Abstract
The composition, ecology and environmental conditions of mesophotic coral ecosystems near the lower limits of their bathymetric distributions remain poorly understood. Here we provide the first in-depth assessment of a lower mesophotic coral community (60–100 m) in the Southern Caribbean through visual submersible surveys, genotyping of coral host-endosymbiont assemblages, temperature monitoring and a growth experiment. The lower mesophotic zone harbored a specialized coral community consisting of predominantly Agaricia grahamae, Agaricia undata and a “deep-water” lineage of Madracis pharensis, with large colonies of these species observed close to their lower distribution limit of ~90 m depth. All three species associated with “deep-specialist” photosynthetic endosymbionts (Symbiodinium). Fragments of A. grahamae exhibited growth rates at 60 m similar to those observed for shallow Agaricia colonies (~2–3 cm yr−1), but showed bleaching and (partial) mortality when transplanted to 100 m. We propose that the strong reduction of temperature over depth (Δ5°C from 40 to 100 m depth) may play an important contributing role in determining lower depth limits of mesophotic coral communities in this region. Rather than a marginal extension of the reef slope, the lower mesophotic represents a specialized community and as such warrants specific consideration from science and management.
Similar content being viewed by others
Introduction
Mesophotic coral ecosystems (MCEs) are light-dependent coral communities that occur in the tropics and subtropics between depths of 30–40 m to over 150 m1. The upper limit of MCEs is arbitrarily defined by the maximum depth limit of conventional SCUBA diving (marking the beginning of a relatively unexplored depth zone), but roughly coincides with the depth where the reef community starts to transition from shallow to deep-water fauna2 and where active reef building generally ceases as bio-erosion rates start to exceed those of reef accretion3. On the other hand, the lower boundary of MCEs is defined by the lower depth limit of light-dependent corals1 and can vary strongly between locations depending on physical and bathymetrical parameters4. Within the boundaries of this mesophotic zone, there is a further subdivision of the “upper mesophotic” (30–60 m) and “lower mesophotic” (>60 m). Most of the recent studies on MCEs have focused on this upper mesophotic depth zone, due to the overlap in species composition and potential connectivity with shallow reef communities. In comparison, the lower mesophotic zone remains understudied due to the increased logistical complexity associated with manned exploration of these depths. Visual surveys with remote camera systems have yielded descriptions at the functional group level (reviewed in Kahng et al.4), but detailed knowledge on the composition and ecology of lower mesophotic coral communities and the abiotic and biotic factors structuring these communities remains limited at present.
The first attempts to explore lower mesophotic coral ecosystems in the Caribbean were made using manned submersibles in the early seventies in Honduras, Jamaica and Belize5,6,7,8, followed by similar efforts in the northwestern Gulf of Mexico and Bermuda during the mid-eighties9,10. Since then, research efforts were sparse, until “inspection class” remotely operated and autonomous underwater vehicles became available as a more affordable alternative to manned submersibles (e.g. Refs. 11,12,13). Subsequent surveys found that lower mesophotic reef systems in the Caribbean share a similar geomorphology, characterized by steep walls that were formed during the Wisconsin Low stillstand14. Lower mesophotic coral communities in the Caribbean are generally dominated by species of the genera Agaricia and Madracis, but other species such as Montastraea cavernosa and Stephanocoenia intersepta can be locally abundant4. For the Southern Caribbean, benthic observations in the lower mesophotic zone are limited to anecdotal observations from deep SCUBA dives, reporting a community15,16,17 similar to those observed for the Northern Caribbean. Many of the species reported at lower mesophotic depths are also dominant members of shallow reefs, but it is unclear whether the distinct environmental conditions at mesophotic depths have lead to local adaptation or cryptic speciation of such “shallow-water” species18. In addition, lower mesophotic corals may harbor distinct photosynthetic endosymbionts (Symbiodinium)17,19 adapted to the low irradiance level or spectral composition characteristic of these depths. Molecular approaches are required to assess such genetic differences and will ultimately help address the question if lower mesophotic ecosystems represent specialized coral communities or marginal extensions of their shallower counterparts.
Surveys from other parts of the Caribbean have shown that scleractinian corals are generally abundant down to approximately 60 m, after which they rapidly decrease in abundance with a lower depth limit of ~80 m4. Corals are occasionally found deeper and the deepest zooxanthellate corals recorded for the Western Atlantic were Agaricia spp. growing at 119 m in the Bahamas20. The correlation between observed local depth limits of zooxanthellate corals and extrapolated light attenuation coefficients (with corals being observed down to greater depths with increasing water transparency) has led to the hypothesis that light is the primary factor in determining the lower limit of mesophotic coral ecosystems4. Although light attenuation over depth will ultimately pose a theoretical depth limit to zooxanthellate corals, other environmental factors such as substrate availability, sedimentation and temperature may become limiting prior to that. The accumulation of sediment on near horizontal substrates can limit the occurrence of coral communities at depth21,22 and the presence of strong thermoclines observed on some Indo-Pacific reefs has been linked to the localized absence or degraded state of mesophotic coral communities3,23. Short-term measurements from the Caribbean and other regions indicate that temperatures usually remain conducive to coral growth even below the observed lower depth limit of zooxanthellate corals, questioning the importance of temperature limiting the vertical distribution of coral communities4. However, long-term environmental data from lower mesophotic depths are lacking and it therefore remains unknown whether the occurrence of seasonal temperature variations or episodic cold-water events may be limiting the development of coral communities at depth.
Although MCEs are valued for their ability to act as refugia from certain disturbances that affect shallow reefs, they are certainly not immune to all anthropogenic and natural stressors (reviewed in Bongaerts et al.24). Some disturbances can have an indiscriminate effect over depth affecting both shallow and mesophotic reefs (e.g. sedimentation and nutrient enrichment), whereas others can be confined to deeper communities only (e.g. cold-water bleaching and deep-water macroalgal blooms)24. Nonetheless, the frequency and effect of disturbances on mesophotic reefs is largely unknown as is the capacity of MCEs to recover post-disturbance through external coral recruitment and regrowth of surviving coral colonies25. Studies on coral growth rates in deeper water are limited and generally describe lower growth rates compared to those of similar species living in shallow water26,27,28. For lower mesophotic depths, our knowledge on coral growth rates comes from a single colony of Leptoseris hawaiiensis29 in Hawaii and several Leptoseris fragilis colonies in the Red Sea30. Insight into the growth rates (and therefore recovery potential) of corals living at mesophotic depths is important, particularly given the predominant plate-like morphology and fragile skeletons that make these communities more susceptible to sedimentation and breakage24.
