Abstract
Vanillin dehydrogenase (VDH) is a crucial enzyme involved in the degradation of lignin-derived aromatic compounds. Herein, the VDH from Corynebacterium glutamicum was characterized. The relative molecular mass (Mr) determined by SDS-PAGE was ~51kDa, whereas the apparent native Mr values revealed by gel filtration chromatography were 49.5, 92.3, 159.0 and 199.2kDa, indicating the presence of dimeric, trimeric and tetrameric forms. Moreover, the enzyme showed its highest level of activity toward vanillin at pH 7.0 and 30C and interestingly, it could utilize NAD+ and NADP+ as coenzymes with similar efficiency and showed no obvious difference toward NAD+ and NADP+. In addition to vanillin, this enzyme exhibited catalytic activity toward a broad range of substrates, including p-hydroxybenzaldehyde, 3,4-dihydroxybenzaldehyde, o-phthaldialdehyde, cinnamaldehyde, syringaldehyde and benzaldehyde. Conserved catalytic residues or putative cofactor interactive sites were identified based on sequence alignment and comparison with previous studies and the function of selected residues were verified by site-directed mutagenesis analysis. Finally, the vdh deletion mutant partially lost its ability to grow on vanillin, indicating the presence of alternative VDH(s) in Corynebacterium glutamicum. Taken together, this study contributes to understanding the VDH diversity from bacteria and the aromatic metabolism pathways in C. glutamicum.
Similar content being viewed by others
Introduction
Corynebacterium glutamicum, a fast growing Gram-positive soil bacterium, is one of the most important microorganisms in industrial biotechnology. Since its discovery, C. glutamicum has been widely used for industrial production of amino acids, vitamins, nucleotides and various other bio-based chemicals1. As a soil bacterium, recent studies have demonstrated that C. glutamicum is able to utilize a large variety of lignin derived aromatic compounds (e.g. vanillin, ferulate, p-coumarate, cinnamate, etc.) for growth2,3. The outstanding capability of C. glutamicum in assimilation of aromatic compounds provides it with a distinct advantage in using lignocellulosic hydrolysates as sustainable and inexpensive feedstocks in industrial fermentation, thanks to its capability to detoxify and assimilate great amounts of lignin derived aromatic inhibitors in lignocellulosic hydrolysates as an alternative source to sugars for carbon and energy.
The main lignin-derived aromatic inhibitors in lignocellulosic hydrolysates are ferulate, vanillin, p-coumarate, 4-hydroxybenzoic acid (4-HBA) and vanillic acid and most of which can be assimilated into TCA cycle intermediates by C. glutamicum3,4,5. Catabolism of ferulate follows a CoA-dependent non--oxidative pathway that contains feruloyl-CoA synthetase (Fcs) and enoyl-CoA hydratase/aldolase (Ech), yielding vanillin6. Vanillin is further converted into protocatechuate catalyzed by an aldehyde dehydrogenase (Vdh) and a demethylase (VanAB)7,8. Although some peripheral pathways converting various phenylpropanoids (such as vanillin, vanillate, caffeate, p-coumarate and cinnamate) to protocatechuate have been suggested in C. glutamicum and the genes vanAB encoding vanillate demethylase that catalyzes the conversion of vanillate to protocatechuate have been functionally identified3,6, the upstream vanillin dehydrogenase gene (vdh) has not been experimentally investigated.
The vanillin dehydrogenase is a critical enzyme for the degradation of lignin derived phenylpropanoids (such as vanillin, vanillate, caffeate, p-coumarate and cinnamate) and studies on vanillin dehydrogenase gene (vdh) in Gram-negtive bacteria have been well documented. For instance, vdh has been characterized in Pseudomonas fluorescens9, Pseudomonas putida10,11, Pseudomonas sp. strain HR19912 and Sphingomonas paucimobilis SYK-613,14. When the vdh gene was deleted in Pseudomonas fluorescens, it completely lost the ability to utilize ferulic acid, vanillin, p-coumarate as carbon source but not 4-hydroxybenzaldehyde9. In addition, experimental results have confirmed that the vdh gene is associated with the degradation of vanillin, benzaldehyde, p-hydroxybenzaldehyde, protocatechualdehyde in S. paucimobilis SYK-613,14. However, studies focusing on the vanillin dehydrogenase in Gram-positive bacteria were rare. This study indentified the gene coding for putative vanillin dehydrogenase in C. glutamicum and investigated the enzyme activity, substrate specificity and roles in catabolism of aromatic compounds in C. glutamicum, thus contributing to a deeper understanding of the aromatic metabolism pathways in C. glutamicum.
