Abstract
While Src plays crucial roles in shear stress-induced cellular processes, little is known on the spatiotemporal pattern of high shear stress (HSS)-induced Src activation. HSS (65 dyn/cm2) was applied on bovine aortic endothelial cells to visualize the dynamic Src activation at subcellular levels utilizing a membrane-targeted Src biosensor (Kras-Src) based on fluorescence resonance energy transfer (FRET). A polarized Src activation was observed with higher activity at the side facing the flow, which was enhanced by a cytochalasin D-mediated disruption of actin filaments but inhibited by a benzyl alcohol-mediated enhancement of membrane fluidity. Further experiments revealed that HSS decreased RhoA activity, with a constitutively active RhoA mutant inhibiting while a negative RhoA mutant enhancing the HSS-induced Src polarity. Cytochalasin D can restore the polarity in cells expressing the active RhoA mutant. Further results indicate that HSS stimulates FAK activation with a spatial polarity similar to Src. The inhibition of Src by PP1, as well as the perturbation of RhoA activity and membrane fluidity, can block this HSS-induced FAK polarity. These results indicate that the HSS-induced Src and subsequently FAK polarity depends on the coordination between intracellular tension distribution regulated by RhoA, its related actin structures and the plasma membrane fluidity.
Similar content being viewed by others
Introduction
Src is a 60-kDa non-receptor kinase consisting of a Myristylation site (M), Src Homology (SH) domains, a catalytic domain, a unique domain and a negative regulatory tyrosine residue. When integrin is activated, it can associate with Src via the SH3 domain, thus unmasking the Src kinase domain and activating Src1,2,3. The activated Src affects integrin–cytoskeleton interface to cause dissolution of actin stress fibers and the release of mechanical tensile stress4, which ultimately regulates cell spreading and migration5. Src can also bind to active focal adhesion kinase (FAK) at tyrosine 397 through its SH2 domain to cause further phosphorylation of FAK6. The Src-FAK complex can stimulate Rac1 activation through the recruitment and phosphorylation of the scaffolding protein p130Cas7. This complex can also phosphorylate paxillin and subsequently regulate small GTPases Cdc42 and Rac1, following integrin ligation8.
Shear stress has been shown to activate many signaling proteins in vascular cells9,10,11, including Src and FAK12,13. 10 or 12 dyn/cm2 of fluid shear stress for 60 minutes caused a significant increase in the phosphorylation of Src on Tyr416 in human endothelial cells (ECs), a residue in the enzymatic activation loop reflecting the kinase activation14,15 and also increased the tyrosine phosphorylation and the kinase activity of FAK in a rapid and transient manner in bovine aortic endothelial cells (BAECs)13. This shear stress-induced Src activation may be mediated by the binding of PECAM-1, since PECAM-1 can bind to Src via its cytoplasmic domain and no activation of Src family kinases could be observed upon shear stress application in PECAM-1−/− endothelial cells16. The shear stress-induced Src activation may result in the activation of various signaling pathways and events such as caveolin-1 tyrosine phosphorylation15, MAPK pathways and transcription activities involving AP-1/TRE and Elk-1/SRE in ECs12, while the shear stress-activated FAK plays critical roles in dual activation of ERK and JNK13.
Upon continuous laminar shear stress application, ECs will change the alignment of actin filaments and microtubules to cause the alteration of cell shape and directional migration17,18. This process appears to be regulated by the Rac1-mediated signaling19, supported by the evidence that Rac1 was activated to promote the lamellipodia formation at the downstream side of the cell along the flow direction20. The small GTPase Cdc42 may also be involved in this polarization process as Cdc42 activity was polarized in the direction of flow observed by a biosensor based on fluorescence resonance energy transfer (FRET). This localized activation of Cdc42 can then establish and maintain the polarity by promoting PAR6/PKC-dependent reorientation of the microtubule organizing center (MTOC) in the direction of flow21. Therefore, the cell polarity upon shear stress stimulation may be based on the spatially restricted activation of signal proteins such as Rac and Cdc42. Since Src can phosphorylate p130Cas to regulate both Rac1 and Cdc427, Src activity and its subcellular distribution may play an important role in regulating the shear stress induced cell polarity. However, the spatial distribution of shear stress-induced Src activation remains unclear.
