Abstract
We have developed new methods enabling in vivo localization and identification of metabolites through their 1H NMR signatures, in a drosophila. Metabolic profiles in localized regions were obtained using HR-MAS Slice Localized Spectroscopy and Chemical Shift Imaging at high magnetic fields. These methods enabled measurement of metabolite contents in anatomic regions of the fly, demonstrated by a decrease in β-alanine signals in the thorax of flies showing muscle degeneration.
Similar content being viewed by others
Introduction
High-resolution magic angle spinning (HR-MAS) proton nuclear magnetic resonance (1H NMR) spectroscopy is a very efficient non-invasive analytical tool for investigating the metabolic profiles of a variety of biological systems1,2,3,4,5,6,7,8,9,10,11,12,13,14. This technique, which removes line broadenings due to magnetic susceptibility effects and residual anisotropic interactions in heterogeneous semi-solid samples, provides 1H high resolution NMR spectra revealing the metabolite contents of unprocessed tissue and also of living cells and small organisms. For example, it has been used to study intact tumors6,7,8, brain tissue affected by neurodegenerative disorders9 and to obtain in vivo metabolic profiles of bacteria10, C. elegans worms11,12, or Drosophila melanogaster flies13,14. For such small organisms, 1H HR-MAS NMR offers a direct access to metabolic phenotypes. However, the one-dimensional (1D) spectra of whole organisms remain complex and, while several metabolites can be directly identified and quantified, some 1H peaks cannot be assigned to specific molecules due to spectral overlap. This limitation is usually overcome using appropriate two-dimensional (2D) homonuclear (1H-1H) and/or heteronuclear (1H-13C) correlation experiments which provide enhanced spectral resolution allowing distinct overlapping signals to be discriminated and assigned1,2,3,8,13,14.
Although these HR-MAS methods enable metabolic profiling in small organisms, they do not enable localizing metabolites in different parts of the body or specific organs. Adding spatial resolution provides important additional information, as it allows assigning metabolites to specific regions of the organism and even evidencing subtle change of metabolite concentrations in these regions. However, in vivo localized NMR measurements in small organisms require much higher spatial resolution than that needed to study larger animal models. In this study, we introduce two new 1H HR-MAS NMR methods to spatially localize metabolites in a small living organism and demonstrate their promising applications in in vivo metabolic profiling in the case of drosophila, a widely used genetic model.
Results and Discussion
The methods developed, 1H HR-MAS Slice Localized Spectroscopy (SLS) and HR-MAS Chemical Shift Imaging (CSI), provide direct information about the spatial distribution of metabolites along the anteroposterior body axis of the specimen studied. As mentioned above, localization in small organisms such as drosophila requires increased spatial resolution and thus high sensitivity. The experiments therefore greatly benefit from the use of a high magnetic field (17.6 T) which enhances both sensitivity and spectral resolution. Performing in vivo HR-MAS measurements in drosophila also requires a viable environment minimizing the centrifugal forces induced by rapid sample spinning. For this purpose, the anteroposterior axis of the fly is aligned along the spinning axis by positioning the insect in a shaped insert in the center of the rotor. This allows the use of spinning frequencies up to 2.7 kHz while keeping the fly alive (without observable damage). Under these experimental conditions, spatial localization along the anteroposterior body axis of the living fly is obtained simply using pulsed magnetic field gradients along the MAS axis corresponding to the usual orientation of gradient coils in HR-MAS NMR probes. With HR-MAS SLS, a localized 1D 1H spectrum is obtained by selecting a transverse slice of the fly body with a defined thickness (Fig. 1a), (Figure 1 is not released under a Creative Commons Attribution 4.0 Unported License. This image is licensed under a separate, Creative Commons Attribution-ShareAlike 4.0 Unported License. To view a copy of this licence visit http://creativecommons.org/licenses/by-sa/4.0/), while HR-MAS CSI provides a 2D map of spatially arrayed 1H spectra along the whole fly body (Fig. 2). Regarding sensitivity, these two methods are complementary since the former allows recording the spectrum of a specific region of interest with a high signal-to-noise ratio, while the latter gives complete spatial distribution of metabolites at the cost of experimental time.