Here, we provide the first in-depth assessment of a lower mesophotic coral community (60–100 m) in the Southern Caribbean to answer the following questions: (1) Does the lower mesophotic zone represent a specialized community of corals and associated Symbiodinium? (2) Does the temperature regime in the lower mesophotic differ substantially from shallow depths and could it play a role in governing lower coral depth distribution limits? (3) Are mesophotic coral communities characterized by slow coral growth, that reduces their ability to recover from disturbances? Using a manned submersible (“Curasub”) we visually assessed the community structure of a large reef section (“Seaquarium”) on the leeward side of Curaçao and collected coral specimens for genotyping of coral host and endosymbiont assemblages (using mitochondrial markers for the coral host and both a mitochondrial marker and ITS2/DGGE for the associated Symbiodinium). Temperature loggers were deployed to characterize long-term temperature conditions over depth and a small transplantation experiment was conducted to assess the growth and survival of coral fragments transplanted below and above their observed lower depth limits. We discuss the extent of overlap between upper and lower mesophotic communities, assess the observed growth rates in light of those observed for shallow-reef corals and discuss the potential role of temperature as a factor limiting the depth distribution of mesophotic coral communities in this part of the world.
Results
Coral community structure
The substrate of the lower mesophotic zone (>60 m) at the study site on the leeward side of Curaçao (Fig. 1) was dominated by sediment, interrupted by patches of hard substrate harboring sparse communities of zooxanthellate corals. From the submersible footage, a total of 438 zooxanthellate coral colonies were observed at depths ≥60 m, with the deepest colonies recorded at a depth of 91 m (Madracis pharensis; n = 2). The coral community was dominated by Agaricia grahamae between 60–75 m and M. pharensis between 80–90 m (Fig. 2). M. pharensis could not be distinguished from Madracis senaria in the submersible footage, but collected specimens from ≥60 m depth were all identified as M. pharensis (n = 53) (Fig. 3). Similarly, A. grahamae could not be distinguished from Agaricia undata in the video transects. From the total number of collected Agaricia specimens ≥60 m depth at the study site (n = 65) several specimens at each depth (1 out of 17 at 60 m, 1 out of 19 at 75 m, 6 out of 25 at 80 m and 2 out of 4 at 90 m) were later identified in the lab as A. undata rather than A. grahamae (Fig. 3). In addition to A. grahamae, A. undata and M. pharensis, the only other zooxanthellate corals that were observed in the video transects were Montastraea cavernosa (n = 1), Agaricia lamarcki (n = 2) and Stephanocoenia intersepta (n = 1) at respectively 63, 74 and 81 m depth. Colony size frequency groups (Fig. 2) were not significantly different across depths for Agaricia (Global R = −0.044, P = 0.979) and Madracis (Global R = −0.003, P = 0.531). Colony morphologies of Agaricia consisted of unifacial plates (sometimes encrusting over dead Agaricia skeletons), whereas Madracis exhibited thinly encrusting colonies, usually following the shape of the underlying substrate. No signs of coral bleaching were observed, however human debris was frequently observed between 60–90 m, consisting of glass bottles, fishing line, ropes and small “disposable” anchors.
Location of the study site (“Seaquarium”), comparative site (“Buoy 0/1”) and positioning of Curaçao within the Caribbean (inset).
Map was modified from Bongaerts et al.17 under Creative Commons Attribution License 4.0.
Temperature regimes across a depth gradient
Temperatures were monitored at 10-minute intervals between April 2013 and January 2014 (Fig. 4), with average temperatures varying less than 0.3°C between 10, 25 and 40 m (27.6–27.9°C), but then declining linearly between 40 and 100 m (~1.68°C per 20 m depth increase; R2 = 0.997). Seasonal variation was observed with coldest temperatures measured around January 2014 (last month of data collection). Rapid (and sometimes large) temperature fluctuations occurred frequently (~120 drops of 1–3°C within 10–30 minutes) at upper mesophotic depths (40–60 m), likely due to cold-water influxes originating from deeper water. However, in the spring (April-June 2013) few departures were noted, especially at 40 m (only three recorded drops of ≥1°C within 10–30 minutes) where temperatures were similar to those measured at shallow depths (Fig. 4). At lower mesophotic depths (80–120 m), temperatures were substantially lower (~2–6°C) compared to shallow depths throughout the year. Across each depth interval below 40 m, there was an increase in exposure to colder temperatures (Fig. 5), with exposures to >25°C decreasing from 87 to 3% of the time and exposures to temperatures of <22°C increasing from 0 to 25% of the time between 60 and 100 m depth.
Coral host sequencing analysis
Four mitochondrial haplotypes were identified for mesophotic Agaricia specimens from the study site (“Seaquarium”) and a comparative location (“Buoy 0/1”) using a section of the nad5 region (473 bp including indels). The majority of sequenced lower mesophotic (n = 14) and upper mesophotic (n = 21) A. grahamae specimens shared a single dominant haplotype independent of depth and location (Haplotype 1a; Fig. 6), which was also shared with upper mesophotic A. lamarcki (n = 7) specimens that were included for comparison. The four specimens of A. undata shared a distinct haplotype (Haplotype 1d; Fig. 6). A similar pattern was observed for the cox1-1-rRNA region (649 bp including indels), with the majority of upper and lower mesophotic A. grahamae (and upper mesophotic A. lamarcki specimens) belonging to a single clade regardless of depth and location (“Clade 1”; Fig. 6). A. undata specimens (n = 7) were observed with two haplotypes belonging to a distinct clade (“Clade 2”), although one specimen identified as A. lamarcki was also observed with one of these haplotypes. These patterns confirm the pervasive polyphyly in the genus Agaricia previously observed using the atp6 marker, as well as the inability of mitochondrial markers to distinguish between A. lamarcki and A. grahamae17. Seven of the nine Agaricia colonies that were used for the transplantation experiment were also sequenced, with three different haplotypes observed for the A. grahamae colonies using the cox1-1-rRNA region and the A. undata colony grouping in the separate “undata” clade (“Clade 2”).