While relatively understudied, high shear stress (HSS) can occur under various pathophysiological conditions such as in compensatory flows inside of collateral arteries (65–85 dyn/cm2) where local arterial blockage occurs22. HSS can also have significant impact on angiogenesis and atherosclerosis in collateral arteries near the bifurcation and high curvature regions23,24,25,26. We have recently reported that HSS can induce intracellular Ca2+ increase in two well-coordinated phases mediated by extracellular calcium influx and ER calcium release27. In the present study, we investigated the HSS-induced Src activation at subcellular levels utilizing a membrane-bound Src FRET biosensor. Our results indicate that HSS stimulates a polarized Src activation, which is dependent on the RhoA-mediated actin cytoskeleton and the plasma membrane fluidity. This Src polarization further controls the polarized FAK activation upon HSS application.
Results
Laminar HSS causes Src activation and polarization
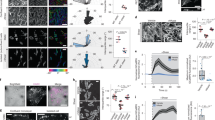
Since Src can be activated at the plasma membrane and plays a crucial role in mechanotransduction of endothelial cells upon mechanical force application4,28, we studied the spatiotemporal characteristics of Src activation under HSS with a membrane-bound Src FRET biosensor (Kras-Src)29, based on the defined polarity of a cell image (Supplemental Methods, supplemental Fig. 1). In BAECs, the Src activity measured by Kras-Src biosensor was observed to increase gradually upon the stimulation of 65 dyn/cm2 shear stress and have achieved a clear increase from baseline after 30 min of this HSS application, with distinct elevations found at different subcellular regions of the representative cell (Fig. 1A, Movie 1). The HSS-induced Src activation, averaged from multiple cells of each group, appeared in a polarized manner with a higher activity concentrated at the side facing the flow (upstream) (Fig. 1B). This HSS-induced FRET response can be eliminated if the biosensor SH2 domain is mutated to disable the FRET function but leave the fluorescent proteins intact (Src-RV)4 (Fig. 1C), or if the cells were pretreated with 50 μmol/l of a Src inhibitor PP1 as previous report27. These results suggest that the FRET responses observed with the wild-type biosensor are specifically due to the Src activation, but not to non-specific signals such as those originated from the morphological changes of the cells upon mechanical loading. No significant alternation in intensity of a GFP-fused Src kinase was observed upon HSS application (Fig. 1C), suggesting that the polarized Src activation induced by HSS is due to differentially localized enzymatic activation, but not to the translocation or local accumulation of Src kinase.
Laminar HSS caused a polarized Src activation and cell membrane fluidity.
(A) The representative ECFP/YPet emission ratio images (upper panel) and its time courses (lower panel) of the Kras-Src FRET biosensor to represent the Src activation in different cell areas under HSS as indicated. (B) Bar graphs (mean ± S.E.M.) represent the averaged values of YPet/ECFP emission ratios of the Kras-Src FRET biosensor in the upstream and downstream sides of BAECs upon 30 min of HSS application (n = 17). * represents a statistically significant P < 0.05 when comparing the two groups. (C) The representative YPet/ECFP emission ratio images of Src-RV mutant biosensor under HSS (upper panel) and the representative GFP intensity images of Src-GFP in pseudo color after 30 min of HSS application(lower panel). (D) Bar graphs (mean ± S.E.M.) represent the averaged values of the lateral diffusion coefficient of DiI in the plasma membrane after HSS application estimated by FRAP and FE-based diffusion model. * represents a statistically significant P < 0.05 when compared between upstream and downstream groups.