Results
Indentification of vdh gene from C. glutamicum genome and phylogenetic analysis
Based on BLAST Search and genome sequence analysis, the gene coding for a putative vanillin dehydrogenase (ncgl2578, named as vdhATCC13032 in this study) was identified, which composed of 1,455bp and encoded a protein of 484 amino acids with a theoretical molecular mass of 51.5kDa. The vdhATCC13032 shares 35%, 41% and 59% amino acid sequence identity with the vdh genes from Pseudomonas aeruginosa DK2, Rhodococcus jostii RHA1 and Pseudomonas fluorescens, respectively. To further assess the phylogenetic relationship between vdhATCC13032 and vdh genes from other bacteria, a multiple-sequence alignment was conducted using ClustalX 1.83 (Fig. 1). The results showed that the vdhATCC13032 from C. glutamicum forms an independent cluster on the phylogenetic tree and exhibits clear evolutionary distance with already verified vdh genes from other bacteria. These results suggested that vdh from C. glutamicum may therefore represent a new vanillin dehydrogenase branch and the vdhATCC13032 represents the first vanillin dehydrogenase characterized in detail within this gene family.
Rooted neihbor-joining tree constructed from the amino acid sequence of 14 vanillin dehydrogenases.
Boot strap confidence limits (expressed as percentage) are shown at nodes. Multiple-sequence alignment was done by using ClustalX 1.83 based on the amino acid sequences of the following enzymes: putative VDH of C. glutamicum ATCC13032 (NP_601867.1), benzaldehyde dehydrogenase of Rhodococcus erythropolis PR438,39, VDH of Rhodococcus jostii RHA140, VDH of Sphingomonas paucimobilis SYK-613,14, benzaldehyde dehydrogenase of Pseudomonas putida CSV8611,41, VDH of Pseudomonas putida WCS35842, VDH of Pseudomonas putida KT244010,43, VDH of Pseudomonas sp. HR19924, VDH of Amycolatopsis sp. ATCC 3911621, VDH of Pseudomonas fluorescens32,44, aldehyde dehydrogenase (NAD+) of Pseudonocardia sp. P1(ZP_08121488.1), benzaldehyde dehydrogenase (NAD+) of Saccharopolyspora erythraea NRRL 2338(YP_001105347.1), benzaldehyde dehydrogenase (NAD+) of Burkholderia cenocepacia AU 1054(YP_621190.1) and aldehyde dehydrogenase of Amycolatopsis mediterranei U32 (AMED_0881). Calculations were performed by using the neighbor-joining method. Enzymes with verified vanillin dehydrogenase activity are shown in boldface type.
Functional characterization of gene vdhATCC13032 in vivo
To further characterize the vanillin dehydrogenase activity of VDHATCC13032, a mutant strain (vdhATCC13032) was obtained by homologous recombination based gene knock-out. Growth analyses of wild type strain and vdhATCC13032 were conducted in liquid media at 30C, using different substrates such as vanillin (8mM), p-hydroxybenzaldehyde (8mM), 3,4-dihydroxybenzaldehyde (5mM), 3-hydroxybenzaldehyde (5mM), ferulic acid (3mM), caffeic acid (3mM), p-cresol (5mM), cinnamyl aldehyde (5mM) and syringaldehyde (2.5mM)) as the sole carbon and energy source. Compared to the wild type, the vdhATCC13032 mutant showed remarkably reduced ability to grow with the above mentioned aromatic compounds (Fig. 2). When complemented with plasmid pXMJ19-vdhATCC13032, the growth ability of the mutant strain could be restored close to that of the wild type (Fig. 2). However, the wild type, the vdhATCC13032 mutant and the complementary strain showed no difference when grown in p-cresol, cinnamyl aldehyde and syringaldehyde (data not shown).
Phenotypic characterization of vdh and the complemented strain vdh(pXMJ19-vdh) grown on mineral salts medium containing 8mM vanillin (A), 5mM 3-hydroxybenzaldehyde (B), 8mM p-hydroxy benzaldehyde (C), 5mM 3,4-dihydroxybenzaldehyde (D), 3mM ferulic acid (E) and caffeic acid (F), respectively.  WT(pXMJ19),
WT(pXMJ19),  vdh(pXMJ19),
vdh(pXMJ19),  vdh(pXMJ19-vdh).
vdh(pXMJ19-vdh).
Heterologous expression and molecular mass (Mr) estimation of VDHATCC13032
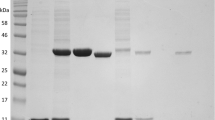
To further study the biochemical function of VDHATCC13032, the gene was PCR-amplified and heterologously expressed in E. coli. The recombinant strain carrying pET28a-vdhATCC13032 showed vanillin dehydrogenase activity and the purified recombinant VDH was estimated to have a relative Mr of about 51.1kDa, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 3A), consistent with the predicted Mr value. However, gel filtration chromatography analysis showed four peaks corresponding to molecular mass of 49.5, 92.3, 159.0 and 199.2kDa, respectively, indicating the native VDHATCC13032 existing as tetramers, trimers and dimers (Fig. 3B). The existence of VDHATCC13032 in tetramer, trimer and dimer was also confirmed by native PAGE analysis (Fig. 3C).
Molecular mass determination of the purified VDHATCC13032.