Phylogenetic trees of Agaricia specimens based on the mitochondrial nad5 region and the mitochondrial cox1-1-rRNA region.
Lower mesophotic specimens (74–88 m) are indicated in red. Bootstrap values are based on maximum likelihood (ML) with only probabilities of main clades over 80% shown. Depth range, number of specimens, location ([Seaq] = “Seaquarium”, [Buoy] = “Buoy 0/1”) and Symbiodinium profile (colored boxes) are given. Asterisks (*) indicate specimens used for the transplantation experiment. Sequences from GenBank are indicated by their Accession Number.
Five distinct mitochondrial haplotypes were observed for the M. pharensis specimens that were sequenced using the atp8 region (953 bp including indels), representing two distinct clades (Fig. 7). One clade (“Clade 2”) only contained shallow specimens (15 m; n = 7), whereas the other clade (“Clade 1”) contained a mix of shallow and mesophotic specimens (15–90 m; n = 40). All lower mesophotic samples shared a single haplotype (n = 14) that was only shared with two specimens from other depths (from 15 and 60 m) (Haplotype 1a). The remaining specimens from upper mesophotic and shallower depths belonged to three other closely-related haplotypes (Haplotypes 1b–d). Within the main M. pharensis clade (“Clade 1”), 37% of molecular variance can be explained by depth zone (i.e. shallow, upper mesophotic and lower mesophotic) (φDEPTH-TOT = 0.381; P = 0.0002) with no significant effect of location (0% of molecular variance; φLOC-DEPTH = −0.044; P = 0.5607).
Phylogenetic tree of Madracis specimens based on the mitochondrial atp8 region.
Lower mesophotic specimens (80–90 m) are indicated in red. Bootstrap values are based on maximum likelihood (ML) with only probabilities over 80% shown. Depth range, number of specimens, location ([Seaq] = “Seaquarium”, [Buoy] = “Buoy 0/1”) and Symbiodinium profile (colored boxes) are given. Sequences from GenBank are indicated by their Accession Number.
Symbiodinium diversity
All sampled colonies of A. grahamae and A. undata (n = 58) harbored the same Symbiodinium ITS2 profile across their depth range (40–88 m). This ITS2 profile matches the “P4” profile previously described for A. grahamae in Curaçao, containing four distinct ITS2 sequences of which one matches with the Symbiodinium C3 and one with C11 sequence17. Similarly, for the Symbiodinium mitochondrial cox1 region, all colonies were found to harbor the same haplotype (GenBank Accession Number KP178810).
M. pharensis colonies harbored either Symbiodinium B7 or B1519, with B7 only observed in shallow colonies (15 m; n = 7) and colonies at depths beyond 25 m hosting exclusively B15 (n = 40). All of the M. pharensis colonies harboring B7 belonged to host “Clade 2” (based on the mitochondrial atp8 region), whereas all of the colonies harboring B15 belonged to one of the “Clade 1” haplotypes (Fig. 7), with 98% of the molecular variance explained by symbiont type (φSYM-TOT = 0.981; P = 0.0001).
Transplantation experiment
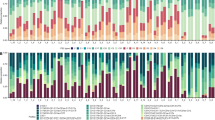
Coral fragments originating from eight A. grahamae colonies and one A. undata colony at ~80 m depth were transplanted to racks at 60, 80 and 100 m (n = 18 per rack). The fragments were visually assessed using the submersible 6–7 days after initial deployment and no signs of bleaching or partial mortality were observed. After 5 months, all fragments except for one on the 80 m rack showed signs of bleaching and/or mortality. At the end of the experiment (after ~10 months), all but three fragments on the 80 m rack had died (Fig. 8) and the surviving corals showed negligible growth (linear increase ranging from 1.8–2.4 mm) (Supplement Table 3, 4). On the 100 m rack, all but one of the fragments were found to be fully pigmented with no signs of bleaching or mortality after 5 months, however after 10 months most corals were still alive but almost all showed signs of bleaching and/or partial mortality. Again, growth was negligible (linear increase ranging from 0.0–3.7 mm) (Supplement Table 3, 4). On the 60 m rack, most fragments (13 out of 15) were still alive after 10 months (the 60 m rack was not checked after 5 months) and all but two had grown, with the six coral fragments that were still fully pigmented exhibiting an average linear increase in diameter of 19.4 mm (ranging from 5.7 to 27.2 mm) (Supplement Table 3, 4).
Transplantation experiment visual summary: health status of individual fragments at the beginning of the experiment (March 2013), in the middle (August 2013) and at the end (February 2014).
Fragments are grouped in pairs representing “clones” taken from the same A. grahamae colony, with the pair noted in blue coming from the same A. undata colony. Fragments from the same colony are depicted at the same position on each of the “racks” for comparative purposes, although in reality fragments were assigned to random positions. On the right, two examples of fragments from 60 m depth (indicated in the schematic by #1 and #2) are shown to demonstrate the increase in fragment diameter over the duration of the experiment.
Discussion
The lower mesophotic community at our study site was dominated by Agaricia grahamae and Madracis pharensis and to a lesser extent Agaricia undata, confirming previous observations in the Western Atlantic reporting Agaricia and Madracis spp. as the dominant members of the lower mesophotic5,8,9,20,31,32,33. Although A. undata is considered rare in Curaçao17,34, we can confirm early observations by Bak15,21 that A. undata does regularly occur at lower mesophotic depths, accounting for about a quarter of our collected Agaricia specimens (8 out of 29) between 80–90 m. Helioseris cucullata and Montastraea cavernosa are two other species that have been regularly reported at depths in excess of 70 m at other locations in the Caribbean4, but we observed only one colony of M. cavernosa at these depths. H. cucullata was only observed in this study at depths shallower than 60 m and in small numbers, which may be related to the reported strong decline of this species in Curaçao over the past several decades35,36. Both M. cavernosa and Stephanocoenia intersepta are normally relatively abundant at ~60 m depth at other sites around Curaçao, with species such as Madracis formosa, Agaricia lamarcki and Scolymia cubensis occurring at lower densities (Bongaerts and Vermeij, personal observations). The (relative) absence of these species at lower mesophotic depths (≥60 m) at our study site is therefore not necessarily representative for other reefs around Curaçao. Nonetheless, the observations here confirm that deep-water specialist Agaricia species (i.e. A. grahamae and A. undata) dominate the lower mesophotic communities beyond 70 m in Curaçao, with M. pharensis occupying the lowest reaches of the mesophotic.