HR-MAS SLS experiments.
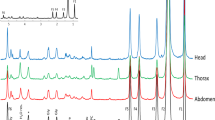
(a) Rotor design allowing the alignment of the anteroposterior axis of the fly body along the MAS axis. The purple arrow indicates the pulsed field gradient direction used for spatial localization. The blue, green and red zones correspond to the head, thorax and selected abdomen slices, respectively. (b) In vivo 1H HR-MAS spectra of a whole Oregon-R female drosophila (W) and corresponding in vivo 1H HR-MAS SLS spectra of the head (H), thorax (T) and abdomen (A) with slice thicknesses of 340, 340 and 680 µm, respectively. The asterisks and the crosses indicate spinning sidebands and unassigned resonances, respectively. The localized spectra of the head, thorax and abdomen were magnified by a factor of 16, 8 and 4, respectively. β-Ala, β-alanine; Gly, glycerol; L = lipids; L1, CH3; L2, (CH2)n; L3, CH2CCO; L4, CH2C = ; L5, CH2CO; L6, CH = CH; PC, phosphocholine; PE, phosphoethanolamine; Tau, taurine; Tre, trehalose. (c) in vivo 1H HR-MAS SLS spectra (1.8–4.2 ppm range) of the thorax of up101 (top) and Oregon-R (bottom) female flies. (d) Integrated intensities of the 1H β-alanine signal (resonance at 2.56 ppm) of the thorax measured for a series of 10 Oregon-R flies (red squares) and 10 up101 mutants (blue diamonds). Each point corresponds to the measurement for a single fly. All spectra were recorded at a magnetic field of 17.6 T with a spinning frequency of 2630 Hz. (Figure 1 is not released under a Creative Commons Attribution 4.0 Unported License. This image is licensed under a separate, Creative Commons Attribution-ShareAlike 4.0 Unported License. To view a copy of this licence visit http://creativecommons.org/licenses/by-sa/4.0/).
HR-MAS CSI experiments.
(a) In vivo1H 2D HR-MAS CSI spectra of female (left, body length of ~ 3 mm) and male (right, body length of ~ 2.5 mm) Oregon-R drosophila. In the 2D maps, the regions corresponding to the head (H), thorax (T) and abdomen (A) are separated by the dashed lines. Sixteen contour levels are plotted with a top contour of 5% of the maximum intensity ((CH2)n resonance at 1.3 ppm) and a dividing factor of 1.22. β-ala: β-alanine; Tau: Taurine; Gly: glycerol. (b) Left:1H 1D HR-MAS spectra of dissected organs of 2 to 5 Oregon-R males. Green, orange and brown spectra correspond to testis, paragonia and penis, respectively. Middle: Scheme of the male reproductive system (dorso-lateral view oriented anterior top, from reference 24). Te: testis, Pa: paragonia, Pe: penis apparatus. Right: 1D sum along the spatial dimension (vertical) of the 2D 1H HR-MAS CSI spectrum of the male fly for the two specific regions corresponding to the middle (black) and posterior (blue) parts of the abdomen. Ac, acetate (or acetyl); Gal, galactoside; L4, CH2C = ; L5, CH2CO; PC, phosphocholine; PE, phosphoethanolamine; Tre, trehalose. All spectra were recorded at a magnetic field of 17.6 T. The spinning frequency was 2630 Hz for in vivo experiments and 4000 Hz for dissected organs.
