University Institutional Review Board determined that the experiments whereby mosquitoes were attracted to LJC’s hand did not meet the criteria for human subjects research and thus, do not require human subjects approval.
Dose of Immune Challenge and Response
For these studies, 3–5 day old females provided an uninfected mouse bloodmeal were divided into two control and three treatment groups. Controls included an unmanipulated group to account for any impact of cold anesthetization and an injury control group that was cold anesthetized and injected with sterile LB. The three treatment groups were all cold anesthetized and challenged with varying doses of heat-killed E. coli directly after the bloodmeal. The low challenge group received a dose of 2,000 heat-killed E. coli, the medium challenge group received 20,000 heat-killed E. coli and the high challenge group received 200,000 heat-killed E. coli. The high dose is equivalent to the concentration used in the timing experiment (Fig. 1b) and in previous experiments using heat-killed E. coli challenge and has been shown to produce similar changes in behaviour to challenge with a P. yoelii-infected bloodmeal2. The responsiveness of these females to human odor was measured using the short-range assay described above (Fig. 1b). In the first replicate, responses were measured during two periods; days 6–7 and days 14–15. In the second and third replicate, we measured responses in an additional intermediate period between these and report responsiveness on days 6–8, 10–12 and 14–16 post initial bloodmeal. We tested a naïve group of 20–25 females per treatment group on each day.
Immune Gene Expression
To quantify the response of females to different doses of heat-killed E. coli, we sampled 10 females 0, 6, 12, 24 and 48 hrs post immune challenge. Females were killed immediately with chloroform and stored in RNAlater RNA stabilization reagent and held at 4 °C. Five females were sampled from each treatment group at each time point (N = 125 females) and individually prepared in B-Mercaptoethanol and RLT lysis buffer (Qiagen, Venlo, Netherlands) for messenger RNA isolation. mRNA was extracted and quantified for each female as described in9,30. cDNA from each experimental sample was quantified by comparing threshold cycle numbers against a standard curve generated from 1:10 serial dilutions of our standard sample (cDNA from a pool of four mosquitoes). Levels of cDNAs for both DEF1 and ribosomal protein S7 (rps7), a housekeeping gene commonly used in a wide range of studies on Anopheles31,32,33, were quantified from individual mosquitoes collected across all experimental treatments relative to a standard curve produced for that gene (Fig. S1). To account for individual differences in background gene expression, rpS7 cDNA counts were included as a covariate in our statistical analyses (see below). Primers and probes were previously described30.
ILP 3 and ILP 4 expression in female challenged with heat-killed E. coli and P. falciparum
Females from control, low, medium and high treatment groups from the dose experiment were sampled on days 6–7 post-challenge and day 14 post-challenge with E. coli, or days 7, 10 and 14 post-infection with P. falciparum (11NF54 strain). We dissected heads and midguts from 20–30 females per treatment into PBS and tissues were stored in Trizol reagent (Invitrogen, Carlsbad, CA). Samples were homogenized by pulse sonication and total RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s protocol. Quantitative RT-PCR was performed on an ABI 7300 Sequence Detection System (Applied Biosystems; Foster City, California) using ILP-specific primers and probes as previously described19. Data were analyzed using the delta-delta Ct method and ILP expression in challenged/infected groups was normalized against rps7 to control for loading then normalized against levels in controls receiving blood only (Fig. 2). Experiments were replicated three times and data are reported as fold change in expression relative to controls.
Knockdown of ILP 3 and ILP4 using Morpholinos
As described in34 Anti-ILP3 (5′ TCGTGGACGACA TCTTGACAGAGGT-3′) or anti-ILP4 (5′-CGTGGAACTTTCATCTCAAGGACCT-3′) morpholinos (Gene Tools, Philometh, OR) at a stock concentration of 500 μM were diluted to 10 μM in saline and ATP (Sigma Aldrich, St. Louis, MO) was added to a final concentration 1 mg/ml as a feeding stimulant. Immediately prior to feeding, morpholino solutions were warmed in a water bath at 37 °C for 10 min. Vivo-morpholino standard control targeting an intron in the human beta-globin gene (5′-CCTCTTACCTCAGTTACAATTTATA-3′) in saline at equal concentrations was used as a matched control. Cohorts of 50–60 newly emerged female mosquitoes were provided with water only (no sucrose) for 24 hours and then provided a meal of either control-morpholino, ILP3-morpholino, or ILP4-morpholino in saline via a Hemotek artificial circulation system (Discovery Workshops, Accrington, UK). After 30 min, any unfed mosquitoes were removed from experimental cohorts.