Our molecular data provides further insights into whether the lower mesophotic zone represents a unique, specialized community or a “marginal” extension of upper mesophotic reef communities (30–60 m). While the upper mesophotic zone still hosts many “depth-generalist” coral species (~25–40%) that are also found in shallow-water habitats21,24, the lower mesophotic community (at the study site) consists mainly of deep-water specialists (i.e. Agaricia grahamae and Agaricia undata). An exception is the species M. pharensis, which is also commonly found in shallow-water16. However, the host phylogeny for this species confirms previous work reporting the presence of two divergent lineages18 (Fig. 7), that likely represent distinct (cryptic) species. These two M. pharensis lineages each associate with a distinct Symbiodinium type (“Clade 1” with Symbiodinium B15 and “Clade 2” with Symbiodinium B7) and exhibit distinct depth distributions, with the B15-associated lineage found predominantly at mesophotic depths18,19 (Fig. 7). Interestingly, a further genetic subdivision was found within this clade, between M. pharensis hosts collected from upper mesophotic (40–60 m) and lower mesophotic depths (80–90 m), with depth zones explaining 37% of molecular variance. However, the extent of genetic divergence between these mesophotic populations remains unclear as only a single mitochondrial marker was used. No genetic subdivision was found between A. grahamae specimens from upper mesophotic and lower mesophotic depths (nor between the species A. grahamae and A. lamarcki) using conserved mitochondrial markers, with all individuals also hosting the same Symbiodinium subtype (assessed for both the ITS2 and cox1 region). The two Symbiodinium types found in association with mesophotic Agaricia and Madracis spp. are rare in shallow reef communities17,19 and may represent Symbiodinium types specialized to deep-water conditions (e.g. low irradiance)17,19,37. These findings corroborate that the upper mesophotic is a transition zone hosting coral-endosymbiont associations that are shared with both the shallow and lower mesophotic reef2,24, whereas the lower mesophotic reef hosts a specialized “deep-water” coral and endosymbiont community.
Fragments of Agaricia colonies were transplanted from their original depth at ~80 m to a shallower depth (60 m), back to their original depth (80 m) and a depth exceeding their natural distribution range at this site (100 m). Surprisingly, survival rates were extremely low on the “control” rack (80 m) compared to the shallower and deeper rack, despite the fact that fragments were transplanted back to their original depth in the direct vicinity of existing Agaricia colonies. Factors explaining this high mortality may be due to unnoted differential handling or positioning of the rack at a location unfavorable to coral growth (e.g. with increased sedimentation), however an obvious cause could not be identified. The high mortality overall might reflect a lowered ability of corals at these depths (due to physiological limitations) to cope with the stress associated with these manipulations. Although no noticeable growth was observed on the 80 and 100 m racks (linear increases of <4 mm), “healthy” A. grahamae fragments left on the 60 m rack (n = 6) grew at an average (extrapolated) rate of 22.0 mm yr−1, with a maximum (extrapolated) rate of 30.8 mm yr−1 (Supplement Table 4). These represent some of the first growth estimates measured at lower mesophotic depths (60–100 m), with other records being those from Leptoseris fragilis in the Red Sea (radial growth of 5–8 mm yr−1)30 and that from a single Leptoseris hawaiiensis colony in Hawaii (radial growth of 11 ± 3 mm yr−1)29. Interestingly, the growth rates we observed at 60 m are similar to the maximum diameter growth rates (24.8–33.6 mm yr−1) previously measured for juvenile and adult A. humilis and A. agaricites colonies at shallow and intermediate depths (5–30 m) in Curaçao38,39,40. Calcification rates are however likely to be lower, as the skeletons of agariciids at lower mesophotic depths are usually much thinner compared to shallow depths29,30. The minimal growth of the few “healthy” fragments left on the 80 and 100 m racks (n = 2 on each rack) could indicate that growth rates may decline rapidly at depths beyond 60 m, but given the low sample sizes this should be assessed further. Nonetheless, this initial assessment demonstrates that the growth rates of corals at mesophotic depths (i.e. 60 m) are not necessarily stunted and can be similar to that observed in shallow water, which implies that the coral communities in this depth zone have some ability to recover from physical disturbances.
At the study site, zooxanthellate corals were observed down to a maximum depth of ~90 m. The extrapolated proportion of surface PAR irradiance reaching these depths (assuming a constant Kd(PAR)) would be 0.13 or 0.30% depending on which reported Kd(PAR) value for Curaçaoan waters is used (0.07219 or 0.06341). Although these values match extrapolated values reported for other parts of the Caribbean (0.15–0.29%), corals have been observed growing at even lower extrapolated values such as 0.007% in Hawaii (reviewed in Kahng et al.4). Extrapolated values only provide very rough estimates, as optical transparency of the water column is often greater in deeper water and irradiance values at depth are strongly influenced by local bathymetric features22. Usually, in the lower mesophotic, a marked decrease in colony size over depth is observed30, or exclusively small colonies are found close to the lower depth distribution42,43,44, which has been attributed to light gradually becoming a limiting factor to coral growth4. At the study site no such pattern was identified for either Agaricia or Madracis, with large colonies of both genera present close to the rather abrupt lower depth limit (Fig. 2). As such, it may be that other environmental factors play a contributing role in determining lower depth distributions of zooxanthellate corals at the study site. Lack of suitable substrate at depth can be an important factor in determining lower depth limits21,22, particularly in the presence of sandy terraces, steep walls or undercut ridges and can prevent the development of coral communities at depths where irradiance levels should still be able to sustain coral growth. Although the slope angle at our study site was mostly very steep at depths beyond 90–100 m, there were several deeper outcrops and ledges consisting of “suitable” hard substrate. Thus, despite the important role of substrate availability and sedimentation in driving the spatial patchiness of mesophotic communities, they cannot explain the observed abrupt depth limit at the study site.