(A) SDS-PAGE analysis of VDHATCC13032. M: protein marker. Lane 1: cell extracts of E. coli/pET28a-ncg12578 (not induced); lane 2: cell extracts of E. coli/pET28a-ncg12578 (induced); Lanes 3: purified VDHATCC13032 after Ni-NTA affinity chromatography. (B) Gel filtration analysis of purified VDHATCC13032. Molecular weight standards from large to small weight: Thyroglobulin (bovine)(670kDa), -globulin (bovine)(158kDa), Ovalbumin (chicken)(44kDa), Myoglobin (horse)(17kDa) and Vitamin B12(1.35kDa). The molecular weight of purified VDHATCC13032 was estimated using the above molecular weight standards. (C) Typical appearance of native-PAGE obtained by loading 40g of purified VDHATCC13032. The position of the monomer (V1), dimer (V2), trimer (V3) and tetramer (V4) of VDH are indicated.
Biochemical properties of VDHATCC13032
Purified enzyme preparations of VDHATCC13032 were found to be stable and could be stored at 4C for 2 weeks without significant loss of activity. The influence of pH and temperature on the activity of VDHATCC13032 was investigated (Fig. 4). The highest activity was demonstrated to be at 30C in accordance with the temperature of physiological habitat of C. glutamicum whereas the optimum pH observed for reaction was pH 7.0 (in 100mM potassium phosphate buffer). Several aldehydes were selected as the potential substrates to test the substrate specificity and measure the activity of purified VDHATCC13032. VDHATCC13032 showed catalytic activity toward a broad range of tested substrates (Table S1), including vanillin, 3,4-dihydroxybenzaldehyde, 3-hydroxybenzaldehyde, p-hydroxybenzaldehyde, p-nitrobenzaldehyde, terephthalaldehyde and 2,4-dichlorobenzaldehyde; and VDHATCC13032 showed lower activities (less than 50% of that toward vanillin) toward o-phthaldialdehyde, cinnamaldehyde, syringaldehyde, benzaldehyde and benzenepropanal. However, phenylacetaldehyde, formaldehyde and aldehyde were not oxidized at detectable rates (rates lower than 5% of the enzyme activity with vanillin).
pH (A) and temperature (B) optimum of purified VDHATCC13032.
(A) The optimum pH was measured at room temperature in buffers with different pHs using vanillin as the substrate. The optimal pH was determined in various pHs of 100mM buffer as follows: glycine-HCl buffer (pH 34), potassium phosphate buffer (pH48), Tris-HCl buffer (pH810) and glycine-HCl buffer (pH1012). (B) The optimum temperature was measured at pH7.0 (100mM potassium phosphate buffer) using vanillin as the substrate.
The catalytic efficiency of VDHATCC13032 toward vanillin, p-hydroyxlbenzaldehyde and 3,4-dihydroxybenzaldehyde was further tested by kinetics analysis (Table S2). The experimental results revealed that VDH had a higher affinity toward vanillin than to the other two substrates and NAD+ was a required cofactor for this reaction, with the same level of activity as replaced with NADP+ (Table S3). This suggested that the enzyme could utilize both NAD+ and NADP+ as cofactors and show no obvious difference to them.
The catalytic activity of VDHATCC13032 was further confirmed by LC-MS analysis using the selected substrates. Purified VDHATCC13032 enabled the conversion of vanillin to vanillate (as shown in Figure 5). The HPLC results indicated that the production of vanillate in the presence of NAD+ and VDHATCC13032 was coupled with the decrease of the vanillin concentration (Fig. 5A). The production of vanillate was also validated by MS analysis (Data not shown). Purified VDHATCC13032 also considerably converted 3,4-dihydroxybenzaldehyde to 3,4-dihydroxybenzoic acid (Fig. 5B).
Site-directed mutagenesis analysis of VDHATCC13032
Based on sequence alignment, E258 and C292 were identified as the candidate conserved catalytic residues whereas N157, K180 and E199 were identified as the candidate cofactor interactive sites in VDHATCC13032 and these residues were demonstrated to be essential for VDH from the hyperthermophilic archaeon Sulfolobus tokodaii15 and Pseudomonas aeruginosa16. To investigate whether these five residues are also important for the VDHATCC13032 catalytic activity, VDHATCC13032 variants were constructed followed by in vitro catalytic assay using vanillin as the substrate in the presence of NAD(P)+. As a result, in the presence of NAD+, all the five variants showed activities of less than 50% of the wild type toward vanillin (Table S4). Interestingly, in the presence of NADP+, the activities of N157A, K180A and C292A decreased to as low as 10% of the wild type enzyme; but for E199A, the catalytic activity still kept at 78% of the wild type enzyme (Table S4). In addition, while other variants showed more than six times higher Km values than the wild type toward NADP+, the E199A variant showed significantly lower Km (Table S5). Moreover, all the mutations showed decreased affinity to NAD+ (Table S5). Thus, it could be speculated that E199 have less influence on binding NADP+, compared with the other residues; however, all these residues play key roles in binding NAD+. Furthermore, mutation of any of the five residues resulted in increased Km values and decreased kcat values toward vanillin (Table S6). Compared with the wild type enzyme, the deficient mutant of C292A, K180A and E258A had very lower affinities to vanillin (the Km values was 49 times higher and the kcat/Km was 1023 times lower, compared with the wild type enzyme). These results indicate that these residues also played important roles in the VDHATCC13032 to the affinity of substrate (e.g. vanillin).
