Abstract
Vaccine efficacy depends on strong long-term development of immune memory and the formation of memory CD8+ T cells is critical for recall responses to infection. Upon antigen recognition by naïve T cells, the strength of the TcR signal influences the subsequent effector and memory cells differentiation. Here, we have examined the role of Itk, a tyrosine kinase critical for TcR signaling, in CD8+ effector and memory T cell differentiation during Listeria monocytogenes infection. We found that the reduced TcR signal strength in Itk deficient naïve CD8+ T cells enhances the generation of memory T cells during infection. This is accompanied by increased early Eomesodermin, IL-7Rα expression and memory precursor effector cells. Furthermore, Itk is required for optimal cytokine production in responding primary effector cells, but not secondary memory responses. Our data suggests that Itk-mediated signals control the expression of Eomesodermin and IL-7Rα, thus regulating the development of memory CD8+ T cells, but not subsequent response of memory cells.
Similar content being viewed by others
Introduction
CD8+ T cells play critical roles in the immune response to pathogens and vaccine efficacy depends on strong long-term development of immune memory in both the B cell and T cell compartment. Memory CD8+ T cells develop following antigenic stimulation over several identifiable phases. Initial antigen recognition initiates clonal expansion of naïve T cells, which develop into effector T cells. Upon antigen clearance, these effector T cells undergo a contraction phase and the development of memory precursor effector cells leading to memory T cells. Using mouse models and model pathogens such as Lymphocytic Choriomeningitis Virus (LCMV) and Listeria monocytogenes (L. monocytogenes), these phases have been identified based on expression of surface markers and transcription factors (for review see ref. 1). The development of memory T cells requires signals from the cytokines IL-15 and IL-7 and expression of the common receptor chain for both cytokines IL-7Rα (CD127)1 and responding naïve CD8+ T cells develop into Short-Lived Effector Cells (SLECs) carrying the phenotype CD127lo/KLRG1hi. Once the pathogen is cleared, some effector cells become Memory Precursor Effector Cells (MPECs) and take on the phenotype CD127hi/KLRG1lo. SLECs express higher levels of transcription factor T-bet and lower levels of Eomesodermin (Eomes) (higher T-bet:Eomes ratio), while MPECs exhibit the reverse behavior, expressing higher levels of Eomes and lower levels of T-bet. Thus KLRG1hi/T-bethi cells are generally SLECs while KLRG1lo/Eomeshi are generally destined to become long-term memory cells1.
The parameters for optimal development of the CD8+ T cell immune response have been of keen interest. The strength of the signal that T cells receive via the TcR is one important factor in generation of effector and memory cells. Antigen dose has been used as a proxy for TcR signal strength and has been shown to be inversely correlated with CD127 expression2. It has also been suggested that short or weak TcR stimuli results in unfit cells that do not survive in vivo3. Bevan and colleagues have also reported that CD8+ T cells that receive short and weak stimuli generate fewer memory T cells, although the resultant populations have the same functional capacity as T cells that received strong signals4,5. These data suggest a direct correlation between TcR signal strength and T cell response for generation of memory. The tyrosine kinase Itk acts downstream of the TcR to regulate TcR signal strength6. As a result, Itk deficiency in mice and humans affects the development and function of CD4+, CD8+ T cells, iNKT cells and γδ T cells7,8,9,10,11,12,13,14,15,16. In addition, Itk−/− CD4+ T cells exhibit reduced Th2 responses and reduced IL-17A production in Th17 cells17,18,19. Furthermore, Itk−/− mice have a population of innate memory CD8+ T cells due to CD8+ T cell extrinsic dysregulated production of IL-420,21. Thus Itk−/− mice have complex phenotypes that affect both CD4+ and CD8+ T cells22.