Temperatures do not appear to decrease dramatically across the shallow-mesophotic depth gradient in many regions that support coral reefs and are therefore not considered to play a role in determining depth limits of zooxanthellate corals at tropical locations4. Similar conclusions might have been drawn for our study site if temperatures were only measured episodically (e.g. at certain times in July 2013 temperatures were identical from 10 to 80 m depth) (Fig. 4), however over longer time intervals it becomes apparent that thermal conditions are in fact very distinct between shallow and mesophotic depths on Curaçao. A steep decrease in temperatures and increased variability was observed at depths below 40 m16,45. Just below the observed depth limit of zooxanthellate corals (100 m depth), temperatures were on average ~5°C lower than at shallow depths (i.e. 22.8°C at 100 m versus 27.6–26.9°C at 10–40 m) and exposures to temperatures <22°C occurred regularly (Fig. 5). Although these temperatures are still well above the known lower temperature limits observed for zooxanthellate corals globally (15–18°C)46, they are much lower than seawater temperatures recorded in shallow waters around Curaçao45,47 and Bonaire48. The last month of our transplantation experiment was also the coldest (minimum temperature recorded at 100 m was 19.8°C) (Fig. 4) and the observed bleaching and partial mortality observed on the 100 m transplant rack (Fig. 8) could be the result of long- or short-term exposure to these colder temperature conditions. Although other causes such as long-term exposure to suboptimal light conditions cannot be ruled out, deep-water bleaching (≥30 m) of Agaricia colonies has previously been observed in Curaçao45 and Bonaire48. This bleaching could be attributed to cold-water influxes likely due to the shallowing of a deep-water thermocline. The bleaching in Curaçao was followed by substantial mortality of Agaricia on the deep reef (Bak, unpublished results), demonstrating that episodic cold-water exposure can be an important selective force for deep reef communities. As such, we hypothesize that temperature is a contributing factor in determining lower depth limits at the study site and may be limiting the development of coral communities at depth through long-term exposure to colder temperatures or short-term exposure to episodic cold-water anomalies49. As cold-water influxes on deep reefs can be dependent on bathymetry50, it could well be that lower depth limits also vary around the island depending on the reef profile.
Overall, this study demonstrates that the lower mesophotic represents a specialized coral community, rather than a marginal extension of the reef slope and should be regarded as a distinct entity within the coral reef ecosystem. Only a few dominant zooxanthellate coral species were found at these depths, but all of them represented deep-specialists, hosting specialist Symbiodinium types, that only rarely occur on the shallow reef. Although our initial findings on growth rates at the transition of the upper and lower mesophotic zone (i.e. 60 m depth) indicate a certain potential for recovery, the dominant flat and plate-like morphologies at lower mesophotic depths make these coral communities susceptible to sedimentation from e.g. coastal development45 and their thin skeletons make them more prone to direct physical disturbances. Indirect effects of artisanal and recreational fisheries can be substantial and we observed a lot of human debris ranging from discarded glass bottles to small “disposable” fishing anchors. Currently, lower mesophotic coral ecosystems in this region do not receive any form of protection as they fall outside of the “Marine Park” boundaries in both Curaçao and Bonaire, which are defined by the 60 m isobaths. We argue that these ecosystems deserve separate consideration during marine conservation planning, taking into account their distinct set of stressors and to provide them with adequate protection despite being located “out-of-sight”.
Methods
Study site, environmental conditions and specimen collections
As part of the “Catlin Seaview Survey”, we assessed the lower mesophotic coral communities (60–100 m) near the “Seaquarium” reef (12°05.067′N, 68°53.900′W) on the leeward site of Curaçao in the Southern Caribbean. Submersible dives were conducted using the “Curasub” operated by the “Substation Curaçao” between March-April 2013 and in February 2014. The manned submersible was equipped with a large front-viewing dome for observations, two hydraulic manipulator arms for gear deployment and sample collection and high-definition video cameras for recording. Roughly 2 kilometres of the study site was explored along depth isobaths, with in total ~15 hours spent within the target depth range (60–100 m). Video fragments were analysed after the dives and observed scleractinian corals were scored for their identity (down to genus level or species level where possible) and categorized into a rough size class (0–15 cm, 20–50 cm, >50 cm) using the chisel or submersible “specimen basket” as a size reference. As surveying was not done along a transect line and effort was not uniform across depth intervals, only relative abundances and size class distributions could be determined. Weighted HOBO temperature loggers were deployed using the submersible at 60, 80, 100 and 120 m on the 21st of March 2013, with additional loggers being placed on SCUBA at 10, 25 and 40 m on the 31st of March. Temperatures were logged every 10 minutes until the 16th of January 2014 (60, 80,100 and 120 m loggers) and 27th of January 2014 (10, 25 and 40 m loggers) and were recovered on the 6th of February 2014. Samples were collected from a total of 123 scleractinian coral colonies between 60–100 m for genetic analyses and to help with taxonomic identification. Additional specimens (including several different Agaricia species) were collected from shallow and upper mesophotic depths (15–40 m) at the same location (n = 21) and shallow and upper mesophotic depths (15–60 m) at another location (n = 57) on the leeward side of the island (“Buoy 0/1”; 12°07.478′N, 68°58.430′W) for comparative purposes. Small fragments were subsampled from each specimen and stored in salt-saturated buffer solution containing 20% DMSO and 0.5 M EDTA, with the remaining specimens cleaned in household bleach solution (4% hypochlorite) for 36–72 h, rinsed in freshwater and dried for taxonomic identification. Coral species were identified following the taxonomic features specified by Veron and Stafford-Smith51 and Humann & Deloach34.