Discussion
The -ketoadipate pathway is the major catabolic route for lignin-derived aromatic compounds in soil bacteria17,18,19. In the present study, we cloned, expressed and functional characterized a vdh gene from C. glutamicum, which channeled a variety of lignin-derived aromatic compounds to the protocatechuate branch of -ketoadipate pathway for further degradation. Based on the genome sequence of C. glutamicum ATCC130325,20, one putative aldehyde dehydrogenase gene, vdhATCC13032, was identified. But this gene does not show a remarkably high level of homology to those with verified vanillin dehydrogenase activity (Fig. 1). Our study suggests that VDHATCC13032 is probably a unique aldehyde dehydrogenase with special catalytic roles, as discussed in the following.
The conclusion that VDHATCC13032 is a vanillin dehydrogenase is based on at least three lines of independent evidence. First, analysis of a vdh deletion mutant revealed a delayed growth when 3, 4-dihydroxy benzaldehyde, 3-hydroxy benzaldehyde, vanillin, or ferulic acid was present as the sole carbon source, suggesting an important role of vdhATCC13032 in assimilation of these compounds. It is known that caffeic acid is not a direct target of VDH. However, when grown in the presence of caffeic acid, the vdh deletion mutant showed a delayed growth as well, supporting the idea that vdh is at the central pathway for catabolism of aromatic compounds. The growth defects of the mutant strain were complemented by expressing wild type vdh (Fig. 2). Thus the resulting phenotype was not due to the polar effects caused by deletion of the vdh gene. The delayed growth observed with the deletion mutant grown on the different substrates may indicate minor alternative pathways for catabolism of aromatic compounds. This is consistent with the Amycolatopsis sp. strain ATCC 39116, in which VDH also plays a significant role in the course of vanillin degradation, but the biotransformation from vanillin to vanillate were still observed when vdh gene was knocked out21.
HPLC-MS was performed to examine the biotransformation products of VDHATCC13032 in the presence of vanillin or 3,4-dihydroxybenzaldehyde. As expected, vanillin and 3,4-dihydroxybenzaldehyde were transformed to vanillate and 3,4-dihydroxybenzoic acid, respectively, confirming the activity of VDHATCC13032. Relatively high catalytic activity of purified VDHATCC13032 was observed with p-hydroxylbenzaldehyde and vanillin, consistent with the fact that vdh gene is at the downstream of ferulate and p-coumarate metabolism pathway. Also, VDHATCC13032 was heterologously expressed and its Mr was estimated by SDS-PAGE (Fig. 3A). Interestingly, the native molecular mass determined by gel filtration showed that VDH from C. glutamicum existed in the form of tetramers, trimers and dimers (Fig. 3B and C). This is consistent with previous reports that VDHs from Micrococcus sp. TA1 and Burkholderia cepacia TM1 exist as tetramers and dimers, respectively22. The enzyme displayed the highest activity at 30C, in accordance with the physiological temperature of C. glutamicum and the results of in vitro expression assay. VDH from most of the studied species showed specificity against vanillin and benzaldehyde compounds. While purified VDH from C. glutamicum ATCC13032 showed catalytic activity toward a relatively broad range of tested substrates (Table S1), oxidation rate for phenylacetaldehyde, formaldehyde and aldehyde were not detected. The observed substrate specificity is consistent with the substrate specificity observed with vanillin dehydrogenases from S. paucimobilis SYK-614 and P. fluorescens9. While most VDHs identified so far tend to use NAD+ as a sole cofactor, VDHATCC13032 could utilize both NAD+ and NADP+, which has been observed with the VDH from P. fluorescens in a previous study9. Taken together, these results have revealed the important roles of VDH in C. glutamicum ATCC13032, whereas substrate specificity may vary from one species to another.