Cell membrane fluidity participates in HSS-induced Src activation and polarity
Src is activated at the plasma membrane and membrane fluidity has been reported to show a polarity upon shear stress application30. We hence examined whether the cell membrane and its fluidity play a role in regulating the polarized Src activation upon HSS stimulation. Indeed, BAECs under HSS also showed a polarized distribution of membrane fluidity, which is represented by the lateral diffusion rate of DiI as determined by fluorescence recovery after photobleaching (FRAP) and a diffusion model31,32. In 5–30 min after the application of HSS on BAECs, the diffusion coefficient at the upstream side (8.96 ± 2.20 μm2/sec) of the cell was significantly higher than that at the downstream side (1.20 ± 0.43 μm2/sec, Fig. 1D). BAECs expressing the Kras-Src biosensor were pretreated with 0.1 mmol/l of Cholesterol (Cho) for 3 hr or 45 mmol/l benzyl alcohol (BA) for 15 min to reduce or enhance the fluidity of cell membrane, respectively30. Cho had no significant effect on the overall Src activation level, nor the Src polarity upon HSS application (Fig. 2). While the BA treatment affected the overall basal Src activity slightly(Fig. 2B), it has no effect on the Src polarity before the HSS application. Interestingly, the BA treatment drastically inhibited the HSS-induced Src polarity (Fig. 2A and 2C, Movie 2). These results indicate that BAECs displayed polarized membrane fluidity under HSS and that cell membrane fluidity plays an important role in the HSS-induced Src polarity.
The HSS-induced Src polarity is regulated by the cytoskeleton and plasma membrane fluidity.
(A) The representative ECFP/YPet emission ratio images of Kras-Src biosensor in BAECs pre-treated for 1 hr with 2 μmol/l CytoD (n = 12), 5 μmol/l ML-7 (n = 10), 1 μmol/l Noco (n = 8), 3 hr with 0.1 mmol/l Cho (n = 9), or 15 min with 45 mmol/l BA (n = 6) before 30 min of HSS application. Bar graphs (mean ± S.E.M.) represent the averaged values of ECFP/YPet emission ratios measuring (B) the overall Src activation in the whole cell and (C) Src polarity (ratio of the averaged FRET signals from upstream and downstream sides) in BAECs with different pretreatments as indicated. * represents a statistically significant P < 0.05 when comparing to the control group.
Cytoskeleton participates in regulating the HSS-induced Src activation and polarity
Cytoskeleton has been shown to provide critical mechanical support for the plasma membrane33, serving as a primary integrator to sense shear stress and mediate mechanotransduction34,35. We hence further explored the role of cytoskeletal structure in mediating the HSS-induced Src polarity. BAECs expressing the Kras-Src biosensor were pretreated with 1 μmol/l of nocodazole (Noco) or 2 μmol/l of Cytochalasin D (CytoD) for 1 hr before HSS application. Significant suppression of the overall HSS-induced Src activation level was observed by either Noco or CytoD application, while the HSS-induced Src polarity were surprisingly enhanced by CytoD but not affected by Noco (Fig. 2, Movie 3). An inhibitor of myosin light chain kinase (MLCK) ML-7 had similar effects as those of Noco (Fig. 2). The actin-GFP transfected BAECs were also exposed to HSS and the decrease of the stress fibers can be observed within 30 min of flow application (Supplemental Fig. 2), consistent with previous observations that a rapid depolymerization of F-actin occurs upon shear stress36. These results suggest that the overall activation level of HSS-induced Src is dependent on the cytoskeletal integrity and intracellular acto-myosin contractility. However, the HSS-induced Src polarity is mainly mediated by actin cytoskeleton.
RhoA, but not Rac, abolishes the HSS-induced Src polarity
RhoA has been shown to play critical roles in regulating actin cytoskeleton and the translocation/activation of Src37,38. It has also been shown that RhoA activity changed prior to the activation of several other signaling molecules induced by shear stress39. We hence examined the effects of HSS on RhoA activity and its role in regulating the Src polarity. BAECs expressing a RhoA FRET biosensor were exposed to HSS. A decrease of RhoA activity can be clearly observed upon HSS application (Fig. 3A–C, Movie 4). This HSS-induced RhoA decrease was not suppressed by 50 μM PP1 pretreatment (Fig. 3B–C), indicating that RhoA may indeed act upstream to Src activation under flow. Consistently, an active RhoA mutant (V14-RhoA) blocked and a negative mutant (N19-RhoA) enhanced the HSS-induced Src polarity (Fig. 3C and 3E, Movie 5). The HSS-induced Src polarity was also blocked by pretreatment for 1 hr with 4 μM lysophosphatidic acid (LPA), a RhoA activator (Fig. 3C–E). Interestingly, neither V14-RhoA nor N19-RhoA affected the overall HSS-induced Src activation level (Fig. 3C–D). These results indicate that HSS may regulate the RhoA activity to control Src polarity, but not overall level of Src activation. Because RhoA has been shown to regulate the stress fibers formation, it may regulate the HSS-induced Src activation via actin stress fiber formation. Indeed, more F-actin was observed when the actin-GFP was co-transfected with V14-RhoA into BAECs (Supplemental Fig. 2), which is also consistent with previous reports40,41.
