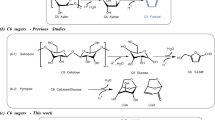
Abstract
For the first time, renewable high density aviation fuels were synthesized at high overall yield (95.6%) by the Guerbet reaction of cyclopentanol which can be derived from lignocellulose, followed by the hydrodeoxygenation (HDO). The solvent-free Guerbet reaction of cyclopentanol was carried out under the co-catalysis of solid bases and Raney metals. Among the investigated catalyst systems, the combinations of magnesium-aluminium hydrotalcite (MgAl-HT) and Raney Ni (or Raney Co) exhibited the best performances. Over them, high carbon yield (96.7%) of C10 and C15 oxygenates was achieved. The Guerbet reaction products were further hydrodeoxygenated to bi(cyclopentane) and tri(cyclopentane) over a series of Ni catalysts. These alkanes have high densities (0.86 g mL−1 and 0.91 g mL−1) and can be used as high density aviation fuels or additives to bio-jet fuel. Among the investigated HDO catalysts, the 35 wt.% Ni-SiO2-DP prepared by deposition-precipitation method exhibited the highest activity.
Similar content being viewed by others
Introduction
With the depletion of fossil energy and the increase of social concern about the environmental problems (such as CO2 and SO2 emissions) from the utilization of fossil energy, the catalytic conversion of renewable biomass to fuels1,2,3 and chemicals4,5,6,7,8 becomes a hot topic. Lignocellulose is the main component of agriculture wastes and forest residues. Jet fuel is one of the most often used transport fuels nowadays. Pioneered by the previous work of Dumesic9,10,11, Huber12,13, Corma14,15 and their groups, the synthesis of jet fuel range alkanes with the lignocellulose derived platform chemicals has drawn tremendous attention16,17,18,19,20.
So far, most of the reported lignocellulosic bio-jet fuels are primarily composed of linear or branched chain alkanes. These alkanes have good thermal stability and excellent combustion efficiency. However, their densities (~0.76 g mL−1) and volumetric heating values are lower than those of conventional jet fuels, because the latter is a mixture of chain alkanes and cyclic hydrocarbons. Due to the strong ring strain, cyclic hydrocarbons (Especially the polycyclic hydrocarbons) have higher densities and volumetric heating values than those of chain alkanes21. In real application, these chain alkanes must be blended with conventional jet fuel to meet the specification of aviation fuel. In order to solve this problem, it is imperative to develop new routes for the synthesis of jet fuel range cyclic hydrocarbons with lignocellulosic platform chemicals22,23,24,25,26.
Cyclopentanol is the aqueous-phase selective hydrogenation product of furfural which has been manufactured on an industrial scale by the hydrolysis-dehydration of the hemicellulose in agriculture wastes and forest residues27. In the recent work of Xiao's group28, it was found that cyclopentanol can be produced at high carbon yield (93.4%) by the aqueous-phase selective hydrogenation of furfural over Cu-MgAlO3 catalyst. Due to its cyclic structure, cyclopentanol can be used as a potential feedstock for the synthesis of high density polycyclic aviation fuel. However, there is no report about the synthesis of high density aviation fuel with cyclopentanol.
In this work, a mixture of bi(cyclopentane) and tri(cyclopentane) with high densities (0.86 g mL−1 and 0.91 g mL−1) was first prepared at high overall carbon yield (95.6%) by the Guerbet reaction of cyclopentanol, followed by the hydrodeoxygenation (HDO) over the SiO2 loaded Ni catalysts under solvent free conditions.
Results and Discussion
Guerbet reaction
The solvent-free Guerbet reaction of cyclopentanol was carried out under the co-catalysis of Raney metal and different solid base catalysts. From the analysis of GC-MS and NMR (see Supplementary Fig. S1–S4), 2-cyclopentyl-1-cyclopentanol (i.e. compound 1 in Fig. 1), 2,5-dicyclopentylcyclopentanone (i.e. compound 2 in Fig. 1) and 2,5-dicyclopentylcyclopentanol (i.e. compound 3 in Fig. 1) were identified as the main products. These oxygenates can be used as the precursors for the lignocellulosic high density jet fuel.