Extensive works have been done to examine the active amino acid residues in the ALDH enzymes and the examined residues have been well documented15,16,23. Sequence alignment revealed conserved sequences in VDHATCC13032 with relatively high amino acid similarity with the active residues identified in ALDH and PaBADH. One strictly conserved residue is Cys-292 in VDHATCC13032, which has been demonstrated to be responsible for the dehydrogenase as well as esterase activities of aldehyde dehydrogenase16,24. In P. aeruginosa, the PaBADH catalytic cysteine (C286, corresponding to C292 in VDHATCC13032) is oxidized to sulfenic acid or forms a mixed disulfide with 2-mercaptoethanol19. The second active residue identified in VDHATCC13032 was Glu-258, which is in the vicinity of the catalytic cysteine, corresponding to E268 in ALDH2, E252 in PaBADH and E258 in VDH. This conserved residue probably functions as a general base in ALDH catalysis25. Lys-180 is another conserved residue in VDHATCC13032 and may have functions similar to that of ALDH (K78) and PaBADH (K162)16,23. Glu-199 in the VDHATCC13032 may also be an conserved residue that would produce a steric clash with the 2-phosphate of NAD(P)+, resulting in a low affinity of ALDH2 for NAD(P)+16. Therefore we speculated that these five conserved sites may be important for the catalytic activity of VDHATCC13032 and they were subjected to further characterization. Mutations of these residues decreased catalytic activities by more than 50% compared with wild type when NAD+ was used as a cofactor. However, when NADP+ was used as a cofactor, mutations of N157A, E258A and C292A caused 90% reduction of catalytic activities compared with wild type, while E199A mutation only reduced the activity by 12%. This indicates that Glu-199 may have less influence on NADP+ binding compared with the other tested residues, whereas all these residues play roles in NAD+ binding. Consistently, Glu-199 showed no effect on the affinity of VDHATCC13032 to NADP+, but affected the affinity of VDHATCC13032 for the substrates such as vanillin. Taken together, the examined residues play critical roles in VDH catalysis, but their mechanisms may be different.
Lignocellulosic hydrolysates for biofuel production usually contain not only fermentable sugars but also non-fermentable growth inhibitors, including furan, weak acids and various lignin-derived aromatic compounds (such as vanillin, ferulic acid, p-coumaric acid, 4-hydroxybenzoic acid, vanillic acid, etc.), which inhibit microbial fermentation to the desired products26,27. C. glutamicum could be applied to both detoxify and assimilate lignin-derived aromatic inhibitors as an alternative source to sugars for carbon and energy. The functional characterization of VDHATCC13032 contributes not only to a systematical understanding of aromatic compound assimilation, but also to develop C. glutamicum as an efficient strain to convert lignocellulose to bioproducts, such as biofuels.
Methods
Bacterial strains, plasmids and culture conditions
All bacterial strains and plasmids used in this study are listed in Table S7. Escherichia coli was cultivated at 37C in Luria-Bertani (LB) medium28. C. glutamicum strains were routinely grown at 30C in LB or in mineral salts medium (MM), which was adjusted to pH 8.4 and supplemented with yeast extract (0.05g L1)4. For generation of mutants and maintenance of C. glutamicum, BHIS (brain heart broth with 0.5M sorbitol) medium was used2. C. glutamicum RES167, a restriction-deficient strain derived from C. glutamicum ATCC 13032 was the parent of all derivatives used in this study. Aromatic compounds were added to the MM medium at suitable final concentrations. Cell growth was monitored photometrically at 600nm12,29. Kanamycin, chloramphenicol were added at final concentrations of 20 and 10g ml1, respectively for both E. coli and C. glutamicum and ampicillin of 100g ml1 for E. coli, whereas nalidixic acid of 40g ml1 for C. glutamicum.
Gene prediction in the C. glutamicum genome
Translated BLAST search (blastx) from the National Center for Biotechnology Information (NCBI) was used to identify genes involved in vanillin catabolism in C. glutamicum ATCC13032 (Accession no. NC 003450). Through the BLAST analyses, homologues of the putative genes encoding vanillin dehydrogenase were identified. The phylogenetic tree including vdhATCC13032 and vdh genes from other bacteria was constructed by ClustalX 1.83 and Molecular Evolutionary Genetics Analysis (MEGA) and the calculation were performed using the neighbor-joining method30.
DNA manipulations and plasmid construction
Genomic DNA of C. glutamicum was isolated according to the method described by Tauch et al.31. Plasmids were isolated with plasmid DNA miniprep spin columns (TIANGEN, Beijing, China) and DNA fragments were purified from agarose gels by EasyPure Quick Gel Extraction Kit (TransGen Biotech, Beijing, China). C. glutamicum was transformed by electroporation according to the method of Tauch et al.31. Competent cells of E. coli were prepared and transformed using the CaCl2 procedure29. Target DNA fragments were PCR amplified and digested by standard methods. All plasmids were constructed based on pK18mobsacB, pXMJ19, or pET28a. Primers used are listed in Table S8. To construct the plasmid for ncgl2578 knock out, the upstream and downstream DNA fragments were amplified using primer pairs ncgl2578upFBamHI/ncgl2578upR and ncgl2578dwF/ncgl2578dwRHindIII, respectively. The upstream and downstream PCR fragments were linked together by overlap PCR and then inserted into pK18mobsacB to generate pK18mobsac-ncgl2578 through restriction enzyme digestion. For complementation, plasmid pXMJ19-ncgl2578 was created by insertion of the PCR-amplified ORF into pXMJ19. Plasmid for expression of the target gene in E. coli was constructed from PCR-amplified gene and pET28a. All the constructed plasmids were confirmed by DNA sequencing.