RhoA mediates the HSS-induced Src polarity, but not overall activation.
(A)The averaged time courses of representative YFP/CFP emission ratios of the RhoA FRET biosensor to represent the RhoA activity in different cell areas under HSS application (n = 17). (B) Bar graphs (mean ± S.E.M.) represent the averaged values of YFP/CFP emission ratios measuring the RhoA activity in the upstream and the downstream sides of BAECs pretreated with or without 50 μmol/l of PP1 before 30 min of HSS application (n = 14). (C) The representative YFP/CFP emission ratio images of (left panels) the RhoA FRET biosensors in BAECs pretreated with or without PP1, or (right panels) pretreated with 4 μmol/l of LPA (N = 7), Kras-Src FRET biosensors co-transfected with V14-RhoA (n = 11) or N19-RhoA (n = 11), or CytoD in V14-RhoA co-expressing BAECs (n = 4) as indicated. (D–E): Bar graphs (mean ± S.E.M.) represent the averaged values of YFP/CFP emission ratios measuring (D) the overall Src activation or (E) the Src polarity in BEACs with different pretreatments as indicated. * represents a statistically significant P < 0.05 when comparing to the control group; # represents a statistically significant P < 0.05 when comparing to the V14-RhoA co-transfected group.
The disruption of actin stress fibers with CytoD can restore the HSS-induced Src polarity in cells expressing V14-RhoA (Fig. 3C and 3E, Movie 6), although a minor suppression of the HSS-induced Src activation was observed (Fig. 3D). These results indicate that RhoA activity may play a pivotal role in regulating the HSS-induced Src polarity, possibly via the intracellular tension distribution based on the acto-myosin contractility.
Rac1 has been shown to affect PDGF-induced Src activation29. While a negative mutant of Rac1 (N17-Rac) suppressed the HSS-induced Src activation (Fig. 4A–B), neither N17-Rac nor an active mutant of Rac1 (V12-Rac) affected the HSS-induced Src polarity (Fig. 4), indicating that Rac1 does not mediate the HSS-induced Src polarity although it may affect the HSS-induced overall Src activation.
Rac1 does not mediate the HSS-induced Src polarity.
(A) The representative images of Kras-Src biosensor in BAECs co-transfected with V12-Rac (n = 8) or N17-Rac (n = 9). Bar graphs (mean ± S.E.M.) represent the averaged values of ECFP/YPet emission ratios measuring (B) the Src activity or (C) Src polarity in BAECs upon 30 min of HSS application with different pretreatments as indicated. *represents a statistically significant P < 0.05 when comparing to the control group.
Laminar HSS causes the FAK activation and polarity
Since Src can form a complex with and indeed recruit FAK to regulate downstream signaling cascades and cellular functions8, the HSS-induced FAK activity was also examined with a membrane-bound FAK FRET biosensor (Lyn-FAK). Averaged from multiple cells of each group, 5 min of HSS application can induce a significant increase of FAK activity with a clear polarity, higher at the upstream part of the cell (Fig. 5, Movie 7). In contrast, no significant change can be observed when the FAK biosensor was mutated to eliminate the FRET function or co-expressed with a FAK negative mutant FAK-related nonkinase (FRNK), or the FAK biosensor is mutated to disable the FRET function but leave the fluorescent proteins intact (FAK-YF) (Supplementary Fig. 3A). These results suggest that HSS can specifically induce a polarized FAK activation to cause the observed FRET signals. A FAK-GFP further revealed that HSS did not cause significant change of distribution in FAK intensity, suggesting that the FAK polarity induced by HSS is due to the differentially localized enzymatic activation of FAK, but not to the FAK translocation (Supplementary Fig. 3B).
Laminar HSS caused a polarized FAK activation.