Figure 2 demonstrates the carbon yields of C10 (i.e. compound 1) oxygenate and C15 oxygenates (i.e. compound 2 and 3) obtained under the co-catalysis of Raney Ni and a series of solid bases. Among these catalysts, the combination of Raney Ni and magnesium-aluminium hydrotalcite (MgAl-HT) exhibited the best catalytic activity. Over it, 86.6% carbon yield of C10 and C15 oxygenates was achieved. The activity sequence for the investigated catalyst systems is: Raney Ni + MgAl-HT > Raney Ni + CaO > Raney Ni + KF/Al2O3 > Raney Ni + MgO-ZrO2 > Raney Ni + MgO > Raney Ni + CeO2. This sequence is consistent with the activity sequence of these solid bases for the self aldol condensation of cyclopentanone22.
The influence of Raney metal was also studied. From the carbon yields of C10 oxygenate (i.e. compound 1) and C15 oxygenates (i.e. compound 2 and 3) demonstrated in Fig. 3, the combinations of Raney Ni (or Raney Co) with MgAl-HT exhibited the best performances among the investigated candidates. This result can be rationalized by the higher activity of Raney Ni (or Raney Co) for the dehydration of cyclopentanol.
Subsquently, the influence of MgAl-HT dosage on the carbon yields of C10 oxygenate (i.e. compound 1) and C15 oxygenates (i.e. compound 2 and 3) was also investigated. From the results shown in Fig. 4, the carbon yield of C10 oxygenate (i.e. compound 1) increased with the increasing of MgAl-HT dosage from 0.2 g to 0.6 g, then leveled off with the further increment of MgAl-HT dosage. In contrast, the carbon yield of C15 oxygenates (i.e. compound 2 and 3) (or the total carbon yield of C10 and C15 oxygenates) monotonously increased with the increment of MgAl-HT dosage from 0.2 g to 1.2 g. When 1.2 g MgAl-HT was used with 0.1 g Raney Ni catalyst, 72.5% carbon yield of C10 oxygenate (i.e. compound 1) and 24.2% carbon yield of C15 oxygenates (i.e. compound 2 and 3) (total carbon yield of C10 and C15 oxygenates: 96.7%) were achieved after reacting at 443 K for 8 h.
To get deeper insight of the individual roles of Raney metal and solid base in the Guerbet reaction of cyclopentanol, the catalytic performances of MgAl-HT and Raney Ni were investigated under the same reaction conditions. In the presence of MgAl-HT, no conversion of cyclopentanol was observed. Likewise, it was observed that Raney Ni only promoted the dehydrogenation of cyclopentanol (with cyclopentanone as the final product), neither C10 oxygenates nor C15 oxygenates was identified in the products. From these results, it can be seen that the synergy effect of solid base and Raney metal is necessary in the Guerbet reaction of cyclopentanol. The Raney metal promotes the dehydrogenation of cyclopentanol to cyclopentanone which further self-condenses to C10 and C15 oxygenates under the catalysis of solid base. As another function, the Raney metal also promotes the hydrogen transfer reactions between the cyclopentanol and the self aldol condensation products of cyclopentanone7,29, which may be the reason for the absence of α,β-unsaturated ketone (i.e. compound 4 and 5 in Fig. 1) in the Guerbet reaction products of cyclopentanol.
Hydrodeoxygenation (HDO)
The solvent-free HDO of the Guerbet reaction products was carried over SiO2 loaded Ni catalysts. From the results shown in Fig. 5, it was found that the preparation method has strong influence on the HDO activity of Ni catalysts. Over the 30 wt.% Ni-SiO2-DP catalyst, evidently higher carbon yields to bi(cyclopentane) (41.4%) and tri(cyclopentane) (12.8%) were achieved by the HDO of Guerbet products of cyclopentanol at 503 K and 6 MPa. These alkanes have high densities (bi(cyclopentane): 0.86 g mL−1; tri(cyclopentane) 0.91 g mL−1)30. In real application, they can be used as high-density aviation fuels or additives to increase the volumetric heating value of bio-jet fuel or bio-diesel. The activity sequence of different Ni catalysts is: 30 wt.% Ni-SiO2-DP > 30 wt.% Ni/SiO2-CIM > 30 wt.% Ni/SiO2-IM. According to the results of H2-chemisorption (see Supplementary Table S2), there is evident correspondence between the activity of Ni catalysts and the metal dispersions over these catalysts. Therefore, the excellent performance of 30 wt.% Ni-SiO2-DP can be rationalized by the higher dispersion of Ni over this catalyst.