Given the critical role that Itk plays in TcR signals, its role in CD8+ T cell responses in infection is of considerable interest and has been examined using mice lacking this kinase. The data suggested that Itk plays a role in CD8+ T cell antiviral responses, with decreased CD8+ T cell cytotoxic function against LCMV, Vaccinia Virus (VV) and Vesicular Stomatitis Virus (VSV). In addition, while viral clearance of VV infection was observed in the absence of Itk, the kinetics was delayed23. However, this work was performed prior to the discovery of the innate memory phenotype CD8+ T cells in the Itk−/− mice14,15,24, prompting re-evaluation of these conclusions about the role of Itk in CD8+ T cell response to infection. We have previously examined the function of the CD8+ innate memory phenotype in mice during the early response to infection with L. monocytogenes and found that Itk−/− mice were able to clear infections with L. monocytogenes more quickly than WT mice. However, this was not antigen specific and primarily due to the elevated numbers of IMP CD8+ T cells that develop in the absence of Itk24. It is therefore very likely that previous studies using Itk−/− mice to examine CD8+ T cell response to infection were affected by the presence of these populations of IMP cells, particularly since it has been shown that preexisting memory cells affect subsequent responses of naïve CD8+ T cells25. Thus the role of Itk in CD8+ antigen specific T cell response to infection as developed from naïve precursors, or in the development of CD8+ T cell memory is unclear. We have therefore reexamined the role of Itk in the activation and differentiation of naïve Itk−/− CD8+ T cells using naive Ovalbumin specific OTI T-cells (on a RAG deficient background), to infection with L. monocytogenes carrying Ovalbumin (LM-OVA).
Results
The absence of other T cell populations allows the development of naïve Itk−/− CD8+ T cells
The ability to specifically examine the role of Itk in the generation of antigen specific CD8+ T cell responses and development of memory is hampered by the presence of altered populations of CD8+ (and CD4+) T cells in mice lacking Itk. To bypass these caveats with using Itk−/− mice, we generated Ovalbumin peptide (SIINFEKL) specific OTI transgenic T cell receptor mice on a Rag−/− background also lacking Itk (Itk−/− OTI/Rag−/−). We found that >95% of the CD8+ T cell population from such mice had a naïve phenotype (CD44lo/CD62Lhi/CD122lo) (data not shown) and thus used naïve T cells from these mice to study the response of antigen specific naïve CD8+ T cells in response to infection.
Itk regulates the quality of the antigen-specific CD8+ T cell cytokine response in vitro in a digital-like manner
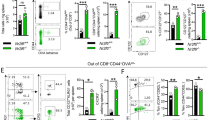
Isolated naïve WT and Itk−/− T cells were co-cultured in vitro with SIINFEKL peptide-pulsed dendritic cells (DCs) at varying concentrations. After 5 days, the cells were restimulated using the same initial concentration of peptide and then analyzed for expression of cytokines and transcription factors as a measure of their response. Given the role of Itk in regulating TcR signals, we were surprised to find that Itk−/− T cells proliferated similarly to, or better than WT T cells as measured by cell numbers and expression of proliferative marker Ki67 (Fig. 1A). However, a higher frequency of WT T cells produced IFN-γ and TNFα, as well as amount of cytokine/cell as measured by the MFI, although for TNFα, this was less pronounced (Fig. 1B, D). The observed difference in proportion of responding cells was more pronounced in those T cells producing IFN-γ and in those higher quality T cells that produced both IFN-γ and TNFα (double producers). Note that there was no difference in the capacity of Itk−/− T cells in producing these cytokines when they were stimulated using PMA/Ionomycin to bypass the TcR (Fig. 1C). Furthermore, while it is possible that differences in cell viability could be responsible for the differences in cytokine production, we think this is less likely since there was no difference in cell numbers between WT and Itk−/− T cells.
Itk regulates the quality of the antigen-specific CD8+ T cell response in vitro.
Naïve WT or Itk−/− T cells were stimulated with the indicated concentration of SEINFEKL peptide for 5 days, followed by restimulation with the original concentration of peptide or P/I for 5 hours in the presence of BFA, fixed and analyzed for cytokine or transcription factor as indicated. (A) Cell number (top panel) and Ki67 expression (bottom panel) of resultant cells as a function of peptide concentration. (B) Percent IFN-γ, TNFα or double producers as a function of peptide concentration. (C) Cells restimulated with P/I and analyzed for percent IFN-γ, TNFα or double producers as a function of peptide concentration. n = 3, 3 independent experiments. (D) MFI of cytokine expressing cells as a function of peptide concentration. (E) Percent of T-bet, Eomesodermin or double expressers as function of peptide concentration. *p < 0.05, n = 3 for 3 independent experiments. All error bars indicate the value of +/−SEM.