(A) The representative ECFP/YPet emission ratio images (left panels), (B) averaged time courses and (C) bar graphs (mean ± S.E.M.) represent the averaged values of the Lyn-FAK FRET biosensor to represent the FAK activation in different cell areas upon 30 min of HSS application (n = 20). * represents a statistically significant P < 0.05 when comparing the upstream and downstream area at the same point.
HSS induces the FAK polarity through Src
When BAECs were pre-incubated with 50 μmol/l of the Src inhibitor PP1, no FAK polarity could be observed anymore upon HSS application although the overall FAK activation was surprisingly enhanced (Fig. 6, Movie 8). In contrast, neither a FAK inhibitor PF271 nor a negative mutant of FAK (FRNK) affected the HSS-induced Src polarity and the overall Src activation (Supplementary Fig. 4). These results indicated that Src plays a role upstream in regulating the HSS-induced FAK polarity.
The HSS-induced FAK polarity is mediated by Src, RhoA and membrane fluidity.
(A) The representative images of Lyn-FAK biosensor in BAECs co-transfected with V14-RhoA (n = 13), pre-treated for 15 min with 45 mmol/l BA (n = 5) or 1 hr with 50 μmol/l PP1 (n = 14) before 30 min of HSS application. (B–C) Bar graphs (mean ± S.E.M.) represent the averaged values of ECFP/YPet emission ratios measuring (B) the overall FAK activity or (C) the FAK polarity in BEACs with different pretreatments as indicated. * represents a statistically significant P < 0.05 when comparing to the control group.
RhoA and cell membrane fluidity also affect the HSS-induced FAK polarity
We then examined the role of RhoA and membrane fluidity in regulating the HSS-induced FAK polarity. Both V14-RhoA and BA can significantly inhibit the HSS-induced FAK polarity and overall FAK activation level (Fig. 6, Movie 9 and 10). These results indicate that RhoA and membrane fluidity can also regulate the HSS-induced FAK polarity by affecting the Src polarity.
Discussion
Our results indicate that HSS may create an asymmetric distribution of the cell membrane fluidity, higher at the upstream side of the cell. This membrane fluidity polarity can be coupled to the subcellular unevenness of the intracellular tension, which leads to a polarized Src activity distribution followed by that of FAK. RhoA activity and actin stress fibers may facilitate the homogeneous dissipation and distribution of intracellular tension, hence antagonizing the polarizing effect of shear stress on the distribution of Src and FAK activities (Fig. 7).
A hypothesized model of the mechanotransduction pathways involved in the HSS-induced Src/FAK polarity in BAECs.
The cell membrane fluidity can perceive the spatial distribution of mechanical cues to cause a polarized intracellular tension distribution with subcellular difference. This polarized intracellular tension may have caused the Src and subsequently FAK polarity. These polarities can be attenuated by RhoA activity and actin stress fibers.
With FRET technology, a rapid distal Src activation and a slower directional wave propagation of Src activation along the plasma membrane were observed by applying laser-tweezer traction on fibronectin-coated beads adhering to the cells. This Src activation is a dynamic process that directs signals via the cytoskeleton to spatial destinations4. Sungsoo Na et al also showed that the local stress induced rapid activation of Src at remote cytoplasmic sites, which is dependent on the cytoskeleton related intracellular tension28. These findings indicate that cytoskeleton and its related intracellular tension may play important roles in regulating the spatial localization of Src activation. Consistently, our current paper also showed that cytoskeleton disruption can inhibit the overall HSS-induced Src activation. It was initially surprising to us that the actin disruption enhanced the HSS-induced Src polarity. It has been shown that actin filaments can be coupled to the plasma membrane and adhesion sites via actinin and membrane proteins/receptors such as integrins42. F-actin system is also one of the key elements for maintaining the intracellular tension33. Actin filaments may hence perceive the mechanical loading on the plasma membrane and facilitate the rearrangement of the intracellular tension to distribute the external mechanical loading into the cells more uniformly at the global level. It makes sense then, when actin filaments are disrupted, the spatially heterogeneous mechanical loading cannot be sufficiently rebalanced and distributed across the whole cells. This may have caused the enhancement of the Src polarity when actin filaments were destroyed by CytoD.