Verification of ILP knockdown
To validate the efficiency of morpholino-mediated peptide knockdown in vitro (Fig. S4), we used an experimental surrogate of tagged AsILP3 and AsILP4 overexpression in rat pancreatic islet or insulinoma cells to infer analogous activity against peptide levels in A. stephensi. These cells express properly processed, mature rat insulin35 and we have confirmed that processed, mature AsILPs can also be produced by these cells following transfection (not shown). Here, rat insulinoma cells (RIN-5F, ATCC, Manassas, VA) were plated at a density of 250,000 cells/ml in RPMI 1640 growth medium (Invitrogen) supplemented with 10% FBS and 5% glucose and allowed to rest until adherent. On day 1, cells were transfected with AsILP3- or AsILP4-encoding plasmid (pcDNA3.1/V5-His, Invitrogen) using Effectene reagent (Qiagen, Limburg, Netherlands) according to the manufacturer’s protocol. The AsILP constructs contained twelve base pairs of the 5′ untranslated region for morpholino binding, the full-length AsILP coding sequence and a C-terminal V5 epitope tag for detection. Constitutive, high level transcription was driven by the cytomegalovirus (CMV) promoter. Empty plasmid was used as a negative control. On day 2, cells were treated with either standard control, anti-AsILP3 or anti-AsILP4 morpholinos (10 μM, Gene Tools) by directly adding morpholinos to the culture medium. On day 3, cells were collected into cell extraction buffer (Invitrogen), mixed with sample loading buffer (125 mM Tris-HCl pH 6.8, 10% glycerol, 10% SDS, 0.006% bromophenol blue) and boiled for 5 minutes. These experiments were replicated with 2 independent preparations of cells. To validate the efficiency of morpholino-mediated peptide knockdown in vivo (Fig. S4), we analyzed AsILP3 and AsILP4 levels in whole mosquitoes fed control or anti-AsILP morpholinos by western blot, as peptide levels in individual tissues are low and difficult to quantify. Three days after feeding of morpholinos, whole mosquitoes were collected into cell extraction buffer (Invitrogen) and homogenized with a pestle by grinding for 2–3 minutes. Homogenates were then passed through a QIAshredder column (Qiagen), cleared by centrifugation at 16,000 g for 10 minutes, mixed with sample loading buffer (125 mM Tris-HCl pH 6.8, 10% glycerol, 10% SDS, 0.006% bromophenol blue) and boiled for 5 minutes. This experiment was performed using 15–20 mosquitoes from 1 morpholino feed. Proteins from in vitro and in vivo experiments were separated on 16.5% Tris-tricine gels (BioRad) and transferred to nitrocellulose membranes (BioRad). Membranes were then fixed with 0.05% glutaraldehyde for 5 minutes and blocked with 5% non-fat dry milk in TBS-T for 2 hours at room temperature. For detection of peptides, membranes were incubated overnight at 4 °C with a 1:5,000 dilution of anti-V5 antibody (Sigma Aldrich) or 1:1,000 dilution of anti-AsILP antibody (Antibodies Inc., Davis, CA) in 5% non-fat dry milk. Membranes were then washed three times with TBS-T for 5 minutes and incubated with a 1:10,000 dilution of secondary HRP-conjugated rabbit anti-mouse antibody (Sigma Aldrich) in 5% non-fat dry milk overnight. Proteins were visualized by incubating membranes with SuperSignal West Dura chemiluminescent reagent (Pierce, Rockford, IL) for 3 minutes and exposing on an Image Station 4000 Pro digital imager (Kodak, Rochester, NY) for 5–10 minutes. Densitometry was performed using the ImageJ gel analysis tool (http://rsbweb.nih.gov/ij/) and values were normalized to Coomassie brilliant blue stain for total protein.