This suggests that Itk does not regulate proliferative capacity in response to antigen, but regulates the ability of responding CD8+ T cells to produce IFN-γ and TNFα upon TcR restimulation. We also noted that the absence of Itk did not affect the EC50 for peptide activation, but affected the maximum achievable at specific concentrations, suggesting a digital-type response. WT and Itk−/− T cells expressed T-bet and Eomes, particularly at higher doses and both populations had elevated frequency of Eomes single positive cells at low dose of peptide, but higher frequency of T-bet and T-bet/Eomes double positive cells at the higher doses (Fig. 1E).
Itk suppresses the development of CD8+ T cell memory during L. monocytogenes infection
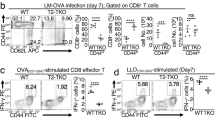
We next examined Itk's role in the CD8+ T cell response in vivo during infection with L. monocytogenes carrying the model antigen Ovalbumin (LM-OVA). We transferred naïve WT or Itk−/− T cells into CD45.1 congenic WT mice, followed by infection with 5 × 105 LM-OVA within 24 hours after adoptive transfer. Blood (and spleens) from infected mice were analyzed over time to monitor the response of the transferred T cells. Infection with LM-OVA resulted in similar expansion of WT and Itk−/− T cells 7–12 post infection, but while WT T cells underwent rapid contraction to a low number by day 21, a significantly higher number of Itk−/− T cells remained at 21 and 30 days post infection (Fig. 2A), indicating that Itk signals suppress the conversion of effector CD8+ T cells to long-term memory cells during this infection. There was no difference in bacterial clearance between recipient mice, which was cleared by day 7 post-infection (Fig. 2B). These data suggest that Itk signals play a negative role in the response of CD8+ T cells during infection with L. monocytogenes.
Itk tunes the expression of Eomes and development of MPECS during infection.
Naïve WT or Itk−/− T cells were transferred separately into WT mice, which were infected with L. monocytogenes. Mice were bled on the indicated days and cells analyzed as indicated. (A) Cell number. (B) Bacterial numbers in liver determined from mice infected for day 2 or day 7, and. n = 4, 3 independent experiments, no bacteria detected. (C) Percent SLEC and MPEC. (D) Percent T-bet and Eomes. *p < 0.05, n = 3–8 mice, 3 independent experiments. (E) Flow cytometric analysis of the expression of CD127 (left panels) or Eomes (right panels) in representative mice. (F) Naïve WT or Itk−/− T cells were stimulated with 20 μg/ml OVA plus antigen presenting cells over 5 days. Cells were analyzed for expression of IRF4 by flow cytometry. n = 3. (G) IRF4 expression was determined by flow cytometry in WT or Itk−/− OTI/Rag−/− T cells following transfer into WT mice and infection with L. monocytogenes for 5 days. n = 3–8 mice, 3 independent experiments. All error bars indicate the value of +/−SEM.
Itk signals negatively regulate the development of MPEC during infection
We followed the differentiation of transferred naïve WT and Itk−/− T cells to SLECs and MPECs by examining their expression of CD127 and KLRG1 during infection with LM-OVA. We found a significantly higher frequency of KLRG1lo/CD127hi MPECs among the Itk−/− T cells, starting at the earliest time point we examined, day 7 (Fig. 2C). This higher frequency of MPEC generation was not observed at later time points as the WT cells caught up. By contrast, there was no difference in the frequency of SLECs (Fig. 2C). Thus the absence of Itk signals results in enhanced generation/survival of KLRG1lo CD127hi MPECs during infection and suggests that signals regulating the development of MPEC are inhibited by Itk signaling.
Itk signals negatively regulate CD127 and Eomesodermin expression during infection
The development of MPECs is controlled in part by the expression of Eomes and we found that a higher frequency of Itk−/− T cells expressed Eomes compared to WT T cells 7–21 days post LM-OVA infection. By contrast, there was little difference in the frequency of T-bet expressing cells (Fig. 2D). Indeed, we found that a higher frequency of Itk−/− T cells expressed higher levels of Eomes and its target CD127 at day 7 and the WT T cells do not catch up until day 21. Thus Itk signals suppress early expression of Eomes and CD127 (Fig. 2E). Berg et al recently suggested that Itk regulates the expression of IRF4, which is a negative regulator of Eomes26. However, we did not see any differences in induction of IRF4 when naïve Itk−/− T cells are activated by antigen in vitro or in vivo, suggesting that mature peripheral T cells may have different requirements for induction of IRF4 via the TcR (Fig. 2F, G). These data suggest one explanation for the more efficient conversion of Itk−/− T cells to MPEC during infection, earlier and enhanced expression of Eomes, potentially leading to enhanced development of MPECs.