RhoA can increase the stress fibers formation and is a key signaling molecule regulating cellular functions in cells40,41. Indeed, dominant negative mutants of RhoA and the inhibition of its downstream target Rho-kinase/ROCK have been shown to inhibit the shear stress–induced cell alignment and stress fiber formation in confluent BAECs43. Constitutively active mutant of RhoA also inhibited the shear stress–induced alignment of BAECs, indicating that a balanced level of RhoA activity is important for the regulation of stress fiber alignment and cell shape20,44. Shear stress can also cause the decrease of RhoA activity accompanied by actin de-polymerization in BAECs44. As such, shear stress may affect RhoA activity and subsequently stress fibers, which regulate the distribution of the intracellular tension to result in the polarized localization of Src activities. Indeed, the constitutively active V14-RhoA and the induction of stress fiber formation by LPA inhibited the HSS-induced Src polarity, which can be restored if stress fibers or intracellular tension are disrupted. Therefore, RhoA may serve as a master signaling molecule in regulating the HSS-induced Src polarity by controlling the stress fiber formation and mediating the subcellular distribution of the intracellular tension under mechanical stimulations.
It has been reported that the plasma membrane fluidity mediates the shear-induced modulation of signaling molecules such as MAPK30. Our results also indicate that the modulation of membrane fluidity by BA can inhibit the HSS-induced Src polarity. It is possible that the plasma membrane and its fluidity may perceive the spatial distribution of the mechanical loading and transmit it into the activation polarity of intracellular signaling molecules, such as Src. Fluid shear stress may cause cell deformation in the direction of flow, thus leading to temporally varying and spatially heterogeneous stresses in the cell membrane to increase the cell membrane fluidity in a polar manner31. The higher cell membrane fluidity at upstream under flow may allow more deformation and force transmission to the cytoskeleton than the downstream and helps to result in the polarized activation of Src. Therefore, the membrane fluidity may couple with RhoA/actin stress fibers to perceive the external mechanical loading and convert them into intracellular tension and signaling activities at subcellular levels.
The autophosphorylation of FAK at tyrosine 397 mediated by integrin can create a binding site for the SH2 domain of Src. The recruited Src can then phosphorylate other tyrosine residues in FAK and promote its maximal kinase activity6. It was reported that a hyper-osmotic stress stimulated FAK phosphorylation at Tyr-397 in a Src-independent manner, whereas the Tyr-577 phosphorylation of FAK can be blocked by the Src family kinase inhibitor PP245. It is hence possible that shear stress induces the initial activation of FAK independent of Src, but may involve Src to regulate the full activation of FAK. It is intriguing that the inhibition of Src by PP1 enhanced the overall FAK activation under HSS. While the Src inhibition may have triggered other signaling cascades to promote the FAK activation under HSS, the mechanism remains largely unclear. However, the Src activity is clearly involved in regulating the polarized distribution of FAK activity. Indeed, the inhibition of Src with PP1 blocked the HSS-induced FAK polarity. In contrast, the manipulation of FAK activity by FRNK or PF271 did not affect the HSS-induced Src polarity, suggesting a role of Src activity upstream of FAK polarity. Consistently, V14RhoA and BA, which inhibited the HSS-induced Src polarity, also suppressed the FAK polarity. Therefore, HSS regulates the FAK polarity by modulating the subcellular distribution of Src activity.
Rac1 has no effect on the HSS-induced Src polarity
Rac1 has been shown to play critical roles in regulating actin network via Arp2/346. Shear stress was also demonstrated to induce a polarized Rac1 activity distribution in endothelial cells20,39,47,48. However, Rac1 is apparently not involved in regulating the HSS-induced Src polarity as neither active or negative mutants of Rac1 had significant effect on the polarized distribution of Src activity. Therefore, the Rac1 and Src polarity under HSS stimulation may have different regulation mechanisms. This is also consistent with a recent report that Rac1 can be activated at the cell periphery under mechanical twisting, independent of Src activation49. Given that Rac1 could be activated at the downstream to promote the lamellipodia formation along the flow direction20, the HSS-induced polarity of Src-FAK complex may coordinate with Rac1 activation in regulating the cell shape and alignment along the flow.