Measurement of Engorgement Success in Morpholino-treated Mosquitoes
Mosquitoes were maintained on cotton pads soaked in 10% sucrose through day 3 post- morpholino feeding, then starved on water for 24 h and subsequently provided a meal of human red blood cells in PBS (Cellgro, Manassas, VA) via Hemotek. Mosquitoes were allowed to engorge on blood for 15 min. After 15 min, mosquitoes were examined for the presence of blood in the midgut, counted and discarded. Experiments were independently replicated three times and data are reported as the proportion of mosquitoes that engorged in 15 min.
Survival of immune challenged females
As described above, 200 females were challenged directly after a bloodmeal (HK-0) on an uninfected mouse with 200,000 heat-killed E. coli (high dose). Each female was individually transferred to a 50 mL falcon tube fitted with a mesh lid and placed in a 27 °C incubator. Females were continually provided 2–5 ml of water for oviposition at the bottom of each tube and a cotton ball soaked in a 2.5% sugar solution. We used this lower concentration of sugar to impose some stress on these females, which otherwise were not subjected to many common sources of stress or mortality likely experienced in the field (long range flight, predation, host defensive behaviour, environmental fluctuation). Female mortality was checked daily. Females in the control group were provided a bloodmeal from an uninfected anesthetized mouse every 4 days (days 4, 8, 12 and 16). Treatment females were offered bloodmeals on days 12 and 16, but skipped the meals associated with down-regulated feeding behaviour in our assays (day 4 and 8 feds). The study was carried out in strict accordance with the recommendations in the guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Care and Use Committee of the Pennsylvania State University (#44512). To increase engorgement rate, sugar was removed the day prior to a bloodmeal and returned following the bloodmeal. Females which did not engorge were removed from analyses. We tracked females for one day beyond the final bloodmeal days (Fig. 3). The experiment was replicated twice giving a final sample size of 400.
Data Analysis
For the timing experiment, we utilized a Generalized Linear Model (GLM) fitted with a binary logistic regression to determine the effect of treatment on the likelihood that females responded positively to the host. We determined the statistical significance of treatment (unmanipulated control/ bloodfed control/ heat-killed only/ heat-killed Day 0/ heat-killed day 2/heat-killed day 4), time period (days 6–8/ days 13–15), day, replicate, wing length and the interactions of these variables. For the dose experiment, we also utilized a GLM fitted with a binary logistic regression. We determine the effect of treatment (unmanipulated control/injured/ 2,000 E. coli, 20,000 E. coli and 200,000 E. coli), test period (days 6–8, 10–12, or 14–16), day, replicate and wing length and the interaction of these variables on the probability that females responded.
We ran a general linear mixed effects model to estimate how DEF1 expression was influenced by dose of immune challenge, the time point the immune system was sampled post-immune challenge and their interaction. We first transformed DEF1 cDNA levels to ensure a normally distributed error structure. We included immune challenge treatment (unmanipulated, injured, 2,000 E. coli, 20,000 E. coli and 200,000 E. coli) and sampling time point (6 hr, 12 hr, 24 hr, 48 hr) post-immune challenge as fixed effects in the model analysis. To control for inter-sample variation introduced through the sampling, mRNA extraction and cDNA conversion processes, we normalized DEF1 expression by including centered rpS7 cDNA levels as a covariate in our model analysis. To control for inter-block variation, biological replicate was included in the model as a random effect. Model fit and assumption of equal variances was confirmed through model residuals.