Itk intrinsically promotes cytokine production during the CD8+ T cell response in vivo in a digital manner
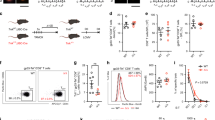
We next examined effector cytokine responses of transferred T cells after LM-OVA infection. We found that both WT and Itk−/− T cells responded to infection with cytokine production upon restimulation with the SIINFEKL peptide in vitro (as measured by percent responding cells and MFI of IFN-γ and TNFα expression). A smaller proportion of Itk−/− T cells produced cytokine (both IFN-γ and TNFα and double producers) (Fig. 3A, B), however, the cells that produced cytokine produced the same amount as WT cells (MFI, Fig. 3A). These results suggest that Itk signaling is critical for determining whether responding cells will produce cytokine, but not how much cytokine they produce, akin to a digital response.
Itk regulates the production of effector cytokines during infection.
Analysis of WT or Itk−/− T cells for cytokine production during infection as indicated. (A) Percent and MFI of cytokine positive cells (IFN-γ, left panels), (TNFα, right panel). (B) Percent IFN-γ/TNFα double positive cells. *p < 0.05, n = 3–8 mice, 3 independent experiments. All error bars indicate the value of +/−SEM.
Itk is not required for antigen specific recall response following infection
Since the absence of Itk enhanced the development of MPEC during infection, we asked whether the resultant memory cells exhibited differential response to reinfection. In contrast to the effector cytokine response during the primary T cell response, there was no difference in effector cytokine production, including frequency or amount/cell (Fig. 4A). Note that the number of cells upon reinfection was lower in the WT mice, in agreement with the lower number of WT MPECs generated (Fig. 4B, see Fig. 2), however, there was no difference in fold expansion between Itk−/− and WT cells (ratio Itk−/−:WT cell numbers), indicating that reinfection induced similar expansion of memory cells that developed (Fig. 4B). Indeed, transfer of equal numbers of WT and Itk−/− memory CD8+ T cells into naïve recipients, followed by similar infection with LM-OVA also resulted in statistically similar expansion between the WT and Itk−/− memory CD8+ T cells, although it is possible that the inclusion of more mice in this experiment would reveal a significant difference (Fig. 4C). Thus, while Itk signals are important in enhancing the frequency of cytokine producing cells within the responding population during a primary T cell response, as well as in delaying the timing of the development of MPECS, these signals are not important for the recall response of the fully differentiated memory population.
Itk signals are not required for CD8+ T cell memory responses.
Naïve WT or Itk−/− T cells were transferred into WT mice, which were infected with LM-OVA. After 30 days, mice were infected with 10× the initial dose of bacteria and analyzed 3 days post-infection. (A) Percent IFN-γ, TNFα and IFN-γ/TNFα double positive cells (left panel), as well as MFI IFN-γ or TNFα positive cells (right panel). (B) Cell number (left panel). *p < 0.05, n = 7 mice, 3 independent experiments. Fold difference in cell numbers between Itk−/− and WT T cells (expressed as ratio Itk−/−:WT T cells) after the primary response (day 28, data from Fig. 2A), or after the recall response (data from left panel). All error bars indicate the value of +/−SEM. n.s. = no statistically significant difference in fold expansion between Itk−/− and WT T cells between the primary response and secondary response. (C) Naïve WT or Itk−/− T cells were transferred into WT mice, which were infected with LM-OVA. After 30 days, memory donor CD8+ T cells were isolated, then transferred (40,000/mouse) into naïve mice, which were infected with 10× the initial dose of bacteria and number of donor CD8+ T cells analyzed 3 days post-infection. All error bars indicate the value of +/−SEM. n = 4–5 mice. Experiment performed 1×.