Methods
BAECs transfected with various FRET biosensors or DNA plasmids were starved with 0.5% FBS of Dulbecco's Modified Eagle Medium(DMEM) for 24 hr before 65 dyn/cm2 of HSS application. All images were obtained only on isolated single cell by using Zeiss Axiovert inverted microscope equipped with FRET system. Time lapse fluorescence images were acquired and quantified by MetaFluor 6.2 software (Universal Imaging) and the data of FRET efficiency was analysis by Excel (Microsoft).
Additional details are available in the Supplement.
References
Arias-Salgado, E. G. et al. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A 100, 13298–13302 (2003).
Huveneers, S. et al. Integrin alpha v beta 3 controls activity and oncogenic potential of primed c-Src. Cancer Res 67, 2693–2700 (2007).
Takada, Y., Ye, X. & Simon, S. The integrins. Genome Biol 8, 215 (2007).
Wang, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005).
Felsenfeld, D. P., Schwartzberg, P. L., Venegas, A., Tse, R. & Sheetz, M. P. Selective regulation of integrin--cytoskeleton interactions by the tyrosine kinase Src. Nat Cell Biol 1, 200–206 (1999).
Mitra, S. K. & Schlaepfer, D. D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18, 516–523 (2006).
Chodniewicz, D. & Klemke, R. L. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta 1692, 63–76 (2004).
Huveneers, S. & Danen, E. H. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci 122, 1059–1069 (2009).
Chiu, J. J. et al. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-[alpha] in endothelial cells. Arterioscler Thromb Vasc Biol 24, 73–79 (2004).
Mowbray, A. L., Kang, D. H., Rhee, S. G., Kang, S. W. & Jo, H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem 283, 1622–1627 (2008).
Qin, X. et al. Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells. Cardiovasc Res 74, 506–514 (2007).
Jalali, S. et al. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol 18, 227–234 (1998).
Li, S. et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem 272, 30455–30462 (1997).
Helmke, S., Lohse, K., Mikule, K., Wood, M. R. & Pfenninger, K. H. SRC binding to the cytoskeleton, triggered by growth cone attachment to laminin, is protein tyrosine phosphatase-dependent. J Cell Sci 111 (Pt 16), 2465–2475 (1998).
Radel, C. & Rizzo, V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol 288, H936–945 (2005).
Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005).
Hsu, P. P. et al. Effects of flow patterns on endothelial cell migration into a zone of mechanical denudation. Biochem Biophys Res Commun 285, 751–759 (2001).
Braddock, M. et al. Fluid Shear Stress Modulation of Gene Expression in Endothelial Cells. News Physiol Sci 13, 241–246 (1998).
Li, S., Huang, N. F. & Hsu, S. Mechanotransduction in endothelial cell migration. J Cell Biochem 96, 1110–1126 (2005).
Tzima, E. et al. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. Embo J 21, 6791–6800 (2002).
Tzima, E., Kiosses, W. B., del Pozo, M. A. & Schwartz, M. A. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem 278, 31020–31023 (2003).
Sho, E. et al. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp Mol Pathol 73, 142–153 (2002).
Okano, M. & Yoshida, Y. Influence of shear stress on endothelial cell shapes and junction complexes at flow dividers of aortic bifurcations in cholesterol-fed rabbits. Front Med Biol Eng 5, 95–120 (1993).
Metaxa, E. et al. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 295, H736–742 (2008).
Meng, H. et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38, 1924–1931 (2007).
Meng, H. et al. A model system for mapping vascular responses to complex hemodynamics at arterial bifurcations in vivo. Neurosurgery 59, 1094–1100; discussion 1100–1091 (2006).
Liu, B., Lu, S., Zheng, S., Jiang, Z. & Wang, Y. Two distinct phases of calcium signalling under flow. Cardiovasc Res 91, 124–133 (2011).
Na, S. et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A 105, 6626–6631 (2008).
Ouyang, M., Sun, J., Chien, S. & Wang, Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci U S A 105, 14353–14358 (2008).
Butler, P. J., Tsou, T. C., Li, J. Y., Usami, S. & Chien, S. Rate sensitivity of shear-induced changes in the lateral diffusion of endothelial cell membrane lipids: a role for membrane perturbation in shear-induced MAPK activation. FASEB J 16, 216–218 (2002).
Butler, P. J., Norwich, G., Weinbaum, S. & Chien, S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol 280, C962–969 (2001).