Coral host genotyping
Subsections of the mitochondrial nad5 region and cox1-1-rRNA intron were amplified for a total of 60 Agaricia specimens (Supplement Table 1) using respectively the NAD5_316F/NAD1_157R53 and AGAH/AGAL54 primer pairs. For 45 Madracis specimens, a small portion of the nad5 region, the entire trnW and atp8, an intergenic spacer and a small portion of cox1 were amplified (Supplement Table 2) using the FNAD5-RCOI3 primer pairs55. PCR amplifications were performed with concentrations and cycling protocol as reported for the atp6 marker in Bongaerts et al.17. PCR products were run on agarose gels, cleaned using ExoSAP-IT and sequenced in both the forward and reverse directions (ABI BigDye Terminator chemistry, Australian Genome Research Facility). All chromatograms were analyzed using Codoncode Aligner, with sequences being aligned with MUSCLE and blasted on GenBank (http://www.ncbi.nlm.nih.gov/BLAST). Phylogenetic analyses of sequences were performed using maximum likelihood in MEGA 656 under the delayed transition setting and calculation of bootstrap support values based on 1000 replicates, using additional sequences retrieved from GenBank as outgroups. The best-fit model of molecular evolution was selected by hierarchical Akaike information criterion (AIC) using MEGA 656 with a HKY+I, K2+G and T92 model best describing respectively the nad5, cox1-1-rRNA and atp8 data under a log likelihood optimality criterion.
Symbiodinium genotyping
Symbiodinium identity was established for specimens that were sequenced for the coral host (n = 105), by amplifying the internal transcribed spacer (ITS2) region using Symbiodinium-specific primers57 as described in Bongaerts et al.58. To identify the dominant Symbiodinium types in each sample, the amplified ITS2 fragments were separated using denaturing gradient gel electrophoresis (DGGE) on a CBScientific System following conditions outlined in Sampayo et al.59 and compared to samples with known profiles from previous studies17,19. In addition, the Symbiodinium cox1 region was amplified for 44 Agaricia specimens using the COX1_FOR2 and COX1_REV1 primers60 to test for potential variability not detected using ITS2/DGGE following the PCR, sequence and alignment protocol as outlined above for the host sequences.
Transplantation experiment
A total of 9 Agaricia colonies were collected between 79–84 m depth on the 20th of March 2013 using the submersible and brought up to a depth of 40 m, where divers transferred the colonies from the submersible collection basket to a large plastic container with a lid. The colonies were then brought to the surface in the dark (to avoid light-stress in the shallow) and transferred to a blacked out, temperature-controlled laboratory. There, six roughly equal-sized fragments were taken from each of the colonies using bonecutters or a hammer and chisel, with two fragments randomly assigned to each of three transplantation racks. From the remaining coral material, small fragments were subsampled and stored in 20% DMSO and 0.5 M EDTA for DNA analyses and a skeletal voucher was bleached for taxonomic identification. Fragments were mounted onto calcium carbonate plugs using underwater epoxy putty and cyanoacrylate glue and randomly assigned to positions on a PVC rack. Transplantation racks were kept in dark conditions in large plastic containers containing a small powerhead to provide some water circulation overnight. In the morning of the 21st of March 2013, the closed-lid containers containing the transplant racks were brought back to a depth of 40 m by divers, where they were taken out of the containers and placed directly onto the sand. The racks were photographed using a still camera system mounted on a PVC frame that fitted into the transplant rack (ensuring a perpendicular angle) and a ruler in the plane of the coral colonies for scale. The three transplant racks were then picked up using the manipulator of the submersible and slowly brought to the respective depths of 60, 80 and 100 m where they were deployed. After 6–7 days, the three transplant racks were revisited using the submersible to visually assess the status of the fragments. After ~5 months (6th of August 2013), two of the transplant racks (80 and 100 m) were revisited using the submersible (the 60 m transplant rack was not revisited due to time restrictions) and photographed using the on-board camera and lights. After ~10 months, the transplant racks were recovered by the submersible (6th of February 2014) and brought to a sandy patch at a depth of ~25 m, where they were rephotographed in the same way as during the initial deployment.
Growth rate measurements
Surface area measurements of the colonies were carried out using ImageJ52 for the beginning (t = 0) and end of the experiment (t = 46 weeks/322 days). Coral fragments were encircled with the polygon tool and surface area was measured in square millimeters using the photographed scale for reference. Surface areas were then converted to a linear diameter (following van Moorsel39) and linear growth over the 46 weeks was determined by subtracting the two linear diameters (at the beginning and the end of the experiment) and then linearly extrapolated for samples that were still alive to a yearly linear growth rate (for comparison against previously published growth rates).
Statistical analyses
An analysis of similarity (One-way ANOSIM) was conducted for both Agaricia and Madracis to test for differences in relative colony size groups between depth zones using a Sorensen resemblance measure in the software package PRIMER v6. The relation between average temperature values (determined across the monitoring period) and depth was assessed using linear regression. The contribution of symbiont type on the molecular variance of M. pharensis nad5 haplotypes was assessed under the AMOVA framework using GenAlEx v661, as was the contribution of depth zone (i.e. shallow 15–25 m, upper mesophotic 40–60 m and lower mesophotic 70–90 m) and location (with location nested within depth zone).
Additional information
Accession codes Sequences have been deposited in NCBI GenBank under Accession Numbers KP178810-KP178821 and KP178901-KP178912.
References
Hinderstein, L. M. et al. Theme section on Mesophotic coral ecosystems: characterization, ecology and management. Coral Reefs 29, 247–251 (2010).
Kahng, E., Copus, M. & Wagner, D. Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr. Opin. Envir. Sust. 7, 72–81 (2014).
Grigg, R. W. Depth limit for reef building corals in the Au'au Channel, SE Hawaii. Coral Reefs 25, 77–84 (2006).
Kahng, S. E. et al. Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29, 255–275 (2010).
Hartman, W. D. Beneath Caribbean reefs. Discovery 9, 13–26 (1973).
Lang, J. C. Biological Zonation at the Base of a Reef: Observations from the submersible Nekton Gamma have led to surprising revelations about the deep fore-reef and island slope at Discovery Bay, Jamaica. Am. Sci. 272–281 (1974).
Ginsburg, R. N. & James, N. P. British Honduras by submarine. Geotimes 18, 23–24 (1973).