Discussion
Early antigen stimulation of CD8+ T cells via the TcR has been proposed to be an important parameter in regulating the nature of the response, as well as generation of memory. This early TcR signal can be regulated by antigen dose, antigen affinity (for TcR) or intracellular signaling controlling TcR signal strength. TcR signal strength, antigen dose and antigen affinity have sometimes been used interchangeable, however, they are different parameters. For example, antigen affinity seems to act in an analog manner, altering the EC50 of peptide required for functional activation as illustrated by Bevan and colleagues5. In that case antigen affinity does not affect whether a CD8+ T cell response is generated and effector function is not affected. However, there is reduced proliferation and the timing of the T cell contraction is shifted earlier with low affinity antigen. The expression of CD8 can also control the response of CD8+ T cells in an analog-like fashion, with differences in CD8 altering the EC50 of peptide required for functional activation in vitro27. By contrast, changes in the expression of the protein tyrosine phosphatase SHP-1, which regulates TcR signal strength28, does not affect the EC50 for antigen, but affects the magnitude of the response at the same antigen concentrations in vitro (digital-like)27. In vivo SHP-1 regulates the magnitude of the peak of the CD8+ T cell response, along with the frequency of SLECs generated, without affecting the effector cytokine or MPECs generated29. Our results indicate that as a regulator of TcR signaling in the vein of SHP-1, Itk acts in digital-like manner for the cytokine response, with little effect on the effectiveness of antigen activation (EC50), but on the likelihood that responding cells will produce cytokine. Our results also suggest that Itk acts in a “tuning” capacity in regulating the ability of cells to differentiate to MPECs and thus generate memory cells.
Stimulating CD8+ T cells carrying a TcRβ chain that reduces signaling results in reduced development of memory, without affecting development of effector responses30 and reducing the affinity of the TcR ligands can rescue the development of memory in these cells31. This rescue of memory development in these T cells is suggested to be due to NFκB signaling31. These data suggests that reduced TcR signaling leads to reduced development of effector and memory cells. Our data with Itk deficient T cells, which also exhibit reduced TcR signaling, provides a different view, in that we observe normal effector cell development, but enhanced memory development. Of course, while CD8+ T cells carrying the altered TcRβ chain do exhibit reduced TcR signaling, they may also have other signaling pathways that are altered, that may affect effector and memory cell development, particularly since memory development in these cells can be rescued by using a lower affinity ligand30,31. The groups of Jordan as well as of Teixeiro have previously shown that weak TCR signaling favors the generation of memory CD8 T cells31,32. Indeed, the work of Smith-Garvin et al use, among others, a SLP-76 Y145F mutant which regulates its interaction with Itk and our work with Itk leads to similar conclusions regarding the role of this pathway on regulating the development of memory CD8+ T cells. Thus our data are in support of the idea that weak TCR signals preferentially allow the development of CD8+ memory T cells.
We found that Itk−/− T cells exhibit early expression of Eomes and the mechanism is of considerable interest. Berg et al recently suggested that Itk regulates the expression of IRF4, which is a negative regulator of Eomes26. However, these experiments were performed using Itk−/− thymocytes, which, as discussed earlier, may have developmental differences compared to WT T cells. In our experiments we have not seen any differences in induction of IRF4 when naïve Itk−/− OTI/Rag−/− T cells are activated by antigen in vitro or in vivo, suggesting that mature peripheral T cells may have different requirements for induction of IRF4 via the TcR. Of significant interest is the recent report that Eomes is regulated by the small GTPase, N-Ras and that the absence of N-Ras in naïve T cell results in reduced development of MPECs33. As Itk deficient T cells exhibit the inverse phenotype, this would suggest that Itk negatively regulates N-Ras activity and in its absence, there is elevated expression of Eomes. As previous studies have shown, Itk regulates the activation of the Ras/MAPK pathway downstream of the TcR34, although this regulation may be more complex. This differential regulation of Eomes may endow Itk−/− T cells with the ability to generate early MPECs.
During the immune response, an increase in SLEC has been suggested to reduce the availability of precursors able to become MPECs35. This increase in SLEC is suggested to reduce the availability of precursors able to become MPECs. Our data suggest that these may be separable events and that enhanced early MPEC development can occur in the face of apparently normal SLEC development. While it can be difficult to untangle the effects of differences in bacterial clearance and the corresponding effects on inflammation, we note that there is no difference in bacterial clearance between WT and Itk−/− T cell recipients, suggesting that this is not likely to be a factor in the differential development of MPECs. Our work therefore supports a model where weaker TCR signals due to lack of Itk in CD8+ T cells contributes to the promotion of earlier MPEC development.