For analysis of ILP expression, raw data were rank transformed and analyzed by two-way ANOVA to examine the effects of both time and dose on ILP expression in E. coli-challenged mosquitoes. Statistical analysis of ILP expression in P. falciparum-infected mosquitoes consisted of non-parametric, one way ANOVA followed by Tukey’s multiple comparison test. Assays of mosquito engorgement were replicated three times and control groups from each replicate were compared by chi-squared test to control for variation between cohorts. No significant differences between the three replicates were found (P > 0.05). Data from all replicates were then combined for chi-squared goodness of fit analysis to determine differences in the proportion of mosquitoes engorged in control and anti-ILP morpholino-treated groups.
Finally, the survival of immune-challenged females under different feeding regimes was compared using a Cox Regression. We tested for the effect of feeding treatment, replicate and interaction between these variables.
In all cases, full models were reduced through stepwise elimination of non-significant interactions and terms. Significance values reported for significant terms were those taken from the final model. Significance values reported for non-significant terms were those computed in the final step prior to removal of the term from the model. Non-significant values were only reported if they had a significant interaction with another parameter.
Data Deposition: All data has been deposited in the Dryad Online Repository doi:10.5061/dryad.qn765.
Additional Information
How to cite this article: Cator, L. J. et al. Immune response and insulin signalling alter mosquito feeding behaviour to enhance malaria transmission potential. Sci. Rep. 5, 11947; doi: 10.1038/srep11947 (2015).
References
Cator, L. J., Lynch, P. A., Read, A. F. & Thomas, M. B. Do malaria parasites manipulate mosquitoes? Trends Parasitol. 28, 466–470 (2012).
Cator, L. J. et al. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. R. Soc. B. 280, 20130711 (2013).
Smallegange, R. C. et al. Malaria infected mosquitoes express enhanced attraction to human odor. PLoS One 8, e63602 (2013).
Cator, L. J., Lynch, P. A., Thomas, M. B. & Read, A. F. Alterations in mosquito behavior by malaria parasites: potential impact on force of infection. Malar. J. 13, 1–11 (2014).
Schwartz, A. & Koella, J. C. Trade-offs, conflicts of interest and manipulation in Plasmodium-mosquito interactions. Trends Parasitol. 17, 189–194 (2001).
Moore, J. Parasites and the Behavior of Animals. (Oxford University Press, USA, 2002).
Rossignol, P. A., Ribeiro, J. M. & Spielman, A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am. J. Trop. Med. Hyg. 33, 17–20 (1984).
Lefevre, T. et al. Malaria Plasmodium agent induces alteration in the head proteome of their Anopheles mosquito host. Proteomics 7, 1908–1915 (2007).
Murdock, C. C., Moller-Jacobs, L. L. & Thomas, M. B. Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proc. Biol. Sci.B. 280, 20132030 (2013).
Root, C. M., Ko, K. I., Jafari, A. & Wang, J. W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–144 (2011).
Marquez, A. G. et al. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 173, 303–312 (2011).
Hogg, J. C. & Hurd, H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s. l. in north east Tanzania. Parasitology 114, 325–331 (1997).
Lefèvre, T. et al. Exploiting host compensatory responses: the ‘must’ of manipulation? Trends Parasitol. 24, 435–439 (2008).
Ricklefs, R. E. & Wikelski, M. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 (2002).
Okech, B. et al. Influence of sugar availability and indoor microclimate on survival of Anopheles gambiae (Diptera: Culicidae) under semifield conditions in Western Kenya. J. Med. Entomol. 40, 657–663 (2003).
Straif, S. & Beier, J. Effects of sugar availability on the blood-feeding behavior of Anopheles gambiae. J. Med. Entomol. 33, 608–612 (1996).
Rera, M., Azizi, M. & Walker, D. Organ-specific mediation of lifespan extension: more than a gut feeling? Ageing Res. Rev. 12, 436–444 (2013).
Erion, R. & Sehgal, A. Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 4, 1–6 (2013).
DiAngelo, J. R., Bland, M. L., Bambina, S., Cherry, S. & Birnbaum, M. J. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 20853–20858 (2009).
Nässel, D. R. Insulin-producing cells and their regulation in physiology and behaviour of Drosophila. Can. J. Zool. 90, 476–488 (2012).
Pakpour, N. et al. Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum. Infect. Immun. 80, 2141–2149 (2012).