James, N. P. & Ginsburg, R. N. The Seaward Margin Of The Belize Barrier And Atoll Reefs (Blackwell, Oxford, 1979).
Rezak, R., Bright, T. J. & McGrail, D. W. Reefs And Banks Of The Northwestern Gulf Of Mexico: Their Geological, Biological, And Physical Dynamics (Wiley, New York, 1985).
Fricke, H. & Meischner, D. Depth limits of Bermudan scleractinian corals: a submersible survey. Mar. Biol. 88, 175–187 (1985).
Jarrett, B. D. et al. Strange bedfellows–a deep-water hermatypic coral reef superimposed on a drowned barrier island; southern Pulley Ridge, SW Florida platform margin. Mar. Geol. 214, 295–307 (2005).
Locker, S. D. et al. Geomorphology of mesophotic coral ecosystems: current perspectives on morphology, distribution and mapping strategies. Coral Reefs 29, 329–345 (2010).
Sherman, C. et al. Geomorphology and benthic cover of mesophotic coral ecosystems of the upper insular slope of southwest Puerto Rico. Coral Reefs 29, 347–360 (2010).
Liddell, W. D. & Ohlhorst, S. L. Geomorphology and community composition of two adjacent reef areas, Discovery Bay, Jamaica. J. Mar. Res. 39, 791–804 (1981).
Bak, R. P. M. Ecological aspects of the distribution of reef corals in the Netherlands Antilles. Bijdr. Dierkd. 45, 181–190 (1975).
Vermeij, M. J. A. & Bak, R. P. M. Species-specific population structure of closely related coral morphospecies along a depth gradient (5–60 m) over a Caribbean reef slope. B. Mar. Sci. 73, 725–744 (2003).
Bongaerts, P. et al. Sharing the slope: depth partitioning of agariciid corals and associated Symbiodinium across shallow and mesophotic habitats (2–60 m) on a Caribbean reef. BMC Evol. Biol. 13, 205 (2013).
Frade, P. R. et al. Semi-permeable species boundaries in the coral genus Madracis: introgression in a brooding coral system. Mol. Phylogenet. Evol. 57, 1072–1090 (2010).
Frade, P. R., De Jongh, F., Vermeulen, F., van Bleijswijk, J. & Bak, R. P. M. Variation in symbiont distribution between closely related coral species over large depth ranges. Mol. Ecol. 17, 691–703 (2008).
Reed, J. K. Deepest distribution of Atlantic hermatypic corals discovered in the Bahamas. Proc. 5th Int. Coral Reef Symp. 6, 249–254 (1985).
Bak, R. P. M. Coral reefs and their zonation in Netherlands Antilles. Stud. Geol. 4, 3–16 (1977).
Sheppard, C. Coral populations on reef slopes and their major controls. Mar. Ecol-Prog. Ser. 7, 83–115 (1982).
Wolanski, E., Colin, P., Naithani, J., Deleersnijder, E. & Golbuu, Y. Large amplitude, leaky, island-generated, internal waves around Palau, Micronesia. Estuar. Coast. Shelf S. 60, 705–716 (2004).
Bongaerts, P., Ridgway, T., Sampayo, E. M. & Hoegh-Guldberg, O. Assessing the ‘deep reef refugia'hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327 (2010).
Bongaerts, P., Muir, P., Englebert, N., Bridge, T. C. L. & Hoegh-Guldberg, O. Cyclone damage at mesophotic depths on Myrmidon Reef (GBR). Coral Reefs 32, 935–935 (2013).
Dustan, P. Growth and form in the reef-building coral Montastrea annularis. Mar. Biol. 33, 101–107 (1975).
Hughes, T. P. & Jackson, J. B. C. Population dynamics and life histories of foliaceous corals. Ecol. Monogr. 55, 142–166 (1985).
Leichter, J. J. & Genovese, S. J. Intermittent upwelling and subsidized growth of the scleractinian coral Madracis mirabilis on the deep fore-reef slope of Discovery Bay, Jamaica. Mar. Ecol-Prog. Ser. 316, 95–103 (2006).
Kahng, S. E. Growth rate for a zooxanthellate coral (Leptoseris hawaiiensis) at 90 m. Galaxea, J. Coral Reef Stud. 15, 39–40 (2013).
Fricke, H. W., Vareschi, E. & Schlichter, D. Photoecology of the coral Leptoseris fragilis in the Red Sea twilight zone (an experimental study by submersible). Oecologia 73, 371–381 (1987).
Goreau, T. F. & Wells, J. W. The shallow-water Scleractinia of Jamaica: revised list of species and their vertical distribution range. B. Mar. Sci. 17, 442–453 (1967).
Phillips, N. W., Gettleson, D. A. & Spring, K. D. Benthic biological studies of the southwest Florida shelf. Am. Zool. 30, 65–75 (1990).
Culter, J. K. et al. Pulley reef: a deep photosynthetic coral reef on the West Florida Shelf, USA. Coral Reefs 25, 228–228 (2006).
Humann, P. & DeLoach, N. Reef Coral Identification: Florida, Caribbean, Bahamas, Including Marine Plants (New World Publications, 1996).
Bak, R. P. M. & Engel, M. S. Distribution, abundance and survival of juvenile hermatypic corals (Scleractinia) and the importance of life history strategies in the parent coral community. Mar. Biol. 54, 341–352 (1979).
Vermeij, M. J., Bakker, J., Hal, N. V. D. & Bak, R. P. M. Juvenile coral abundance has decreased by more than 50% in only three decades on a small Caribbean island. Diversity 3, 296–307 (2011).
Lesser, M. P. et al. Photoacclimatization by the coral Montastraea cavernosa in the mesophotic zone: light, food and genetics. Ecology 91, 990–1003 (2010).
Bak, R. P. M. The growth of coral colonies and the importance of crustose coralline algae and burrowing sponges in relation with carbonate accumulation. Neth. J. Sea Res. 10, 285–337 (1976).
van Moorsel, G. Disturbance and growth of juvenile corals (Agaricia humilis and Agaricia agaricites, Scleractinia) in natural habitats on the reef of Curaçao. Mar. Ecol-Prog. Ser. 24, 99–112 (1985).
van Moorsel, G. W. M. Early maximum growth of stony corals (Scleractinia) after settlement on artificial substrata on a Caribbean reef. Mar. Ecol-Prog. Ser. 50, 127–135 (1988).