The findings reported in this work, along with our recently reported findings on the development of innate memory phenotype T cells (or IMP cells), suggest a general model for the ability of TcR signals to tune cytokine-mediated signals. We have recently shown that development of IMP CD8+ T cells driven by IL-4 signals is tuned by TcR signals traveling via Itk, such that the absence of Itk enhances the ability of IL-4 to drive development of IMP CD8+ T cells21. We suggest that similar to the development of IMP CD8+ T cells driven by IL-4, development of MPECs driven by cytokines regulating this process may be tuned by TCR signals traveling via Itk.
In contrast to developing Itk−/− effector T cells, Itk−/− CD8+ memory T cells were able to respond equivalent to WT cells in both frequency and cytokine expression upon bacterial rechallenge. This supports previous work showing that TcR signaling is different between naive and memory T cells (see review36), such that there may be less of a dependence on Itk signals for cytokine expression. Based on these findings, there is a strong potential for the use of Itk inhibitors in manipulating the CD8+ T cell response to enhance early development of memory without compromising memory recall responses. TcR signal strength has been proposed as a parameter that determines the CD8+ T cell response, including memory development1. We have referred here to the behavior of Itk in regulating these pathways as acting in a digital manner (in regulating cytokine production) and as a tuner (in regulating memory cell development). We refer to the digital response with regards to the cytokine response, in that while the percent of cells that produce cytokine in vitro and in vivo is different between WT and Itk−/− T cells, the amount of cytokine that they make (as read out by MFI) is not different. This suggests that Itk signals control whether the cells will respond (digital), but not the amount of cytokine that the responding cells make (which we propose would be an analog response). We refer to Itk as a tuner in regulating memory cell development since the absence of Itk enhances memory cell development in a seeming analog manner, not whether they will develop into memory cells, suggesting an analog like behavior controllable by a tuner. Thus we have uncovered a tuning role for Itk in regulating TcR signal strength leading to expression of Eomes during the primary response and to control of the development of memory precursor cells. While our experiments have only examined this idea in a model of bacterial infection, our findings suggest that manipulating early TcR signals by manipulating Itk activity will have a significant impact on subsequent development of memory cells during infections with other pathogens.
Methods
Mice
All mice used are in C57BL6 background. Itk−/− OTI/Rag−/− mice were generated by crossing OTI/Rag1−/− (Taconic) and Itk−/− mice. CD45.1 mice or Thy1a (Jackson Laboratory) mice were used as recipients in the adoptive transfer experiments. All experiments were reviewed and approved by the Cornell University Institutional Animal Care and Use committee (IACUC), carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health.
Adoptive cell transfers and Listeria monocytogenes infection
105 T cells/mouse (WT or Itk−/− OTI/Rag−/− T cells) were transferred via retro-orbital injection. This results in an ~10% take so that 104 cells/mouse are available for immune response5. This was followed by IP infection with 5 × 105 CFU/mouse Listeria monocytogenes carrying the model antigen Ovalbumin on the ActA- mutant background (LM-OVA, a gift from Dr. Hao Shen, University of Pennsylvania) within 24 h of T cell transfer. For reinfection studies mice were infected with 5 × 106 CFU/mouse via IP injection. At the indicated time after LM-OVA infection, mice were sacrificed and blood, spleen and lymph nodes were harvested. For memory T cell expansion studies, purified naïve CD45.1 WT or Itk−/− T cells were transferred into CD45.1 congenic WT mice. Recipient mice were then infected with LM-OVA (5 × 106 CFU/mouse) as previously described. After 30 days, memory donor CD45.2 CD8+ T cells were isolated by FACS, then transferred (40,000/mouse) into naïve mice, which were infected with 10× the initial dose of bacteria and number of donor CD45.2 CD8+ T cells analyzed 3 days post-infection.