To illustrate the efficiency of the 1H HR-MAS Slice Localized Spectroscopy experiment, we recorded spatially-resolved spectra for the three main anatomical parts of the fly. As shown in Figure 1b, the 1D localized spectra of the head, thorax (340 µm slice thickness each) and abdomen (680 µm slice thickness) clearly evidence significant differences in 1H signal intensities reflecting variations in metabolite contents, with some appearing mainly localized in a specific region of the body. In drosophila, β-alanine is required in hard tissues for cuticle melanization and also for vision and inactivation of biogenic amines in the nervous system15,16. 1H HR-MAS SLS measurements reveal here that the largest amount of β-alanine observed in soft tissues was specifically localized in the thorax of the fly (Fig. 1b). This in vivo observation was further confirmed by recording conventional 1H HR-MAS spectra of dissected thorax (Supplementary Fig. 1). Localized 1H HR-MAS experiments were also performed for a drosophila model of muscle degeneration. A mutant in upheld gene (up101) showing degenerative muscle hypercontraction of indirect flight muscles was used17,18. To highlight changes in metabolic profiles resulting from muscle degeneration, the 1H HR-MAS SLS spectrum of the thorax was recorded in vivo. A significant decrease in the β-alanine concentration in the thorax (about four times less) of the mutant with respect to Oregon-R was observed (Fig. 1c). Experiments were performed for 10 up101 mutants (males or females) and this result was found to be highly reproducible (Fig. 1d). Therefore, the marked decrease in the β-alanine content in the thorax of mutants, evidenced by the localized measurements, appeared as a clear metabolic signature of muscle degeneration in the model used.
Spatial localization of tissue metabolites in a living drosophila was also achieved using 1H HR-MAS Chemical Shift Imaging. As shown in Figure 2, HR-MAS CSI spectra directly reflect the metabolite distribution along the fly's anteroposterior body axis (spatial resolution of 189 µm). In these 2D maps, the 1H NMR spectroscopic signature along the horizontal axis is correlated with the spatial position along the vertical axis. The 1H peaks associated with the same molecule thus appear on the same ordinate, in the same way as the 1H signals of different molecules localized in the same region. The three main anatomic parts of the drosophila (head, thorax and abdomen) are easily distinguishable in the 2D CSI spectra confirming that some metabolites are predominantly localized in one of these specific regions (Fig. 2a). For example, the spectra of both male and female Oregon-R flies show that β-alanine and taurine are mainly localized in the thorax, while glycerol is mostly found in the abdomen and to a less extend in the head. These measurements also reveal that significant concentrations of phosphoethanolamine (PE), acetate (and/or acetyl) group (Ac), as well as several 1H peaks assigned to a galactoside19 (Gal), are specifically localized in male but not in female abdomens suggesting that they correspond to metabolites characteristic of the male reproductive tract. Moreover, the HR-MAS CSI spectrum of the male fly evidences that the highest contents of PE and Ac are localized in the same region of the male abdomen. This region is different to that specifically associated with galactoside (Fig. 2b), indicating that these molecules are not present in the same organ of the male reproductive tract. The male reproductive system consists of gonads (testis), accessory structures (paragonia, seminal vesicles) and genital ducts (ejaculatory duct, sperm pump and penis apparatus) (Fig. 2b).1H HR-MAS spectra of isolated dissected organs (testis, paragonia and penis apparatus) were recorded and the 1H peaks characteristic of Ac and PE were mainly observed in the penis apparatus, while intense signals of galactoside and phosphocholine were detected in the paragonia and testis, respectively (Fig. 2b). The distribution of metabolites along the anteroposterior axis of the fly body observed in the 2D HR-MAS CSI spectra thus clearly reflects the male abdomen anatomy. This method therefore proves to be sensitive enough to identify a fingerprint of an organ, suggesting that it could be used for metabolomic studies of pathologies at the organ level.
In summary, these spatially-resolved 1H HR-MAS NMR methods localize metabolites in a living drosophila efficiently. The 1D HR-MAS SLS experiment provides the signature of the most abundant metabolites localized in a single slice of the fly and 2D HR-MAS CSI enables complete mapping of metabolites in the whole individual. These methods, using spatial encoding along the anteroposterior axis of the fly body, have been demonstrated to be sensitive enough to evidence a metabolic fingerprint of a specific organ. Using a conventional gradient HR-MAS probe or a standard MAS probe in a three-axis field gradient system, these methods offer a unique opportunity to highlight variation of metabolite contents in specific regions of living small animal models. Further applications in the study of drosophila models of human diseases are in progress.