Carbon yields of bi(cyclopentane) (black bar), tri(cyclopentane) (dark grey bar), C10 oxygenates (light grey bar) and C15 oxygenates (white bar) over different Ni catalysts (the Ni contents in the catalysts are ~30 wt.%).
Reaction conditions: 503 K, 6 MPa; 1.8 g of the Ni catalyst; Guerbet reaction products flow rate: 0.04 mL min−1; hydrogen flow rate: 120 mL min−1.
The effect of Ni content on the catalytic performance of Ni-SiO2-DP was investigated. From the results shown in Fig. 6, the carbon yields of bi(cyclopentane) and tri(cyclopentane) increase with the increment of Ni content. The activity of Ni-SiO2-DP catalyst reaches the maximum value at the Ni content of 35 wt.%. Over the 35 wt.% Ni-SiO2-DP catalyst, the Guerbet products of cyclopentanol (i.e. compound 1, 2 and 3 in Fig. 1) were completely converted, high carbon yield (98.9%) to bi(cyclopentane) and tri(cyclopentane) was achieved.
Carbon yields of bi(cyclopentane) (black bar), tri(cyclopentane) (dark grey bar), C10 oxygenates (light grey bar) and C15 oxygenates (white bar) over Ni-SiO2-DP as the function of Ni content.
Reaction conditions: 503 K, 6 MPa; 1.8 g of the catalyst; Guerbet reaction products flow rate 0.04 mL min−1; hydrogen flow rate: 120 mL min−1.
Conclusions
Renewable high density aviation fuels were first synthesized at high overall yield (95.6%) by the solvent-free Guerbet reaction of cyclopentanol under the co-catalysis of Raney metal and solid base, followed by the hydrodeoxygenation (HDO) over Ni catalyst. Among the investigated catalyst systems, the combinations of Raney Ni (or Raney Co) and MgAl-HT exhibited the highest activity for the Guerbet reaction of cyclopentanol. The Guerbet products were further hydrodeoxygenated to bi(cyclopentane) and tri(cyclopentane) over a series of SiO2 loaded Ni catalysts. Among them, the 35 wt.% Ni-SiO2-DP catalyst prepared by deposition precipitation method exhibited the best catalytic performance.
Methods
Preparation of catalysts
Magnesium-aluminium hydrotalcite (MgAl-HT) with Mg/Al atomic ratio = 2 was prepared according to the literature9. Before being used in Guerbet reaction, the hydrotalcite was calcined in N2 flow at 723 K for 8 h. CaO, MgO and CeO2 were commercial available. MgO-ZrO2 was prepared according to the literature31. Before the activity test, the CaO, MgO, CeO2 and MgO-ZrO2 catalysts were calcined in N2 flow at 873 K for 3 h. KF/Al2O3 catalyst was prepared according to the literature32 by the incipient wetness impregnation of γ-Al2O3 with an aqueous solution of KF, followed by drying in N2 flow at 393 K overnight. The KF content in the KF/Al2O3 catalyst is 40% by weight (denoted as 40 wt.%). Raney Co, Raney Fe, Raney Cu and Raney Ni were commercial available. Before being used in the Guerbet reaction, these Raney metal catalysts were washed with deionized water for several times until the pH of water was 7.
The SiO2 loaded Ni catalysts used in the hydrodeoxygenation (HDO) of Guerbet reaction products were prepared by the methods of impregnation, complexion impregnation and deposition-precipitation, respectively. The detail information for the preparation of these Ni catalysts was described in supplementary information.