Cell isolation and purification
Naïve T cells were purified from spleen or lymph nodes of WT and Itk−/− OTI/Rag−/− mice by negative selection using: biotin-anti-Fc block, biotin-anti-F4/80, biotin-anti-CD11c, biotin-anti-CD11b, biotin-anti-CD49b, biotin-anti-DX5, biotin-anti-Ter119, biotin-anti-Gr-1 and biotin-anti-CD25 (eBiosciences Inc). Negative selection of purified naïve T cells was done by anti-biotin beads and MACS columns and verified by FACS.
Cell culture and stimulation conditions
Complete RPMI (Invitrogen Inc) with 10% FBS was used for cell culture. Purified naïve T cells were cultured at a concentration of 106/ml with mitomycin C-treated splenocytes (as APCs) from Rag−/− mice (ratio 1:5). OVA257–264 peptide (SIINFEKL, Peptides International Inc.) at the indicated concentrations (10−8 μM to 1 μM) was used and cells cultured for five days. On the 5th day, cells were restimulated with SIINFEKL peptide at the same concentration used for the original culture, or PMA (20 ng/ml)/Ionomycin (2 μM) (P/I) for 4–6 hours in the presence of Brefeldin A (BFA, 100 μg/ml, Sigma Aldrich, Inc). For cells from LM-OVA infection, cells were stimulated with 1 μM SIINFEKL peptide or P/I in the presence of BFA for 4–6 hours.
FACS analysis
Blood samples were collected in 50 U/ml heparin (Sigma Aldrich, Inc.) and ACK (Ammonium-Chloride-Potassium) lysis buffer before FACS analysis. The following antibodies were used: FITC-anti-KLRG1, APC-anti-CD45.1, PECy7-anti-CD127, Pacific Blue-anti-CD122, PerCP-Cy 5.5-anti-EOMES, PECy7-anti-IFN-γ (eBiosciences Inc.), PE-anti-CD62L, PE-anti-Thy1a, APC-Cy7-anti-CD45.2, Alexa-Fluor-700-anti-CD62L, Horizon-V500-anti-CD44, PE-anti-TNFα, APC-anti-T-bet, PE-CF594-CD8α, ACP-Cy7-TCRVα2 and AlexaFluor700-anti-Ki67 (BD Biosciences, Inc.), ECD-Texas Red-anti-CD8α (Invitrogen, Inc.), Fc Block, FITC-CD45.2 and AF700-CD45.2 (Biolegend). MFI was determined by gating on cytokine positive populations. All transferred cells analyzed were gated by CD8+ and CD45.2+, CD45.1+ or Thy1.2+ markers.
Statistical analysis
Data are presented as a representative experiment and 3–5 mice per group were used for each experiment. Student's t test and ANOVA were performed using Prism to evaluate statistical significance with p < 0.05 considered statistically significant.
References
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12, 749–761 (2012).
Hammerbeck, C. & Mescher, M. Antigen controls IL-7R alpha expression levels on CD8 T cells during full activation or tolerance induction. J Immunol. 180, 2107–2116 (2008).
Gett, A. V., Sallusto, F., Lanzavecchia, A. & Geginat, J. T cell fitness determined by signal strength. Nat Immunol 4, 355–360 (2003).
Prlic, M., Hernandez-Hoyos, G. & Bevan, M. J. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med 203, 2135–2143 (2006).
Zehn, D., Lee, S. Y. & Bevan, M. J. Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009).
Berg, L. J. Strength of T cell receptor signaling strikes again. Immunity 31, 529–531 (2009).
Felices, M., Yin, C., Kosaka, Y., Kang, J. & Berg, L. Tec kinase Itk in gammadelta T cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A. 106, 8308–8313 (2009).
Felices, M. & Berg, L. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production and survival. J Immunol. 180, 3007–3018 (2008).
Atherly, L., Brehm, M., Welsh, R. & Berg, L. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol. 176, 1571–1581 (2006).
Atherly, L. et al. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 25, 79–91 (2006).
Qi, Q., Kannan, A. K. & August, A. Tec family kinases: Itk signaling and the development of NKT alphabeta and gammadelta T cells. FEBS J 278, 1970–1979 (2011).
Qi, Q. et al. Interleukin-2-inducible T cell kinase (Itk) network edge dependence for the maturation of iNKT cell. J Biol Chem. 286, 138–146. Epub 2010 Oct 2029. (2011).