Construction of the vdh mutant and complementary strain in C. glutamicum
To construct the vdh mutant, the plasmid pK18mobsacBncgl2578 was transformed into C. glutamicum RES167 by electroporation and chromosomal integration was selected by plating on LB agar plates supplemented with kanamycin. The ncgl2578 deletion mutant was subsequently screened on LB agar plates containing 10% sucrose and confirmed by PCR and sequencing as previously described20. For complementation, pXMJ19-ncgl2578 was transformed into the mutant strain and vdh gene expressed in C. glutamicum was induced by addition of 0.5mM isopropyl-D-thiogalactopyranoside (IPTG) to the culture broth.
Expression and purification of recombinant VDH in E. coli
To express the His6-VDH protein, recombinant plasmid pET28a-ncgl2578 was electroporated into E. coli BL21 (DE3). When the growth of recombinant E. coli reached OD600 = 0.4, the expression of recombinant protein was initiated by addition of 0.5mM IPTG and the culture was then shaked overnight at 22C11. Cells were harvested by centrifugation at 10,000g for 10min at 4C, washed twice with ice-cold phosphate-buffered saline (PBS). Harvested cells were disrupted by sonication and purified with the HisBind Ni-NTA resin (Novagen, WI, USA) following the manufacturer's instructions. Purified recombinant protein was dialyzed against PBS overnight at 4C and stored at 80C until use. Protein concentrations were determined using the Bradford assay according to the manufacturer's instructions (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
SDS-PAGE and determination of molecular mass of the purified enzyme
SDS-PAGE was conducted with 5% stacking gels and 10% resolving gel and run in a Mini-PROTEIN II Electrophoresis Cell (Bio-Rad) according to the manufacturer's instructions. After electrophoresis, the protein bands were visualized by Coomassie brilliant blue staining. Apparent molecular mass was estimated according to the relative mobility of Blue Plus II protein markers, with molecular masses ranging from 14 to 120kDa. The native molecular mass of the VDH was estimated by gel filtration chromatography on a Superdex 200 10/300GL column (1.0cm 30 31cm) (Amersham BioSciences) eluted at a flow rate of 0.25ml min1 with 100mM potassium phosphate buffer containing 150mM NaCl (pH 7.2). Molecular weight standards used are: hyroglobulin (bovine) (670kDa), -globulin (bovine) (158kDa), Ovalbumin (chicken) (44kDa), Myoglobin (horse) (17kDa) and Vitamin B12 (1.35kDa). To characterize the molecular configuration of the purified VDH, we employed PAGE under nondenaturing conditions. Gels consisted of a separating gel (15% acrylamide) and a stacking gel (5% acrylamide) and the loading buffer was also under nondenaturing condition, which contained 0.5M Tris-HCl buffer (pH 6.8), 0.1% bromophenol blue and 10% glycerin. The Sample containing 40g protein was loaded and then electrophoresis was carried out at 4C in Tris-glycine buffer (25mM Tris-base, 250mM Glycine). Apparent molecular mass was estimated according to the relative mobility of protein markers Protein Rule IV (TransGen Biotech, Beijing, China), with molecular masses ranging from 30 to 200kDa.
Enzyme assays
The VDH enzyme assay was performed based on described methods32,33,34 in 100mM potassium phosphate buffer (pH 7.0). The activity of vanillin dehydrogenase was monitored spectrophotometrically by measuring the rate of decrease in absorption at 340nm due to oxidation of NAD(P)H and was calculated with an extinction coefficient of 6,220M1 cm1. The reaction mixture (1ml) contained substrate (1mM), NAD(P)+ (0.5mM) and an appropriate amount of enzyme in potassium phosphate buffer (100mM, pH 7.0). The optimal pH was determined in various pHs of 100mM buffer as follows: glycine-HCl buffer (pH 34), potassium phosphate buffer (pH48), Tris-HCl buffer (pH810) and glycine-HCl buffer (pH1012). One unit of enzyme activity was defined as the amount of enzyme producing 1mmol NAD(P)H min1 as previously described15,33. Specific activities were given as unit mg1 of protein35. Protein concentrations were determined using the Bradford assay with bovine serum albumin as standard.
Biotransformation of aromatic compounds with recombinant VDH
To further analyze the biotransformation of aromatic compounds, the purified VDH from C. glutamicum strains was added to the reaction mixture (1ml) containing substrate (1mM) and NAD+ (0.5mM) in potassium phosphate buffer (100mM, pH 7.0). The reaction was performed at room temperature for 30min and a parallel mixture without VDH enzyme was used as a negative control. The products were analyzed by high-performance liquid chromatography-mass spectrometry (HPLC-MS) as previously described36,37. To confirm the vanillate and 3,4-dihydroxybenzoic acid as products in the two test reactions, standard compound were used as controls and the molecular weight of the products were verified based on MS analysis. The wavelength for UV detection was set at 275nm.
Site-directed mutagenesis
To identify the potential active residues in the VDH from C. glutamicum ATCC13032, genes from ALDH (aldehyde dehydrogenase) family with known active sites, such as ALDH2 and PaBADH (Pseudomonas aeruginsa betaine aldehyde) were used as references for alignment analysis. The alignment suggested that the five known residues were also present in the VDHATCC13032. Subsequently overlap PCR was performed to construct site-directed mutants of VDHATCC13032 protein using five pairs of primers according to the standard PCR-based mutagenesis. In detail, the following mutants were designed: N157A, K180A, E199A, E258A and C292A. Activities and kinetics parameters of all the mutants were determined with the experimental procedures of wild type enzyme described above15.