Activity test
The solvent-free Guerbet reaction of cyclopentanol was conducted in a Teflon lined batch reactor. Typically, 4.0 g, 46.44 mmol cyclopentanol, 0.8 g solid base catalyst, 0.1 g Raney metal were used. Before reaction, the reactor was purged with argon for 3 times. After stirring at 443 K for 8 h, the reactor was quenched to room temperature. The products were taken out from the reactor, filtrated and analyzed by an Agilent 7890 A GC. The HDO of the Guerbet reaction products was carried out at 503 K in a 316 L stainless steel tubular flow reactor described in our previous work18,22,33,34,35. For each test, 1.8 g catalyst was used. Before the HDO process, the catalysts were reduced in-situ by hydrogen flow at 773 K for 2 h. After the reactor temperature was cooled down to 503 K and kept at this value for 0.5 h, the Guerbet reaction products (purified by vacuum distillation) were pumped into the reactor at 0.04 mL min−1 from the bottom along with hydrogen at a flow rate of 120 mL min−1. After coming out from the tubular reactor, the products became two phases in a gas-liquid separator. The gaseous products flowed through a back pressure regulator to maintain the system pressure at 6 MPa and were analyzed online by an Agilent 6890 N GC. The liquid products were drained periodically from the gas-liquid separator and analyzed by an Agilent 7890 A GC.
References
Huber, G. W., Iborra, S. & Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts and engineering. Chem. Rev. 106, 4044–4098 (2006).
Alonso, D. M., Bond, J. Q. & Dumesic, J. A. Catalytic conversion of biomass to biofuels. Green Chem. 12, 1493–1513 (2010).
Matson, T. D., Barta, K., Iretskii, A. V. & Ford, P. C. One-Pot Catalytic Conversion of Cellulose and of Woody Biomass Solids to Liquid Fuels. J. Am. Chem. Soc. 133, 14090–14097 (2011).
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Besson, M., Gallezot, P. & Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 114, 1827–1870 (2013).
Zheng, M., Pang, J., Wang, A. & Zhang, T. One-pot catalytic conversion of cellulose to ethylene glycol and other chemicals:From fundamental discovery to potential commercialization. Chin. J. Catal. 35, 602–613 (2014).
Wang, X. & Rinaldi, R. Exploiting H-transfer reactions with RANEY® Ni for upgrade of phenolic and aromatic biorefinery feeds under unusual, low-severity conditions. Energy Environ. Sci. 5, 8244–8260 (2012).
Macala, G. S. et al. Hydrogen Transfer from Supercritical Methanol over a Solid Base Catalyst: A Model for Lignin Depolymerization. ChemSusChem 2, 215–217 (2009).
Huber, G. W., Chheda, J. N., Barrett, C. J. & Dumesic, J. A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 308, 1446–1450 (2005).
Kunkes, E. L. et al. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 322, 417–421 (2008).
Bond, J. Q., Alonso, D. M., Wang, D., West, R. M. & Dumesic, J. A. Integrated Catalytic Conversion of γ-Valerolactone to Liquid Alkenes for Transportation Fuels. Science 327, 1110–1114 (2010).
Xing, R. et al. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions. Green Chem. 12, 1933–1946 (2010).
Olcay, H. et al. Production of renewable petroleum refinery diesel and jet fuel feedstocks from hemicellulose sugar streams. Energy Environ. Sci. 6, 205–216 (2013).
Corma, A., de la Torre, O., Renz, M. & Villandier, N. Production of High-Quality Diesel from Biomass Waste Products. Angew. Chem. Int. Ed. 50, 2375–2378 (2011).
Corma, A., de la Torre, O. & Renz, M. Production of high quality diesel from cellulose and hemicellulose by the Sylvan process: catalysts and process variables. Energy Environ. Sci. 5, 6328–6344 (2012).
Xia, Q.-N. et al. Pd/NbOPO4 Multifunctional Catalyst for the Direct Production of Liquid Alkanes from Aldol Adducts of Furans. Angew. Chem. Int. Ed. 53, 9755–9760 (2014).
Liu, D. & Chen, E. Y. X. Integrated Catalytic Process for Biomass Conversion and Upgrading to C12 Furoin and Alkane Fuel. ACS Catal. 4, 1302–1310 (2014).
Li, G. et al. Synthesis of renewable diesel with the 2-methylfuran, butanal and acetone derived from lignocellulose. Bioresour. Technol. 134, 66–72 (2013).