Qi, Q. et al. Enhanced development of CD4+ {gamma}{delta} T cells in the absence of Itk results in elevated IgE production. Blood. 114, 564–571. Epub 2009 May 2014. (2009).
Broussard, C. et al. Altered development of CD8+ T cell lineages in mice deficient for the tec kinases Itk and Rlk. Immunity. 25, 93–104 (2006).
Dubois, S., Waldmann, T. & Muller, J. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci U S A. 103, 12075–12080 (2006).
Huck, K. et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest 119, 1350–1358 (2009).
Miller, A., Wilcox, H., Lai, Z. & Berg, L. Signaling through Itk Promotes T Helper 2 Differentiation via Negative Regulation of T-bet. Immunity 21, 67–80 (2004).
Gomez-Rodriguez, J. et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 31, 587–597 (2009).
Schaeffer, E. et al. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol 2, 1183–1188 (2001).
Weinreich, M., Odumade, O., Jameson, S. & Hogquist, K. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 11, 709–716. Epub 2010 Jul 2014. (2010).
Huang, W., Kannan, A., Huang, F., Hu, J. & August, A. ITK tunes IL-4 induced development of innate memory CD8+ T cells in a γδ and invariant NKT cell independent manner. J. Leuk. Biol., In press, (2014).
Berg, L. J. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol 7, 479–485 (2007).
Bachmann, M., Littman, D. & Liao, X. Antiviral immune responses in Itk-deficient mice. J. Virol. 71, 7253–7257 (1997).
Hu, J., Sahu, N., Walsh, E. & August, A. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 37, 2892–2899 (2007).
Joshi, N. S. et al. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J Immunol 187, 4068–4076 (2011).
Nayar, R. et al. TCR signaling via Tec kinase ITK and interferon regulatory factor 4 (IRF4) regulates CD8+ T-cell differentiation. Proc Natl Acad Sci U S A 109, E2794–2802 (2012).
Feinerman, O., Veiga, J., Dorfman, J. R., Germain, R. N. & Altan-Bonnet, G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science 321, 1081–1084 (2008).
Stefanova, I. et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol 4, 248–254 (2003).
Fowler, C. C., Pao, L. I., Blattman, J. N. & Greenberg, P. D. SHP-1 in T cells limits the production of CD8 effector cells without impacting the formation of long-lived central memory cells. J Immunol 185, 3256–3267 (2010).
Teixeiro, E. et al. Different T cell receptor signals determine CD8+ memory versus effector development. Science 323, 502–505 (2009).
Knudson, K. M., Hamilton, S. E., Daniels, M. A., Jameson, S. C. & Teixeiro, E. Cutting edge: The signals for the generation of T cell memory are qualitatively different depending on TCR ligand strength. J Immunol 191, 5797–5801 (2013).
Smith-Garvin, J. E. et al. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood 116, 5548–5559 (2010).
Iborra, S. et al. N-ras couples antigen receptor signaling to Eomesodermin and to functional CD8+ T cell memory but not to effector differentiation. J Exp Med 210, 1463–1479 (2013).
Andreotti, A. H., Schwartzberg, P. L., Joseph, R. E. & Berg, L. J. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol 2, a002287 (2010).
Joshi, N. S. et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Kannan, A., Huang, W., Huang, F. & August, A. Signal transduction via the T cell antigen receptor in naive and effector/memory T cells. Int. J. Biochem. & Cell Biol. 44, 2129–2134 (2012).
Acknowledgements
We thank Amie Wood for animal care, Misty Pocwierz for genotyping and members of the August lab for comments. This work is funded by NIH grant AI051626 & AI073955 to A.A. and USDA-NIFA postdoctoral fellowship to F.H.
Author information
Authors and Affiliations
Contributions
F.H., W.H., J.B., T.C., S.D. and Y.B. performed experiments. F.H., W.H. and A.A. interpreted data. F.H., W.H. and A.A. prepared figures and wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Huang, F., Huang, W., Briggs, J. et al. The tyrosine kinase Itk suppresses CD8+ memory T cell development in response to bacterial infection. Sci Rep 5, 7688 (2015). https://doi.org/10.1038/srep07688
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07688
This article is cited by
-
ITK independent development of Th17 responses during hypersensitivity pneumonitis driven lung inflammation
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.