Methods
Sample preparation
Drosophila melanogaster strain Oregon-R and mutant strain up101 were obtained from Bloomington Drosophila Stock Center (Indiana University 1001 E. Third St., Bloomington, IN 47405-7005 USA). Fly stocks were maintained in our laboratory by mass culture on standard medium at 22°C (362g cornmeal, 200g dry yeast, 60g agar and 150mL of a 10% solution of methyl-4-hydroxybezoate in ethanol, water qsp 4 L). Experiments were performed on adult flies (7 to 10 days old).
To perform all in vivo HR-MAS experiments, a fly was placed in a cylindrical PTFE insert (outer diameter of 2.6 mm, external length of 7 mm, inner diameter of 1.2 mm and internal length of 4 mm) located in the center of the rotor, such that the anteroposterior axis of the fly was aligned along the spinning axis. A petri dish, filled with ice was covered with aluminum foil and the fly was put on this foil to cool for anesthesia purposes. The aluminum foil was perfectly dry to avoid the fly sticking to it. The anesthetized drosophila was then placed in the insert with small tweezers under a magnifying glass. The 1H NMR experiments were performed at 3°C to keep the fly anesthetized and alive20. After the experiments, the insert was opened to check that the fly was alive without observable damage.
To study the dissected parts of drosophila, flies (2 to 5) were first dissected in saline solution (9g/L NaCl in D2O) under a stereomicroscope. The organs, heads or thorax were then placed in a 4 mm HR-MAS rotor containing the same saline solution. 1H HR-MAS experiments were performed at 3°C.
NMR experiments
All 1H HR-MAS NMR experiments were carried out on a Bruker AVANCE III spectrometer operating at a magnetic field of 17.6 T (corresponding to 1H Larmor frequency of 750.13 MHz). In vivo measurements were performed using a Bruker Micro 2.5 gradient system (3 axis, 2.5 Gauss/cm/A, 40A/axis), with a Bruker 3.2 mm double resonance MAS microimaging probe. Proton-free commercial rotors (zirconia), caps (PTCFE) and home-made shaped inserts (PTFE) were employed. For localized experiments, a pulsed field gradient was applied along the MAS axis using a combination of the three orthogonal gradients (Gx, Gy, Gz) of the microimaging system such that  . All in vivo measurements were conducted at a spinning frequency of 2630 Hz. The spectra of the dissected parts of the fly were recorded at a spinning rate of 4000 Hz using a Bruker 4.0 mm double resonance MAS probe and commercial HR-MAS rotors and caps.
. All in vivo measurements were conducted at a spinning frequency of 2630 Hz. The spectra of the dissected parts of the fly were recorded at a spinning rate of 4000 Hz using a Bruker 4.0 mm double resonance MAS probe and commercial HR-MAS rotors and caps.
For all experiments, the spectral width was set to 10000 Hz and the 90° and 180° pulse durations were 25 µs and 50 µs, respectively (rf-field strength of 10 KHz). 1H chemical shifts were referenced using the resonance of the (CH2)n of drosophila fatty acids at 1.3 ppm13 as an internal reference. Water suppression (WS) was achieved using a selective presaturation of the H2O resonance (low-power pulse duration of 1 s). Data were processed with zero filling of twice the number of real points. Exponential apodization with a 2 Hz line broadening was applied prior Fourier transform. Uncertainties on isotropic chemical shift measurements are 0.02 ppm (i.e. 15 Hz).
The 1H HR-MAS spectra of living drosophila and of the dissected parts of the fly were recorded using a spin echo sequence (echo time TE = 0.19 ms). 512 transients were acquired with a recycle delay of 2 s, corresponding to an experimental time of ~ 20 min. Assignment of the 1H resonances was performed using 2D through-bond 1H homonuclear correlation HR-MAS spectra and data from the literature13,19,21. (Supplementary Fig. 2, Supplementary Table 1) The 2D 1H–1H correlation spectra were obtained using the adiabatic TOBSY pulse sequence22. The mixing time was set to 57 ms (corresponding to 150 rotor periods) with a rf-field strength of 15.8 kHz. 1024 rotor-synchronized time increments with 32 transients each were recorded using a recycle delay of 1.5 s.