Brown, M. et al. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 105, 5716–5721 (2008).
Badisco, L., Van Wielendaele, P. & Vanden Broeck, J. Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol. 4, 1–16 (2013).
Luckhart, S. et al. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathog 9, e1003180 (2013).
Hauck, E. et al. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect 15, 775–787 (2013).
Nyasembe, V. O. et al. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr. Biol. 24, 217–221 (2014).
Anderson, R. A., Koella, J. C. & Hurd, H. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc. R. Soc. Lond. B Biol. Sci. 266, 1729–1733 (1999).
Koella, J. C. & Packer, M. J. Malaria parasites enhance blood-feeding of their naturally infected vector Anopheles punctulatus. Parasitol. 113, 105–110 (1996).
Read, A. F. et al. How do make evolution-proof insecticides for malaria control. PLoS Biol. 7, e1000058 (2009).
Murdock, C. C. et al. Complex effects of temperature on mosquito immune function. Proc. Biol. Sci. B. 279, 3357–3366 (2012).
Oliveira, G. A., Lieberman, J. & Barillas-Mury, C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science 335, 856–859 (2012).
Drexler, A. et al. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog 10, e1004231 (2014).
Coggins, S. A., Estévez-Lao, T. Y. & Hillyer, J. F. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Dev. Comp. Immunol. 37, 390–401 (2012).
Pietri, J. E., Cheung, K. W. & Luckhart, S. Targeted knockdown of mitogen-activated protein kinase (MAPK) signaling in the midgut of Anopheles stephensi mosquitoes using antisense morpholinos. Insect Mol. Biol. 23, 558–565 (2014).
Gazdar, A. F. et al. Continuous, clonal insulin- and somatostatin-secreting cell lines established a transplantable rate islet cell tumor. Proc. Natl. Acad. Sci. U.S.A. 77, 3519–3523 (1980).
Paaijmans, K. P., Read, A. F. & Thomas, M. B. Understanding the link between malaria risk and climate. Proc. Natl. Acad. Sci. U.S.A. 106, 13844–13849 (2009).
Acknowledgements
We thank Janet Teeple, Derek Sim and Matthew Jones for technical support. This work was supported by NIH grants AI096036-01 to MBT, AI073745 and AI073745 to SL, AI107263 and NSF dissertation grant 1310194 to JEP.
Author information
Authors and Affiliations
Contributions
The behaviour experiments were conceived of by L.J.C., A.F.R. and M.B.T., designed by L.J.C., C.C.M., A.F. and M.B.T., performed and analyzed by L.J.C. and C.C.M. and funded by M.B.T. The ILP experiments were conceived of by J.E.P., S.L. and E.L., funded by S.L., designed by S.L., J.E.P. and E.L. and performed by J.E.P. and analyzed by J.E.P. and E.L. Survival experiments and analyses were conceived of by J.R.O., L.J.C., A.F.R. and M.B.T. J.R.O. performed survival experiments. All authors wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cator, L., Pietri, J., Murdock, C. et al. Immune response and insulin signalling alter mosquito feeding behaviour to enhance malaria transmission potential. Sci Rep 5, 11947 (2015). https://doi.org/10.1038/srep11947
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11947
This article is cited by
-
Evaluation of insemination, blood feeding, and Plasmodium vivax infection effects on locomotor activity patterns of the malaria vector Anopheles darlingi (Diptera: Culicidae)
Parasitology Research (2024)
-
Use of novel lab assays to examine the effect of pyrethroid-treated bed nets on blood-feeding success and longevity of highly insecticide-resistant Anopheles gambiae s.l. mosquitoes
Parasites & Vectors (2022)
-
Mosquito host-seeking diel rhythm and chemosensory gene expression is affected by age and Plasmodium stages
Scientific Reports (2022)
-
Agent-based modelling of complex factors impacting malaria prevalence
Malaria Journal (2021)
-
Avian malaria alters the dynamics of blood feeding in Culex pipiens mosquitoes
Malaria Journal (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.