References
Lee, J. et al. Succinate production from CO2-grown microalgal biomass as carbon source using engineered Corynebacterium glutamicum through consolidated bioprocessing. Sci Rep 4, 5819 (2014).
Shen, X. H., Jiang, C. Y., Huang, Y., Liu, Z. P. & Liu, S. J. Functional identification of novel genes involved in the glutathione-independent gentisate pathway in Corynebacterium glutamicum. Appl Environ Microbiol 71, 3442 3452 (2005).
Shen, X. H., Zhou, N. Y. & Liu, S. J. Degradation and assimilation of aromatic compounds by Corynebacterium glutamicum: another potential for applications for this bacterium? Appl Microbiol Biotechnol 95, 77 89 (2012).
Huang, Y. et al. Genetic characterization of the resorcinol catabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72, 7238 7245 (2006).
Ikeda, M. & Nakagawa, S. The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62, 99 109 (2003).
Merkens, H. et al. Vanillate metabolism in Corynebacterium glutamicum. Curr Microbiol 51, 59 65 (2005).
Brinkrolf, K., Brune, I. & Tauch, A. Transcriptional regulation of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Genet Mol Res 5, 773 789 (2006).
Havkin-Frenkel, D. & Belanger, F. Biotechnology of Vanillin: Vanillin from Microbial Sources. Handbook of Vanilla Science and Technology 301, (eds Wily-Blackwell) (ISBC press. 2010).
Di Gioia, D. et al. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J Biotechnol 156, 309 316 (2010).
Plaggenborg, R., Overhage, J., Steinbchel, A. & Priefert, H. Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl Microbiol Biotechno 61, 528 535 (2003).
Shaw, J. P. & Harayama, S. Purification and characterisation of TOL plamids-encoded benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase of Pseudomonas putida. Eur J Biochem 191, 705 714 (1990).
Overhage, J., Priefert, H., Rabenhorst, J. & Steinbchel, A. Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase gene. Appl Microbiol Biotechnol 52, 820 828 (1999).
Masai, E. et al. Cloning and Characterization of the Ferulic Acid Catabolic Genes of Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol 68, 4416 4424 (2002).
Masai, E. et al. Characterization of ligV Essential for Catabolism of Vanillin by Sphingomonas paucimobilis SYK-6. Biosci Biotechnol Biochem 71, 2487 2492 (2007).
Liu, T., Hao, L., Wang, R. & Liu, B. Molecular characterization of a thermostable aldehyde dehydrogenase (ALDH) from the hyperthermophilic archaeon Sulfolobus tokodaii strain 7. Extremophiles 17, 181 190 (2013).
Gonzlez-Segura, L., Rudio-Piera, E., Muoz-Clares, R. A. & Horjales, E. The crystal structure of a ternary complex of betaine aldehyde dehydrogenase from Pseudomonas aeruginosa provides new insight into the reaction mechanism and shows a novel binding mode of the 2'-phosphate of NADP+ and a novel cation binding site. J Mol Biol 385, 542 557 (2009).
Davis, J. R. & Sello, J. K. Regulation of genes in Streptomyces bacteria required for catabolism of lignin-derived aromatic compounds. Appl Microbiol Biotechnol 86, 921 929 (2010).
Harwood, C. S. & Parales, R. The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 50, 553 590 (1996).
Shen, X. H. & Liu, S. J. Key enzymes of the protocatechuate branch of the -ketoadipate pathway for aromatic degradation in Corynebacterium glutamicum. Science in China Series C 48, 241 (2005).
Shen, X. H., Huang, Y. & Liu, S. J. Genomic analysis and identification of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Microbes and Environents 20, 160 167 (2005).
Fleige, C., Hansen, G., Kroll, J. & Steinbchel, A. nvestigation of the Amycolatopsis sp. strain ATCC 39116 vanillin dehydrogenase and its impact on the biotechnical production of vanillin. Appl Environ Microbiol 79, 81 90 (2013).
Mitsui, R., Hirota, M., Tsuno, T. & Tanaka, M. Purification and characterization of vanillin dehydrogenases from alkaliphile Micrococcus sp. TA1 and neutrophile Burkholderia cepacia TM1. FEMS Microbiol Lett 303, 41 47 (2010).
Daz-Snchez, A. G. et al. Novel NADPH-cysteine covalent adduct found in the active site of an aldehyde dehydrogenase. Biochem J 439, 443 452 (2011).
Priefert, H., Rabenhorst, J. & Steinbchel, A. Molecular character of Pseudomonas sp. Strain HR199 involved in bioconversion of vanillin to protocachuate. J Bacteriol 179, 2595 2607 (1997).
Wang, X. P. & Weiner, H. Involvement of glutamate-268 in the active-site of human liver mitochondrial (class-2) aldehyde dehydrogenase as probed by site-directed mutagenesis. Biochemistry 34, 237 243 (1995).