The 1H HR-MAS Slice Localized Spectroscopy (SLS) measurements in living drosophila were performed using the pulse sequence shown in Supplementary Figure 3a. It consisted in a spin echo sequence with a refocusing-SNOB23 180° selective pulse applied during the pulsed field gradient period. The appropriate coherence pathway was selected with a 16-step phase cycle. The echo time was 3.04 ms (8 rotor periods) and the gradient was applied during 6 rotor periods at a strength of GMAS = 139 Gauss/cm (i.e. 80 Gauss/cm per axis). The recycle delay was set to 2 s. The frequency and bandwidth of the selective pulse, allowing choice of the position and thickness of the selected slice along the anteroposterior fly axis, were determined from a 1D water density image of the whole fly along its anteroposterior axis. This 1D image (recorded using the sequence in Supplementary Figure 3c with GMAS = 139 Gauss/cm) is not uniform and evidences separate regions attributed to the head, thorax and abdomen of the fly (Supplementary Fig. 4a). 1D water density images of the selected spatial region were acquired with the sequence shown in Supplementary Figure 3d. The 1D profiles of the slices centered on the head and thorax (340 µm thickness each) and on the abdomen (680 µm thickness) are shown in Supplementary Figure 4b. The 1H HR-MAS SLS spectra of these slices were recorded with 2048 transients for the head and thorax and 1024 transients for the abdomen (experimental times of 72 and 36 min, respectively). A comparison of the in vivo1H HR-MAS SLS spectrum of the thorax and the conventional 1H HR-MAS spectrum of a single dissected thorax is shown in Supplementary Figure 1 (wild type male fly).
The 2D 1H HR-MAS Chemical Shift Imaging (CSI) spectra were obtained using the pulse sequence depicted in Supplementary Figure 3b. The echo time was 10.6 ms. 32 gradient increments with 256 transients each were recorded with a recycle delay of 1.5 s. The duration of the GMAS pulse was 400 µs with a maximum strength of 29.5 Gauss/cm. The corresponding field of view was 5 mm (the length of the drosophila being ~ 2.5–3 mm) with a spatial resolution of 189 µm. The total experimental time was ~ 3.5 h.
References
Lindon, J. C., Beckonert, O. P., Holmes, E. & Nicholson, J. K. High-resolution magic angle spinning NMR spectroscopy: Application to biomedical studies. Prog. Nucl. Magn. Reson. Spectrosc. 55, 79–100 (2009).
Beckonert, O. et al. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat. Protoc. 5, 1019–1032 (2010).
Weybright, P., Millis, K., Campbell, N., Cory, D. G. & Singer, S. Gradient, high-resolution, magic angle spinning 1H nuclear magnetic resonance spectroscopy of intact cells. Magn. Reson. Med. 39, 337–345 (1998).
Waters, N. J. et al. High-resolution magic angle spinning (1)H NMR spectroscopy of intact liver and kidney: optimization of sample preparation procedures and biochemical stability of tissue during spectral acquisition. Anal. Biochem. 282, 16–23 (2000).
Swanson, M. G. et al. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn. Reson. Med. 55, 1257–1264 (2006).
Cheng, L. L. Chang, I. W., Louis, D. N. & Gonzalez, R. G. Correlation of high-resolution magic angle spinning proton magnetic resonance spectroscopy with histopathology of intact human brain tumor specimens. Cancer Res. 58, 1825–1832 (1998).
Sitter, B. Sonnewald, U., Spraul, M., Fjosne, H. E. & Gribbestad, I. S. High-resolution magic angle spinning MRS of breast cancer tissue. NMR Biomed. 15, 327–337 (2002).
Martínez-Bisbal, M. C. et al.1H and 13C HR-MAS spectroscopy of intact biopsy samples ex vivo and in vivo 1H MRS study of human high grade gliomas. NMR Biomed. 17, 191–205 (2004).
Cheng, L. L. et al. Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. USA. 94, 6408–6413 (1997).
Righi, V. Constantinou, C., Kesarwani, M., Rahme, L. G. & Tzika, A. A. Live-cell high resolution magic angle spinning magnetic resonance spectroscopy for analysis of metabolomics. Biomed. Rep. 1, 707–712 (2013).
Blaise, B. J. et al. Metabotyping of Caenorhabditis elegans reveals latent phenotypes. Proc. Natl. Acad. Sci. USA 104, 19808–19812 (2007).