Vermeij, M. J. A. & Bak, R. P. M. How are coral populations structured by light? Marine light regimes and the distribution of Madracis. Mar. Ecol-Prog. Ser. 233, 105–116 (2002).
Maragos, J. E. & Jokiel, P. L. Reef corals of Johnston Atoll: one of the world's most isolated reefs. Coral Reefs 4, 141–150 (1986).
Kahng, S. E. & Maragos, J. E. The deepest, zooxanthellate scleractinian corals in the world? Coral Reefs 25, 254–254 (2006).
Englebert, N., Bongaerts, P., Muir, P., Hay, K. B. & Hoegh-Guldberg, O. Deepest zooxanthellate corals of the Great Barrier Reef and Coral Sea. Mar. Biodiv. - in press (2014).
Bak, R. P. M., Nieuwland, G. & Meesters, E. H. Coral reef crisis in deep and shallow reefs: 30 years of constancy and change in reefs of Curacao and Bonaire. Coral Reefs 24, 475–479 (2005).
Jokiel, P. L. & Coles, S. L. Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar. Biol. 43, 201–208 (1977).
NOAA Coral Reef Watch. 2000, updated twice-weekly. NOAA Coral Reef Watch 50-km Satellite Virtual Station Time Series Data for Curacao and Aruba. Data set accessed 2014-09-01 at http://coralreefwatch.noaa.gov/satellite/vs/index.html.
Kobluk, D. R. & Lysenko, M. A. Ring” Bleaching in Southern Caribbean Agaricia agaricites During Rapid Water Cooling. B. Mar. Sci. 54, 142–150 (1994).
Kahng, S. E. Temperature related depth limits of warm-water corals. Proc. 12th Int. Coral Reef Symp. 9C (2012).
Leichter, J. J., Deane, G. B. & Stokes, M. D. Spatial and temporal variability of internal wave forcing on a coral reef. J. Phys. Oceanogr. 35, 1945–1962 (2005).
Veron, J. E. N. & Stafford-Smith, M. Corals Of The World (Australian Institute of Marine Science, Townsville, 2000).
Abràmoff, M. D., Magalhães, P. J. & Ram, S. J. Image processing with ImageJ. Biophot. Int. 11, 36–43 (2004).
Concepcion, G. T., Medina, M. & Toonen, R. J. Noncoding mitochondrial loci for corals. Mol. Ecol. Notes 6, 1208–1211 (2006).
Terraneo, T. I. et al. Pachyseris inattesa sp. n. (Cnidaria, Anthozoa, Scleractinia): a new reef coral species from the Red Sea and its phylogenetic relationships. Zookeys 433, 1–30 (2014).
Flot, J. -F., Magalon, H., Cruaud, C., Couloux, A. & Tillier, S. Patterns of genetic structure among Hawaiian corals of the genus Pocillopora yield clusters of individuals that are compatible with morphology. Cr. Biol. 331, 239–247 (2008).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
LaJeunesse, T. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141, 387–400 (2002).
Bongaerts, P. et al. Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLoS One 5, e10871 (2010).
Sampayo, E. M., Franceschinis, L., Hoegh-Guldberg, O. & Dove, S. Niche partitioning of closely related symbiotic dinoflagellates. Mol. Ecol. 16, 3721–3733 (2007).
Pochon, X., Putnam, H. M., Burki, F. & Gates, R. D. Identifying and characterizing alternative molecular markers for the symbiotic and free-living dinoflagellate genus Symbiodinium. PLoS One 7, e29816 (2012).
Peakall, R. & Smouse, P. E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research - an update. Bioinformatics 28, 2537–2539 (2012).
Acknowledgements
We thank Adriaan “Dutch” Schrier, Laureen Schenk and the submersible team at Substation Curaçao: Barbara van Bebber, Bruce Brandt, Tico Christiaan, Rob Loendersloot, Barry Brown, Manuel Jove and Joe Oliver. We would also like to thank Margaux “Margootje" Carmichael, Bettina “Supernixe” Glasl and Sonja “Sonnchen” Schweinhammer for their help with the transplantation experiment. The study is “Ocean Heritage Foundation/Curaçao Sea Aquarium/Substation Curaçao” (OHF/CSA/SC) contribution #11. Submersible dives were funded by “The Explorers Club - Eddie Bauer Grant for Expeditions”. The study was part of the “Catlin Seaview Survey” funded by Catlin Group Limited, with support from the Global Change Institute.
Author information
Authors and Affiliations
Contributions
P.B., P.R.F., K.B.H. and N.E. conceived the idea and research expedition and processed all samples. P.B., P.R.F., K.B.H., N.E. and M.J.H.V. conducted the transplantation experiment. K.L. and P.B. conducted the molecular work and analyses. P.B., P.R.F., R.P.M.B. and M.J.H.V. interpreted the data. P.B. and O.H.G. conceived the funding. P.B. wrote the main text and prepared figures and all other authors reviewed and edited the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Electronic Supplementary Material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Bongaerts, P., Frade, P., Hay, K. et al. Deep down on a Caribbean reef: lower mesophotic depths harbor a specialized coral-endosymbiont community. Sci Rep 5, 7652 (2015). https://doi.org/10.1038/srep07652
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07652
This article is cited by
-
Calcification rates in the lower photic zone and their ecological implications
Coral Reefs (2023)
-
Differing lesion recovery rates of two Caribbean stony coral species across a shallow water to mesophotic depth gradient suggest different sensitivity to repeated disturbance
Coral Reefs (2023)
-
Styles and rates of mesophotic reef accretion on a Caribbean insular slope
Coral Reefs (2023)
-
Breviolum and Cladocopium Are Dominant Among Symbiodiniaceae of the Coral Holobiont Madracis decactis
Microbial Ecology (2022)
-
Scleractinian diversity in the upper mesophotic zone of Ludao (Taiwan): a museum collection with new records from Taiwanese waters
Marine Biodiversity (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.