Almeida, J. R. et al. NADH- vs NADPH-coupled reduction of 5-hydroxymethyl furfural (HMF) and its implications on product distribution in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 78, 939 945 (2008).
Klinke, H. B., Thomsen, A. B. & Ahring, B. K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66, 10 26 (2004).
Achterholt, S., Priefert, H. & Steinbchel, A. Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 54, 799 807 (2000).
Overhage, J., Priefert, H. & Steinbchel, A. Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65, 4837 4847 (1999).
Saitou, N. & Nei, M. The neighbor-joining method. A new method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406 425 (1987).
Tauch, A., Kassing, F., Kalinowski, J. & Phler, A. The Corynebacterium xerosis composite transposon Tn5432 consists of two identical insertion sequences, designated IS1249, flanking the erythromycin resistance geneermCX. Plasmid 34, 119 131 (1995).
Bar, G., Swiatkowski, T., Moukil, A., Gerday, C. & Thonart, P. Purification and characterization of a microbial dehydrogenase: a vanillin:NAD(P)+ oxidoreductase. Appl Biochem Biotechnol 98, 415 428 (2002).
Peng, X. et al. Characterization of Sphingomonas aldehyde dehydrogenase catalyzing the conversion of various aromatic aldehydes to their carboxylic acids. Appl Microbiol Biotechnol 69, 141 150 (2005).
Yang, W. et al. Characterization of two Streptomyces enzymes that convert ferulic acid to vanillin. PloS one 8, e67339 (2013).
MacKintosh, R. W. & Fewson, C. A. Benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus. Purification and preliminary characterization. Biochem J 250, 743 751 (1988).
Kaur, B., Chakraborty, D. & Kumar, B. Phenolic biotransformations during conversion of ferulic acid to vanillin by Lactic acid bacteria. Biomed Res Int 2013, 590359 (2013).
Saa, L., Jaureguibeitia, A., Largo, E., Llama, M. J. & Serra, J. L. Cloning, purification and characterization of two components of phenol hydroxylase from Rhodococcus erythropolis UPV-1. Appl Microbiol Biotechnol 86, 201 211 (2010).
Plaggenborg, R. et al. Potential of Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol. Appl Microbiol Biotechnol 72, 745 755 (2006).
Sekine, M. et al. Sequence analysis of three plasmids harboured in Rhodococcus erythropolis strain PR4. Environ Microbiol 8, 334 346 (2006).
Chen, H. P. et al. Vanillin catabolism in Rhodococcus jostii RHA1. Appl Environ Microbiol 78, 586 588 (2012).
Shrivastava, R., Basu, A. & Phale, P. S. Purification and characterization of benzyl alcohol- and benzaldehyde- dehydrogenase from Pseudomonas putida CSV86. Arch Microbiol 193, 553 563 (2011).
Venturi, V., Zennaro, F., Degrassi, G., Okeke, B. C. & Bruschi, C. V. Genetics of ferulic acid bioconversion to protocatechuic acid in plantgrowth-promoting Pseudomonas putida WCS358. Microbiology 144, 965 973 (1998).
Nelson, K. E. et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4, 799 808 (2002).
Gasson, M. J. et al. Metabolism of Ferulic Acid to Vanillin A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J Biol Chem 273, 4163 4170 (1998).
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 program, grant 2013AA102802), National Natural Science Foundation of China (31270078), Key Science and Technology R&D Program of Shaanxi Province, China (2014K02-12-01) and Fundamental Research Funds for the Central Universities, Northwest A&F University (Z111021006).
Author information
Authors and Affiliations
Contributions
W.D., W.Z., S.C. and X.S. wrote the main manuscript. W.D., M.S., W.Z., Y.Z., C.C., L.Z., Z.L. and X.S. designed and performed the experiments. W.D., M.S., W.Z., S.C. and X.S. analyzed the data. W.D. and C.C. prepared samples. All authors discussed and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Ding, W., Si, M., Zhang, W. et al. Functional characterization of a vanillin dehydrogenase in Corynebacterium glutamicum. Sci Rep 5, 8044 (2015). https://doi.org/10.1038/srep08044
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08044
This article is cited by
-
Metabolic engineering of Corynebacterium glutamicum for fatty alcohol production from glucose and wheat straw hydrolysate
Biotechnology for Biofuels and Bioproducts (2023)
-
Application of Corynebacterium glutamicum engineering display system in three generations of biorefinery
Microbial Cell Factories (2022)
-
Small protein Cgl2215 enhances phenolic tolerance by promoting MytA activity in Corynebacterium glutamicum
Stress Biology (2022)
-
Vanillic acid and methoxyhydroquinone production from guaiacyl units and related aromatic compounds using Aspergillus niger cell factories
Microbial Cell Factories (2021)
-
Lignin bioconversion into valuable products: fractionation, depolymerization, aromatic compound conversion, and bioproduct formation
Systems Microbiology and Biomanufacturing (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.