Wong, A. et al. μHigh resolution-magic-angle spinning NMR spectroscopy for metabolic phenotyping of Caenorhabditis elegans. Anal. Chem. 86, 6064–6070 (2014).
Righi, V. et al. In vivo high-resolution magic angle spinning magnetic resonance spectroscopy fo Drosophila melanogaster at 14.1T shows trauma in aging and in innate immune-deficiency is liked to reduced insulin signaling. Int. J. Mol. Med. 26, 175–184 (2010).
Righi, V. et al. In vivo high-resolution magic angle spinning proton NMR spectroscopy of Drosophila melanogaster flies as a model system to investigate mitochondrial dysfunction in Drosophila GST2 mutantsInt J. Mol. Med. 34, 327–333 (2014).
Jacobs, M. E. Influence of beta-alanine on ultrastructure, tanning and melanization of Drosophila melanogaster cuticles Biochem. Genet. 18, 65–76 (1980).
Wright, T. R. The genetics of biogenic amine metabolism, sclerotization and melanization in Drosophila melanogaster. Adv. Genet. 24, 127–222 (1987).
Fyrberg, E. A. Fyrberg, C. C., Beall, C. & Saville, D. L. Drosophila melanogaster troponin-T mutations engender three distinct syndromes of myofibrillar abnormalities. J. Mol. Biol. 216, 657–675 (1990).
Nongthomba, U., Cummins, M., Clark, S., Vigoreaux, J. O. & Sparrow, J. C. Suppression of muscle hypercontraction by mutations in the myosin heavy chain gene of Drosophila melanogaster. . Genetics 164, 209–222 (2003).
Chen, P. S., Fales, H. M., Levenbook, L., Sokoloski, E. A. & Yeh, H. J. Isolation. and characterization of a unique galactoside from male Drosophila melanogaster. . Biochemistry 16, 4080–4085 (1977).
Czajka, M. C. & Lee, R. E. Jr. A rapid cold-hardening response protecting against cold shock injury in Drosophila melanogaster. J Exp Biol. 148, 245–54 (1990).
Wishart, D. S. et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 37, D603–10 (2009).
Andronesi, O. C. Mintzopoulos, D., Struppe, J., Black, P. M. & Tzika, A. A. Solid-state NMR adiabatic TOBSY sequences provide enhanced sensitivity for multidimensional high-resolution magic-angle-spinning 1H MR spectroscopy. J. Magn. Res. 193, 251–258 (2008).
Kupce, E., Boyd, J. & Campbell, I. D. Short selective pulses for biochemical applications. J. Magn. Res. B 106, 300–303 (1995).
Hartenstein, V. in Atlas of Drosophila development. 50–51 (Cold Spring Harbor Laboratory Press, New York., 1993).
Acknowledgements
Financial support from the TGIR-RMN-THC FR3050 CNRS for conducting research is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
V.S.K. and F.F. developed the experiments. V.S.K., N.J, F.L., M.Y., F.S, M.D., J.C.B performed the NMR experiments. M.D. performed the laboratory growth of the drosophila and dissection for the ex vivo analyses S.M. and D.M. contributed to analysis of results and discussion. V.S.K., F.F., M.D, J.C.B. wrote the manuscript.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sarou-Kanian, V., Joudiou, N., Louat, F. et al. Metabolite localization in living drosophila using High Resolution Magic Angle Spinning NMR. Sci Rep 5, 9872 (2015). https://doi.org/10.1038/srep09872
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09872
This article is cited by
-
NMR spectroscopy for metabolomics in the living system: recent progress and future challenges
Analytical and Bioanalytical Chemistry (2024)
-
Spatially-resolved metabolic profiling of living Drosophila in neurodegenerative conditions using 1H magic angle spinning NMR
Scientific Reports (2020)
-
Spatial molecular-dynamically ordered NMR spectroscopy of intact bodies and heterogeneous systems
Communications Chemistry (2020)
-
Simultaneous metabolic mapping of different anatomies by 1H HR-MAS chemical shift imaging
Analytical and Bioanalytical Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.