Sutton, A. D. et al. The hydrodeoxygenation of bioderived furans into alkanes. Nature Chem. 5, 428–432 (2013).
Harvey, B. G. & Quintana, R. L. Synthesis of renewable jet and diesel fuels from 2-ethyl-1-hexene. Energy Environ. Sci. 3, 352–357 (2010).
Harvey, B. G., Wright, M. E. & Quintana, R. L. High-Density Renewable Fuels Based on the Selective Dimerization of Pinenes. Energy & Fuels 24, 267–273 (2009).
Yang, J. et al. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem. Commun. 50, 2572–2574 (2014).
Yang, Y. et al. Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts. Green Chem. 15, 1932–1940 (2013).
Zhao, C., Camaioni, D. M. & Lercher, J. A. Selective catalytic hydroalkylation and deoxygenation of substituted phenols to bicycloalkanes. J. Catal. 288, 92–103 (2012).
Zhao, C., He, J. Y., Lemonidou, A. A., Li, X. B. & Lercher, J. A. Aqueous-phase hydrodeoxygenation of bio-derived phenols to cycloalkanes. J. Catal. 280, 8–16 (2011).
Wang, X. & Rinaldi, R. Solvent Effects on the Hydrogenolysis of Diphenyl Ether with Raney Nickel and their Implications for the Conversion of Lignin. ChemSusChem 5, 1455–1466 (2012).
Nakagawa, Y., Tamura, M. & Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 3, 2655–2668 (2013).
Zhou, M., Zeng, Z., Zhu, H., Xiao, G. & Xiao, R. Aqueous-phase catalytic hydrogenation of furfural to cyclopentanol over Cu-Mg-Al hydrotalcites derived catalysts: Model reaction for upgrading of bio-oil. J. Energy Chem. 23, 91–96 (2014).
Wang, X. & Rinaldi, R. A Route for Lignin and Bio-Oil Conversion: Dehydroxylation of Phenols into Arenes by Catalytic Tandem Reactions. Angew. Chem. Int. Ed. 52, 11499–11503 (2013).
Goheen, G. E. The Synthesis of Multicyclopentyls1. J. Am. Chem. Soc. 63, 744–749 (1941).
Barrett, C. J., Chheda, J. N., Huber, G. W. & Dumesic, J. A. Single-reactor process for sequential aldol-condensation and hydrogenation of biomass-derived compounds in water. Appl. Catal. B: Environ. 66, 111–118 (2006).
Li, J. T., Yang, W. Z., Chen, G. F. & Li, T. S. A facile synthesis of α,α'-bis(substituted benzylidene) cycloalkanones catalyzed by KF/Al2O3 under ultrasound irradiation. Synth. Commun. 33, 2619–2625 (2003).
Li, G. et al. Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan. Chem. Commun. 49, 5727–5729 (2013).
Li, G. et al. Synthesis of High-Quality Diesel with Furfural and 2-Methylfuran from Hemicellulose. ChemSusChem 5, 1958–1966 (2012).
Li, G. et al. Synthesis of renewable diesel range alkanes by hydrodeoxygenation of furans over Ni/Hβ under mild conditions. Green Chem. 16, 594–599 (2014).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21106143; 21277140; 21202163; 21476229), 100-talent project of Dalian Institute of Chemical Physics.
Author information
Authors and Affiliations
Contributions
T.Z. and N.L. designed the experiments. X.S. carried out the experiments with the help of G.L., W.W. and J.Y. A.W., X.W. and Y.C. analysed the data. All authors discussed the results and wrote the the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sheng, X., Li, N., Li, G. et al. Synthesis of high density aviation fuel with cyclopentanol derived from lignocellulose. Sci Rep 5, 9565 (2015). https://doi.org/10.1038/srep09565
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09565
This article is cited by
-
Insight into the production of aviation fuel by aldol condensation of biomass-derived aldehydes and ketones followed by hydrogenation
Biomass Conversion and Biorefinery (2024)
-
Calcinated conch shells combined with Raney Ni as high-performance catalyst for cyclopentanol Guerbet reaction
Journal of Material Cycles and Waste Management (2021)
-
Synthesis of jet fuel range branched cycloalkanes with mesityl oxide and 2-methylfuran from lignocellulose
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.