Lu, S. et al. The spatiotemporal pattern of Src activation at lipid rafts revealed by diffusion-corrected FRET imaging. PLoS Comput Biol 4, e1000127 (2008).
Janmey, P. A. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev 78, 763–781 (1998).
Choi, C. K. & Helmke, B. P. Short-Term Shear Stress Induces Rapid Actin Dynamics in Living Endothelial Cells. Mol Cell Biomech 5, 247–258 (2008).
Davies, P. F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6, 16–26 (2009).
Osborn, E. A., Rabodzey, A., Dewey, C. F., Jr & Hartwig, J. H. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am J Physiol Cell Physiol 290, C444–452 (2006).
Fincham, V. J. et al. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J Cell Biol 135, 1551–1564 (1996).
Sandilands, E. et al. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell 7, 855–869 (2004).
Wojciak-Stothard, B. & Ridley, A. J. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol 161, 429–439 (2003).
Jaffe, A. B., Aspenstrom, P. & Hall, A. Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways. Mol Cell Biol 24, 1736–1746 (2004).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11, 633–643 (2010).
Laukaitis, C. M., Webb, D. J., Donais, K. & Horwitz, A. F. Differential dynamics of alpha 5 integrin, paxillin and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol 153, 1427–1440 (2001).
Li, S. et al. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest 103, 1141–1150 (1999).
Tzima, E., del Pozo, M. A., Shattil, S. J., Chien, S. & Schwartz, M. A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. Embo J 20, 4639–4647 (2001).
Lunn, J. A. & Rozengurt, E. Hyperosmotic stress induces rapid focal adhesion kinase phosphorylation at tyrosines 397 and 577. Role of Src family kinases and Rho family GTPases. J Biol Chem 279, 45266–45278 (2004).
Hall, A. & Lalli, G. Rho and Ras GTPases in axon growth, guidance and branching. Cold Spring Harb Perspect Biol 2, a001818 (2010).
Kraynov, V. S. C. C., Bokoch, G. M., Schwartz, M. A., Slabaugh, S. & Hahn, K. M. Localized Rac activation dynamics visualized in living cells. Science 290, 333–337 (2000).
Zaidel-Bar, R., Kam, Z. & Geiger, B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J Cell Sci 118, 3997–4007 (2005).
Poh, Y. C. et al. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One 4, e7886 (2009).
Acknowledgements
This work was supported in part by grants from National Institute of Health (NIH) HL098472, HL109142, HL121365, National Science Foundation (NSF) CBET0846429, CBET1344298, DMS1361421, National Natural Science Foundation of China (NSFC) 10972139, 31271014 and Natural Science Key Foundation Project of CQ in China (CSTC2012JJB0097).
Author information
Authors and Affiliations
Contributions
B.L. and Y.W. designed research; B.L., S.L., Y.H. and X.L. performed research and analyzed data; B.L., S.L., M.O. and Y.W. wrote the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental information
Supplementary Information
Movie 1
Supplementary Information
Movie 2
Supplementary Information
Movie 3
Supplementary Information
Movie 4
Supplementary Information
Movie 5
Supplementary Information
Movie 6
Supplementary Information
Movie 7
Supplementary Information
Movie 8
Supplementary Information
Movie 9
Supplementary Information
Movie 10
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Liu, B., Lu, S., Hu, Yl. et al. RhoA and Membrane Fluidity Mediates the Spatially Polarized Src/FAK Activation in Response to Shear Stress. Sci Rep 4, 7008 (2014). https://doi.org/10.1038/srep07008
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07008
This article is cited by
-
Matrix stiffness, endothelial dysfunction and atherosclerosis
Molecular Biology Reports (2023)
-
Cell membrane rupture: a novel test reveals significant variations among different brands of tissue culture flasks
BMC Research Notes (2021)
-
FRET biosensor allows spatio-temporal observation of shear stress-induced polar RhoGDIα activation
Communications Biology (2018)
-
Functional impairment triggered by altertoxin II (ATXII) in intestinal cells in vitro: cross-talk between cytotoxicity and mechanotransduction
Archives of Toxicology (2018)
-
FRET-based Visualization of PDGF Receptor Activation at Membrane Microdomains
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.